
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mirela Nicoleta Dinca | + 1668 word(s) | 1668 | 2020-09-10 08:48:02 | | | |
| 2 | Conner Chen | -75 word(s) | 1593 | 2020-09-17 05:25:51 | | |
Video Upload Options
Bioenergy represents energy from biomass and plays an important role in promoting renewable alternatives. LC biomass is one of the most generous renewable bioresources in nature containing lignin, cellulose, and hemicelluloses. Lignocellulosic materials are the best sources used for biofuel production, such as biogas, and include residues from agriculture and forests, energy crops, and municipal and food waste. According to the latest statistical report for biogas, in Europe, almost 72% of the feedstocks used in the anaerobic digestion (AD) process for biogas production come from the agricultural sector, such as energy crops, manure, and other agricultural residues. The main issue of using lignocellulosic (LC) biomass for the biogas production is biomass recalcitrance, which represents biomass resistance to chemical and biological breakdown.
1. Structure of Lignocellulosic Biomass
In order to produce biogas, we need substrate, the most important material used as food for methanogen microorganisms. Substrates are the organic feeding material for all AD applications [1]. The substrate used for biogas production can be of various types like crop residues, animal manure and slurries, organic waste from households, organic fraction of municipal solid waste, and organic waste from dairy production, food industries, and agro-industries. Furthermore, algal biomass, and micro and macroalgae, are being used for studies given their biogas yielding potential [1]. A substrate type that has not been mentioned until recently is represented by energy crops that in recent years have become more and more used for obtaining an alternative source of energy.
Co-digestion is another popular method, where two or more substrates are used in the AD process, with the purpose to balance the C/N ratio of the feedstock. An example for this process can be given by giant cane in combination with pig slurry, co-digested in a continuously stirred tank lab scale reactor [2]. Substrate properties influence the efficiency and stability of the anaerobic process used for obtaining biogas. Moreover, its composition affects the quality and the quantity of the gas obtained.
Since the desire and the necessity to reduce global warming, scientists have studied different types of alternative sources of energies than can be applied, thus mentioning that LC biomass can be of great interest due to the fact that it is the most abundant organic source (forest residues, industrial activities, agricultural activities, or energy crops) [3].
The LC biomass is composed from three major fractions—cellulose, hemicellulose, and lignin. The structure formed by these three major components is resistant to lignocellulosic material bioconversion and that is why a pretreatment process is used to disintegrate the composition in order to obtain a higher biogas production rate.
1.1. Lignocellulosic Biomass Composition
The two main carbohydrate polymers that can be found in the composition of LC biomass are, cellulose and hemicellulose, and lignin, but also other elements found in smaller percentages like other carbohydrates, ash, pectin, and proteins. Given the fact that lignocellulosic material is the principal component of plant cell walls, it is considered an abundant organic source in the world. The average amount of cellulose, hemicellulose, and lignin in most LC biomass is 30 to 60% cellulose, 20 to 40% hemicellulose, and 15 to 25% lignin, but these percentages vary for different materials [4].
There are also studies that give different percentage intervals for lignocellulose biomass composition, such as hemicellulose 10–40%, lignin 5–25%, and cellulose 40–80% [5].
1.2. Cellulose
As part of the lignocellulosic material, cellulose is a polymer, type polysaccharide of glucose disaccharide units, with cellobiose tightly connected by b-1, 4-glycoside bonds [6]. Cellulose is also the principal compound of plant cell walls, making it an abundant source of organic compound on earth [7]. Hydrogen bons link the molecules of cellulose with different orientations, leading to diverse levels of crystallinity, which has an important part in the biodegradation of cellulose, with a higher crystallinity leading to problems in the process [8].
1.3. Hemicellulose
Hemicellulose is branched heterogeneous polymer of diverse polysaccharides like pentoses, hexoses, and sugar acids [6]. Given its branched nature, it allows the formation of almost indestructible bonds with cellulose and lignin, increasing lignocellulosic material rigidity [9].
1.4. Lignin
The second abundant compound of LC biomass on earth is lignin. Lignin is made out of phenylpropane units. Lignin links cellulose and hemicellulose in order to form a solid three-dimensional structure of the plant cell wall [8].
1.5. Lignocellulose Structure
Looking at the structure of lignocellulosic matter, we can see that a skeleton is formed out of cellulose and by hemicellulose and lignin, similar to a matrix, and binding materials [10]. The high resistance of lignocellulose to biological degradation is linked to a high degree of refractory lignin presence [11]. An amorphous matrix material is formed by hemicellulose since it is non-covalently bonded to the external area of the surface of cellulose fibrils [12]. Even though it is the compound that has the weakest bond compound and it has a high chemical sensitivity, it plays a crucial role in strengthening the structure of lignocellulose. Lignin is a macromolecule that is crosslinked and relatively hydrophobic and aromatic, but it has a high resistance to biological degradation [13]. Cellulose is hydrophilic, because of the existence of groups R-OH, and internal hydrogen bonds, but not highly soluble in water because its large size [14]. To express better the composition of the lignocellulosic matter by high lightening the structure we added data that it is presented in Table 1. The chemical composition is mentioned for each structure cellulose, hemicellulose and lignin. Thus, the table below presents the composition of different lignocellulosic substrates.
Table 1. Chemical composition of different lignocellulosic substrates. (Data from—see reference column).
| Source | Cellulose | Hemicellulose | Lignin | References |
|---|---|---|---|---|
| Hardwood | 40–55 | 24–40 | 18–25 | [15] |
| Eucalyptus | 44.9 | 28.9 | 26.2 | [16] |
| Oak | 43.2 | 21.9 | 35.4 | [17] |
| Rubber wood | 39.56 | 28.42 | 27.58 | [18] |
| Softwood | ||||
| Pine | 45.6 | 24 | 26.8 | [17] |
| Japanese cedar | 52.7 | 13.8 | 33.5 | [16] |
| Grasses | ||||
| Bamboo | 46.5 | 18.8 | 25.7 | [19] |
| Bamboo leaves | 31.14 | 25.55 | 35.03 | [20] |
| Bamboo stem | 43.04 | 22.13 | 27.14 | [5] |
| Amur silver-grass | 42 | 30.15 | 7 | [21] |
| Natural hay | 44.9 | 31.4 | 12 | [22] |
| Hemp | 53.86 | 10.6 | 8.76 | [21] |
| Rye | 42.83 | 27.86 | 6.51 | [21] |
| Rye straw | 38 | 36.9 | 17.6 | [23] |
| Reed | 49.40 | 31.5 | 8.74 | [21] |
| Stalk of giant reed | 33.1 | 18.5 | 24.5 | [24] |
| Leaves of giant reed | 20.9 | 17.7 | 25.4 | [24] |
| Sunflower | 34.06 | 5.18 | 7.72 | [21] |
| Sunflower stalk | 31 | 15.6 | 29.2 | [24] |
| Silage | 39.27 | 25.96 | 9.02 | [21] |
| Agro-industrial waste | ||||
| Walnut shell | 23.3 | 20.4 | 53.5 | [22] |
| Groundnut shell | 37 | 18.7 | 28 | [25] |
| Pistachio shell | 15.2 | 38.2 | 29.4 | [25] |
| Almond shell | 27 | 30 | 36 | [26] |
| Pine nut shell | 31 | 25 | 38 | [26] |
| Hazelnut shell | 30 | 23 | 38 | [26] |
| Nut shells | 25–30 | 25–30 | 30–40 | [15] |
| Coconut coir | 44.2 | 22.1 | 32.8 | [25] |
| Cotton stalk | 67 | 16 | 13 | [27] |
| Hemp stalk | 52 | 25 | 17 | [27] |
| Acacia pruning | 49 | 13 | 32 | [27] |
| Sugarcane peel | 41.11 | 26.4 | 24.31 | [20] |
| Sugarcane | 25 | 17 | 12 | [28] |
| Rice husk | 40 | 16 | 26 | [29] |
| Barley straw | 35.4 | 28.7 | 13.1 | [30] |
| 37.5 | 25.3 | 26.1 | [23] | |
| Maize straw | 38.33 | 29.76 | 3.82 | [5] |
| Rice straw | 38.14 | 31.12 | 26.35 | [20] |
| 32 | 24 | 13 | [31] | |
| Wheat straw | 38.2 | 21.2 | 23.4 | [32] |
| 43.4 | 26.9 | 22.2 | [5] | |
| Corn stover | 43.97 | 28.94 | 21.82 | [20] |
| 37.5 | 22.4 | 17.6 | [33] | |
| Miscanthus | 36.3 | 22.16 | 22.55 | [5] |
| Switchgrass | 31–45 | 20–31 | 12–18 | [32] |
| Sugarcane | 25 | 17 | 12 | [28] |
| Sorghum straw | 26.93 | 32.57 | 10.16 | [5] |
| Willow sawdust | 35.6 | 21.5 | 28.7 | [5] |
AD is a well known method for converting organic materials into bioenergy, and its effectiveness and sustainability have been demonstrated by different research papers and industrial investigations [34]. The AD process uses microorganisms to degrade organic material and convert it to biogas. Degradation of lignocellulosic matter is dependent on enzymes produced by microorganisms. AD is separated in four main sequential steps: hydrolysis, acidogenesis, acetogenesis, and methanogenesis [35].
Even though AD is a proven method of converting organic matter into biomethane, the result was not the same in the case of LC biomass rigidity [9], due to the fact that LC biomass properties and composition inhibits the property of microorganisms and enzymes involved in AD to decompose the organicmaterial. This is because of lignin, which is like a rigid cover for cellulose and hemicellulose [36]. Multiple research papers have outlined the fact that greater lignin content in the organic fraction results in reduced biogas and methane yield [37]. Therefore, in order to have a productive AD process on lignocellulosic material, high in lignin content, different pretreatment steps are needed to expedite the hydrolysis of LC biomass, enhancing the biogas production.
To better illustrate the way LC biomass is structured, we can observe Figure 1. The molecules for lignin, cellulose, and hemicellulose are presented, as well as the three lignin monomers, coniferyl (G) alcohols, coumaryl (H) and sinapyl (S), and, with a detailed image of the crystalline cellulose.
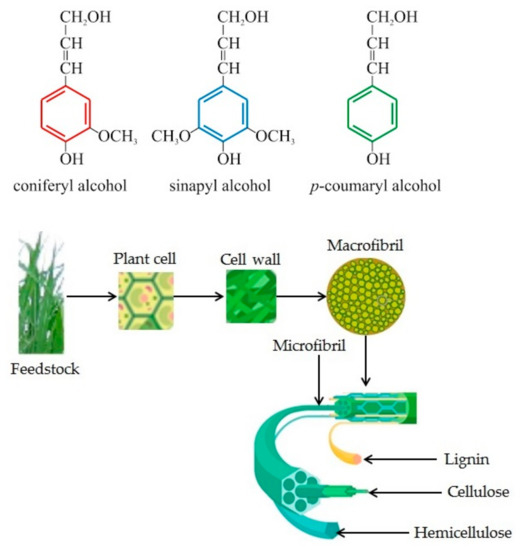
Figure 1. Lignocellulosic biomass substrate structure, representing plant structure and lignin, cellulose, hemicellulose molecule and carbohydrate polymers and aromatic polymers of lignocellulosic biomass (adapted from [38][39]).
Using enzymes in the LC biomass pretreatment is a common way to increase biogas yield, but its effect depends on the enzymes being used, and also the structure of the treated biomass, given enzyme specificity [40]. As an example, there is an enzyme called laccase, derived from phenoloxidase, which works by catalyzing phenol, anilines, and aromatic thiols oxidation in the feedstock, leading to microbial growth and fermentation ability being enhanced in the AD [41].
Efficiency of different enzymes for the AD process depend on several factors, including substrate composition, incubationperiod, temperature, pH levels, and reactor design [42].
Barley shell, pearl millet husk, rice straw, wheat bran, and wheat straw where subjected to a xylanases enzyme that was synthesized from Aspergillus niger. The results reported a saccharification of approximately 34.5% for rice straw after 8 h of incubation [43].
BP uses a variety of microorganisms and enzymes to selectively degrade lignin and hemicelluloses, resulting in enhanced biomass saccharification and biomass yield [44]. The best microorganisms for BP are the species of white-rot fungi from the Basidiomycetes class. Phanerochaete chrysosporium gives maximum efficiency due to its high growth rate and lignin degradation capabilities [45]. Microorganisms from the natural environment (Mcons), like soil, cow dung, goat dung, etc., are used for pretreating lignocellulosic material, having the ability to degrade both cellulose and hemicellulose.
Zhang et al. [46] created thermophilic Mcons from different sources, such as thermophilic landfill and decaying straw. This mixture was mixed with distillery wastewater in order to pretreat cassava waste at 55 °C for 12 h, resulting in a 96% higher methane yield.
Most pretreatment methods for lignocellulosic AD processes require high amounts of energy and chemical input in order to properly work, but they also create detrimental impacts [47]. Compared to these methods, BP is highly economical regarding energy and chemical requirements, and also does not produce inhibitory byproducts. BP is realized by the external addition of microorganisms and/or industrial and lignolytic enzymes and cellulase, to break down the lignocellulosic components [48]. Due to the fact that microbial enzymes are efficient in degrading lignocellulosic compounds, BP is known as one of the best ways of degrading this type of biomass, even though the enzymatic reaction rate is very slow [49].
References
- Zhang, Q.; Hu, J.; Lee, D.J. Biogas from anaerobic digestion processes: Research updates. Renew. Energy 2016, 98, 108–119, doi:10.1016/j.renene.2016.02.029.
- Luca, C.; Pilu, R.; Tambone, F.; Scaglia, B.; Adani, F. New energy crop giant cane (Arundo donax L.) can substitute traditional energy crops increasing biogas yield and reducing costs. Bioresour. Technol. 2015, 191, 197–204, doi:10.1016/j.biortech.2015.05.015.
- Frigon, J.C.; Mehta, P.; Guiot, S.R. Impact of mechanical, chemical and enzymatic pretreatments on the methane yield from the anaerobic digestion of switchgrass. Biomass Bioenerg. 2012, 36, 1–11, doi:10.1016/j.biombioe.2011. 02.013.
- Nanda, S.; Azargohar, R.; Dalai, A.K.; Kozinski, J.A. An assessment on the sustainability of lignocellulosic biomass for biorefining. Renew. Sustain. Energy Rev. 2015, 50, 925–941, doi:10.1016/j.rser.2015.05.058.
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721, doi:10.3390/app9183721.
- Hendriks, A.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18, doi:10.1016/j.biortech.2008.05.027.
- Moglia, E.S. Enzymatic Pre-treatment of Cellulose Rich Biomasses for Use in the Biogas Process; Swedish University of Agricultural Sciences: 2008; Uppsala, Sweden.
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53, doi:10.1016/j.pecs.2014.01.001.
- Hosseini, K.E.; Dahadha, S.; Bazyar, L.A.A.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review. J. Environ. Manag. 2019, 233, 774–784, doi:10.1016/j.jenvman.2018.09.106.
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech. 2015, 5, 337–353, doi:10.1007/s13205-014-0246-5.
- Gao, J.; Chen, L.; Yuan, K.; Huang, H.; Yan, Z. Ionic liquid pretreatment to enhance the anaerobic digestion of lignocellulosic biomass. Bioresour. Technol. 2013, 150, 352–358, doi:10.1016/j.biortech.2013.10.026.
- Brandt, A.; Gräsvik, J.; Halletta, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583, doi:10.1039/C2GC36364J.
- Kulkarni, M.B.; Ghanegaonkar, P.M. Pretreatment methods in anaerobic digestion for biogas generation: A review. Int. J. New Innov. Eng. Technol. 2015, 4, 14–18.
- Laureano-Perez, L.; Teymouri, F.; Alizadeh, H.; Dale, B.E. Understanding factors that limit enzymatic hydrolysis of biomass: Characterization of pretreated corn stover. Appl. Biochem. Biotechnol. 2005, 24, 1081–1099, doi:10.1385/ABAB:124:1–3:1081.
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476, doi:10.1016/j.rser.2011.11.035.
- Muranaka, Y.; Nakagawa, H.; Hasegawa, I.; Maki, T.; Hosokawa, J.; Ikuta, J.; Mae, K. Lignin-based resin production from lignocellulosic biomass combining acidic saccharification and acetone-water treatment. Chem. Eng. J. 2017, 308, 754–759, doi:10.1016/j.cej.2016.09.117.
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 2017, 191, 140–149, doi:10.1016/j.fuel.2016.11.057.
- Khan, A.S.; Man, Z.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Shah, F.U.; Muhammad, N. Efficient conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids. Carbohydr. Polym. 2017, 181, 208–214, doi:10.1016/j.carbpol.2017.10.064.
- Chen, W.; Chen, Y.; Yang, H.; Xia, M.; Li, K.; Chen, X.; Chen, H. Co-pyrolysis of lignocellulosic biomass and microalgae: Products characteristics and interaction effect. Bioresour. Technol. 2017, 245A, 860–868, doi:10.1016/j.biortech.2017.09.022.
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109, doi:10.1016/j.serj.2016.04.012.
- Raud, M.; Tutt, M.; Olt, J.; Kikas, T. Dependence of the hydrolysis efficiency on the lignin content in lignocellulosic material. Int. J. Hydrog. Energy 2016, 41, 16338–16343, doi:10.1016/j.ijhydene.2016.03.190.
- De Caprariis, B.; De Filippis, P.; Petrullo, A.; Scarsella, M. Hydrothermal liquefaction of biomass: Influence of temperature and biomass composition on the bio-oil production. Fuel 2017, 208, 618–625, doi:10.1016/j.fuel.2017.07.054.
- Monlau, F.; Barakat, A.; Trably, E.; Dumas, C.; Steyer, J.P.; Carrère, H. Lignocellulosic materials into biohydrogen and biomethane: Impact of structural features and pretreatment. Crit. Rev. Environ. Sci. Technol. 2013, 43, 260–322, doi:10.1080/10643 389.2011.604258.
- Monlau, F.; Barakat, A.; Steyer, J.P.; Carrere, H. Comparison of seven types of thermo-chemical pretreatments on the structural features and anaerobic digestion of sunflower stalks. Bioresour. Technol. 2012, 120, 241–247, doi:10.1016/j.biortech.2012.06.040.
- Subhedar, P.B.; Ray, P.; Gogate, P.R. Intensification of delignification and subsequent hydrolysis for the fermentable sugar production from lignocellulosic biomass using ultrasonic irradiation. Ultrason. Sonochem. 2017, 40, 140–150, doi:10.1016/j.ultsonch.2017.01.030.
- Álvarez, A.; Cachero, S.; González-Sánchez, C.; Montejo-Bernardo, J.; Pizarro, C.; Bueno, J.L. Novel method for holocellulose analysis of non-woody biomass wastes. Carbohydr. Polym. 2018, 189, 250–256, doi:10.1016/j.carbpol.2018.02.043.
- Kim, H.; Ahn, Y.; Kwak, S.-Y. Comparing the influence of acetate and chloride anions on the structure of ionic liquid pretreated lignocellulosic biomass. Biomass Bioenergy 2016, 93, 243–253, doi:10.1016/j.biombioe.2016.07.022.
- Karthikeyan, O.P.; Visvanathan, C. Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: A review. Rev. Environ. Sci. Bio. Technol. 2012, 12, 257–284, doi:10.1007/s11157-012-9304-9.
- Daza Serna, L.V.; Orrego Alzate, C.E.; Alzate, C.A.C. Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 113–120, doi:10.1016/j.biortech.2015.09.078.
- Liu, X.; Hiligsmann, S.; Gourdon, R.; Bayard, R. Anaerobic digestion of lignocellulosic biomasses pretreated with Ceriporiopsis subvermispora. J. Environ. Manag. 2017, 193, 154–162, doi:10.1016/j.jenvman.2017.01.075.
- Ye, J.; Li, D.; Sun, Y.;Wang, G.; Yuan, Z.; Zhen, F.; Wang, Y. Improved biogas production from rice straw by co-digestion with kitchen waste and pig manure. Waste Manag. 2013, 33, 2653–2658, doi:10.1016/j.wasman.2013.05.014.
- Brown, D.; Shi, J.; Li, Y. Comparison of solid-state to liquid anaerobic digestion of lignocellulosic feedstocks for biogas production. Bioresour. Technol. 2012, 124, 379–386, doi:10.1016/j.biort ech.2012.08.051.
- Li, Y.; Zhang, R.; He, Y.; Zhang, C.; Liu, X.; Chen, C.; Liu, G. Anaerobic co-digestion of chicken manure and corn stover in batch and continuously stirred tank reactor (CSTR). Bioresour. Technol. 2014, 156, 342–347, doi:10.1016/j.biortech.2014.01.054.
- Romano, R.T.; Zhang, R.; Teter, S.; McGarvey, J.A. The effect of enzyme addition on anaerobic digestion of Jose Tall Wheat Grass. Bioresour. Technol. 2009, 100, 4564–4571, doi:10.1016/j.biortech.2008.12.065.
- Appels, L.; Baeyens, J.; Degreve, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781, doi:10.1016/j.pecs.2008.06.002.
- Schroyen, M.; Vervaeren, H.; Vandepitte, H.; Van Hulle, S.W.; Raes, K.; Effect of enzymatic pretreatment of various lignocellulosic substrates on production of phenolic compounds and biomethane potential. Bioresour. Technol. 2015, 192, 696–702, doi:10.1016/j.biortech.2015.06.051.
- Teghammar, A.; Yngvesson, J.; Lundin, M.; Taherzadeh, M.J.; Horváth, I.S.; Pretreatment of paper tube residuals for improved biogas production. Bioresour. Technol. 2010, 101, 1206–1212, doi:10.1016/j.biortech.2009.09.029.
- Magalhães Jr., A.I.; de Carvalho, J.C.; de Melo Pereira, G.V.; Karp, S.G.; Câmara, M.C.; Medina, J.D.C.; Soccol, C. R-Lignocellulosic biomass from agro‐industrial residues in South America: Current developments and perspectives. Biofuels Bioprod. Biorefin. 2019, 13, 1505–1519. doi:10.1002/bbb.2048.
- Nunes, C.S.; Kunamneni, A. Laccases—properties and applications. In Enzymes in Human and Animal Nutrition; Academic Press, Elsevier, Amsterdam, Netherlands; 2018, pp. 133–161, doi:10.1016/ B978-0-12-805419- 2.00007-1.
- Michalska, K.; Bizukojć, M.; Ledakowicz, S. Pretreatment of energy crops with sodium hydroxide and cellulolytic enzymes to increase biogas production. Biomass Bioen. 2015, 80, 213–221, doi:10.1016/j.biombioe.2015.05.022.
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668, doi:10.1016/j.rser.2017.05.075.
- Parawira, W. Enzyme research and applications in biotechnological intensification of biogas production. Crit. Rev. Biotechnol. 2012, 32, 172–186, doi:10.3109/07388551.2011.595384.
- Bhardwaj, N.; Kumar, B.; Agarwal, K.; Chaturvedi, V.; Verma, P. Purification and characterization of a thermo-acid/alkali stable xylanases from Aspergillus oryzae LC1 and its application in Xylo-oligosaccharides production from lignocellulosic agricultural wastes. Int. J. Biol. Macrom. 2018, 122, 1191–1202, doi:10.1016/j.ijbiomac.2018.09.070.
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244, doi:10.1016/j.fuproc.2019.106244.
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass–An overview. Bioresour. Technol. 2016, 199, 76–82, doi:10.1016/j.biortech.2015.08.030.
- Zhang, Q.; He, J.; Tian, M.; Mao, Z.; Tang, L.; Zhang, J.; Zhang, H. Enhancement of methane production from cassava residues by biological pretreatment using a constructed microbial consortium. Bioresour. Technol. 2011, 102, 8899–8906, doi:10.1016/j.biortech.2011.06.061.
- Singh, P.; Suman, A.; Tiwari, P.; Arya, N.; Gaur, A.; Shrivastava, A.K. Biological pretreatment of sugarcane trash for its conversion to fermentable sugars. World J. Microbiol. Biotechnol. 2008, 24, 667–673. doi:10.1007/s11274-007-9522-4.
- Lopez, M.J.; Vargas-Garcia, M.D.; Suarez-Estrella, F.; Nichols, N.N.; Dien, B.S.; Moreno, J.J. Lignocellulose-degrading enzymes produced by the ascomycete Coniochaeta ligniaria and related species: Application for a lignocellulosic substrate treatment. Enzyme Microb. Technol. 2007, 40, 794–800, doi:10.1016/j.enzmictec.2006.06.012.
- Galbe, M.; Zacchi, G. Pretreatment of lignocellulosic materials for efficient bioethanol production. Biofuels 2007, 108, 41–65. doi:10.1007/10_2007_070.




