Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yu Chi Liu | + 1060 word(s) | 1060 | 2022-01-18 06:55:50 | | | |
| 2 | Catherine Yang | Meta information modification | 1060 | 2022-03-08 02:30:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, Y.C. Keratoconus Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/20295 (accessed on 07 February 2026).
Liu YC. Keratoconus Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/20295. Accessed February 07, 2026.
Liu, Yu Chi. "Keratoconus Disease" Encyclopedia, https://encyclopedia.pub/entry/20295 (accessed February 07, 2026).
Liu, Y.C. (2022, March 07). Keratoconus Disease. In Encyclopedia. https://encyclopedia.pub/entry/20295
Liu, Yu Chi. "Keratoconus Disease." Encyclopedia. Web. 07 March, 2022.
Copy Citation
Keratoconus is the most common primary corneal ectasia characterized by progressive focal thinning. Patients experience increased irregular astigmatism, decreased visual acuity and corneal sensitivity. Corneal collagen crosslinking (CXL), a minimally invasive procedure, is effective in halting disease progression.
keratoconus
corneal nerves
1. Introduction
Keratoconus is an ectatic condition of the cornea that is characterised by progressive thinning and steepening, causing significant visual morbidity. Reported prevalence ranges from 0.3 to 3300 per 100,000, depending on diagnostic criteria and geographic location [1]. The pathophysiology of keratoconus is multifactorial. Environmental (microtrauma), genetics, and biochemical factors play a role in disease [1]. Eye rubbing is one of the important environmental factors of keratoconus. Repetitive, prolonged and greater force of eye rubbing is associated with its progression [2]. Patient factors include atopy such as asthma and hay fever [3], and usage of contact lens wear [4][5]. As for genetic factors, alterations in Lysyl oxidase (LOX), Collagen Type V Alpha 1 Chain (COL5A1), and Forkhead box protein O1 (FOXO1) gene have been correlated to keratoconus pathogenesis [6][7][8]. Other studies have also shown that relatives of patients with keratoconus have a high prevalence of undiagnosed keratoconus [9][10]. In addition, biochemical factors such as increased protease activity cause collagen cross-linkages in the stroma to be broken down [11].
There has been much interest in corneal nerve structure, function and their role in corneal health and disease [12]. Corneal nerves beside their sensory function also secrete neuromediators that are vital to the development and maintenance of the cornea. It is hence important to understand the function and morphology of corneal nerves in diseased states. In keratoconus, attempts to understand corneal nerves were previously confined to ex vivo studies or cornea buttons with severe disease with staining techniques [13]. Most recently, the use of confocal microscopy in analysing keratoconic corneas have been instrumental in understanding the microstructural changes in vivo.
2. Corneal Nerve Function and Anatomy
The cornea is a highly innervated structure. Corneal nerves originate from the ophthalmic branch of the trigeminal nerve [14]. The main stromal nerve bundles enter the human cornea radially at the corneoscleral limbus at a distance of 293 ± 106 µm from the ocular surface and are distributed uniformly throughout the corneal circumference [15]. Soon after entering the cornea, each stromal nerve bundle gives rise through repetitive branching to varying numbers of progressively smaller and smaller stromal nerves that anastomose frequently, often at highly acute branch points, to form a moderately dense midstromal plexus. Most midstromal nerve fibres turn abruptly 90 degrees and continued into the narrow band of anterior stroma located immediately beneath bowman’s membrane, and gives rise to a dense, roughly two-dimensional, subepithelial plexus [16]. The subepithelial plexus has a characteristic plexiform appearance due to the anastomosis of tortuous nerve fibres, with it being denser in the peripheral and intermediate cornea than the central region. Straight fibres from the subepithelial plexus generally penetrate Bowman’s membrane and continued into the corneal epithelium, with other nerves becoming subbasal nerves that course parallel to the ocular surface near the interface of Bowman’s membrane and the basal epithelium (Figure 1). Subbasal nerves form a gentle spiral-like clockwise assemblage of long, curvilinear nerve fibres that converge on an imaginary center, or vortex, located inferior and slightly nasal to the corneal apex. This assembly is believed to be influenced by the electromagnetic fields of the eye [17]. They then form intraepithelial terminals that are distributed abundantly throughout the epithelium.
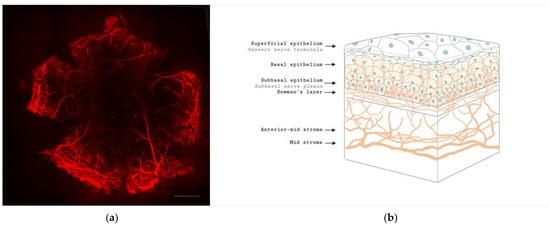
Figure 1. Anatomy of corneal nerves. (a) Whole mount staining with anti-class β III tubulin of mice cornea showing the distributions of corneal nerve. Scale bar: 500 μm. (b) Cross section of corneal nerves. (b) is created by Biorender.
Corneal nerves have afferent and efferent function, conveying touch and pain, as well as producing neuromediators such as neurotrophins and neuropeptides that is thought to play a role in its pathophysiology. These serve as trophic factors in ocular homeostasis and maintaining corneal microstructure. Corneal epithelial, stromal cells and endothelial cells also contribute to the diversity of neuromediators in the cornea by producing neurotrophins [18]. Neurotrophins, such as nerve growth factor (NGF), regulate neuronal development, survival, death and plasticity [12]. In keratoconus, the high affinity receptor of NGF, tyrosine kinase receptor A, was found in high levels and is thought to be due to heterologous upregulation for maintenance of unmyelinated corneal nerves [19]. Another neurotrophin, ciliary neurotrophic factor (CNTF) which is important for protection of the cornea from oxidative radical damage, had a higher expression of its mRNA in keratoconus as compared to normal eyes [19].
Neuropeptides are released slowly, act over an extended period, involved in neurotransmission and have a paracrine function. Calcitonin gene-related peptide (CGRP) plays an important role in the nociceptive pathway in the cornea, by activating factors such as bradykinin and stimulating the release of nitrous oxide [20]. These effects help produce a favorable neurochemical environment that enhances neural activity. Vasoactive intestinal peptide (VIP) is another important neuropeptide, playing a role in corneal wound healing [21] by exerting anti-inflammatory effects in a signaling pathway dependent manner [12][22]. Work by Sacchetti and colleagues analysed 12 keratoconic corneas obtained post keratoplasty and found that keratoconic corneas showed significantly higher CGRP and VIP levels as compared to controls. This increase is thought to be due to an attempt by sensory nerves to counteract degenerative changes in keratoconus [23].
3. Future Applications of IVCM in Keratoconus
IVCM images have been thought to be usable as a screening tool in patients with diabetic corneal neuropathy. Corneal nerve length and thickness have been reported to be early markers of eye involvement in patients with type 2 diabetes [24]. With the incorporation of deep learning techniques, artificial intelligence-based algorithm could provide rapid and good localisation performance for the quantification of corneal nerve biomarkers [25]. At this time of writing, there has not been any articles utilizing artificial intelligence techniques to analyse IVCM images in keratoconus. Although the prevalence of keratoconus is less than diabetes, we believe it could play a supplemental role to the armament of methods used to screen keratoconus.
The evaluation of subclinical or forme fruste keratoconus currently does not have any consensus. Although advances in corneal tomography and biomechanical assessments have made keratoconus diagnosis easier in the early stages, evaluation of these cases remain challenging [26]. Current evidence in the literature using IVCM images of corneal nerves taken from eyes with forme fruste keratoconus is limited. Larger study populations with well-defined inclusion criteria would possibly allow us to better understand nerve changes occurring in this subset of patients with very early keratoconus and possibly provide an opportunity for screening.
References
- Ferrari, G.; Rama, P. The keratoconus enigma: A review with emphasis on pathogenesis. Ocul. Surf. 2020, 18, 363–373.
- Sahebjada, S.; Al-Mahrouqi, H.H.; Moshegov, S.; Panchatcharam, S.M.; Chan, E.; Daniell, M.; Baird, P.N. Eye rubbing in the aetiology of keratoconus: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2057–2067.
- Sugar, J.; Macsai, M.S. What causes keratoconus? Cornea 2012, 31, 716–719.
- Macsai, M.S.; Varley, G.A.; Krachmer, J.H. Development of keratoconus after contact lens wear. Patient characteristics. Arch. Ophthalmol. 1990, 108, 534–538.
- Nauheim, J.S.; Perry, H.D. A clinicopathologic study of contact-lens-related keratoconus. Am. J. Ophthalmol. 1985, 100, 543–546.
- Ates, K.M.; Estes, A.J.; Liu, Y. Potential underlying genetic associations between keratoconus and diabetes mellitus. Adv. Ophthalmol. Pract. Res. 2021, 1, 100005.
- Dudakova, L.; Liskova, P.; Trojek, T.; Palos, M.; Kalasova, S.; Jirsova, K. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp. Eye Res. 2012, 104, 74–81.
- Lu, Y.; Vitart, V.; Burdon, K.P.; Khor, C.C.; Bykhovskaya, Y.; Mirshahi, A.; Hewitt, A.W.; Koehn, D.; Hysi, P.G.; Ramdas, W.D.; et al. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat. Genet. 2013, 45, 155–163.
- Karimian, F.; Aramesh, S.; Rabei, H.M.; Javadi, M.A.; Rafati, N. Topographic evaluation of relatives of patients with keratoconus. Cornea 2008, 27, 874–878.
- Kaya, V.; Utine, C.A.; Altunsoy, M.; Oral, D.; Yilmaz, O.F. Evaluation of corneal topography with Orbscan II in first-degree relatives of patients with keratoconus. Cornea 2008, 27, 531–534.
- Mackiewicz, Z.; Määttä, M.; Stenman, M.; Konttinen, L.; Tervo, T.; Konttinen, Y.T. Collagenolytic proteinases in keratoconus. Cornea 2006, 25, 603–610.
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762.
- Brookes, N.H.; Loh, I.P.; Clover, G.M.; Poole, C.A.; Sherwin, T. Involvement of corneal nerves in the progression of keratoconus. Exp. Eye Res. 2003, 77, 515–524.
- Al-Aqaba, M.A.; Fares, U.; Suleman, H.; Lowe, J.; Dua, H.S. Architecture and distribution of human corneal nerves. Br. J. Ophthalmol. 2010, 94, 784–789.
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492.
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542.
- Dua, H.S.; Gomes, J.A. Clinical course of hurricane keratopathy. Br. J. Ophthalmol. 2000, 84, 285–288.
- Lambiase, A.; Manni, L.; Bonini, S.; Rama, P.; Micera, A.; Aloe, L. Nerve growth factor promotes corneal healing: Structural, biochemical, and molecular analyses of rat and human corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1063–1069.
- Chung, E.S.; Lee, K.H.; Kim, M.; Chang, E.J.; Chung, T.Y.; Kim, E.K.; Lee, H.K. Expression of neurotrophic factors and their receptors in keratoconic cornea. Curr. Eye Res. 2013, 38, 743–750.
- Ceruti, S.; Villa, G.; Fumagalli, M.; Colombo, L.; Magni, G.; Zanardelli, M.; Fabbretti, E.; Verderio, C.; van den Maagdenberg, A.M.; Nistri, A.; et al. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 Knock-in mice: Implications for basic mechanisms of migraine pain. J. Neurosci. 2011, 31, 3638–3649.
- Liu, Y.-C.; Hin-FaiYam, G.; Tzu-YuLin, M.; EriciaTeo; Koh, S.-K.; LuDeng; LeiZhou; LouisTong; Mehta, J.S. Comparison of tear proteomic and neuromediator profiles changes between small incision lenticule extraction (SMILE) and femtosecond laser-assisted in-situ keratomileusis (LASIK). J. Adv. Res. 2021, 29, 67–81.
- Yang LW, Y.; Mehta, J.S.; Liu, Y.C. Corneal neuromediator profiles following laser refractive surgery. Neural Regen. Res. 2021, 16, 2177.
- Sacchetti, M.; Scorcia, V.; Lambiase, A.; Bonini, S. Preliminary evidence of neuropeptides involvement in keratoconus. Acta Ophthalmol. 2015, 93, e315–e316.
- dell’Omo, R.; Cifariello, F.; De Turris, S.; Romano, V.; Di Renzo, F.; Di Taranto, D.; Coclite, G.; Agnifili, L.; Mastropasqua, L.; Costagliola, C. Confocal microscopy of corneal nerve plexus as an early marker of eye involvement in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 142, 393–400.
- Williams, B.M.; Borroni, D.; Liu, R.; Zhao, Y.; Zhang, J.; Lim, J.; Ma, B.; Romano, V.; Qi, H.; Ferdousi, M.; et al. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: A development and validation study. Diabetologia 2020, 63, 419–430.
- Henriquez, M.A.; Hadid, M.; Izquierdo, L., Jr. A Systematic Review of Subclinical Keratoconus and Forme Fruste Keratoconus. J. Refract. Surg. 2020, 36, 270–279.
More
Information
Subjects:
Ophthalmology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
706
Revisions:
2 times
(View History)
Update Date:
08 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




