Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yu Chi Liu and Version 2 by Catherine Yang.
Keratoconus is the most common primary corneal ectasia characterized by progressive focal thinning. Patients experience increased irregular astigmatism, decreased visual acuity and corneal sensitivity. Corneal collagen crosslinking (CXL), a minimally invasive procedure, is effective in halting disease progression.
- keratoconus
- corneal nerves
1. Introduction
Keratoconus is an ectatic condition of the cornea that is characterised by progressive thinning and steepening, causing significant visual morbidity. Reported prevalence ranges from 0.3 to 3300 per 100,000, depending on diagnostic criteria and geographic location [1]. The pathophysiology of keratoconus is multifactorial. Environmental (microtrauma), genetics, and biochemical factors play a role in disease [1]. Eye rubbing is one of the important environmental factors of keratoconus. Repetitive, prolonged and greater force of eye rubbing is associated with its progression [2]. Patient factors include atopy such as asthma and hay fever [3], and usage of contact lens wear [4][5][4,5]. As for genetic factors, alterations in Lysyl oxidase (LOX), Collagen Type V Alpha 1 Chain (COL5A1), and Forkhead box protein O1 (FOXO1) gene have been correlated to keratoconus pathogenesis [6][7][8][6,7,8]. Other studies have also shown that relatives of patients with keratoconus have a high prevalence of undiagnosed keratoconus [9][10][9,10]. In addition, biochemical factors such as increased protease activity cause collagen cross-linkages in the stroma to be broken down [11].
There has been much interest in corneal nerve structure, function and their role in corneal health and disease [12]. Corneal nerves beside their sensory function also secrete neuromediators that are vital to the development and maintenance of the cornea. It is hence important to understand the function and morphology of corneal nerves in diseased states. In keratoconus, attempts to understand corneal nerves were previously confined to ex vivo studies or cornea buttons with severe disease with staining techniques [13]. Most recently, the use of confocal microscopy in analysing keratoconic corneas have been instrumental in understanding the microstructural changes in vivo.
2. Corneal Nerve Function and Anatomy
The cornea is a highly innervated structure. Corneal nerves originate from the ophthalmic branch of the trigeminal nerve [14][27]. The main stromal nerve bundles enter the human cornea radially at the corneoscleral limbus at a distance of 293 ± 106 µm from the ocular surface and are distributed uniformly throughout the corneal circumference [15][28]. Soon after entering the cornea, each stromal nerve bundle gives rise through repetitive branching to varying numbers of progressively smaller and smaller stromal nerves that anastomose frequently, often at highly acute branch points, to form a moderately dense midstromal plexus. Most midstromal nerve fibres turn abruptly 90 degrees and continued into the narrow band of anterior stroma located immediately beneath bowman’s membrane, and gives rise to a dense, roughly two-dimensional, subepithelial plexus [16][29]. The subepithelial plexus has a characteristic plexiform appearance due to the anastomosis of tortuous nerve fibres, with it being denser in the peripheral and intermediate cornea than the central region. Straight fibres from the subepithelial plexus generally penetrate Bowman’s membrane and continued into the corneal epithelium, with other nerves becoming subbasal nerves that course parallel to the ocular surface near the interface of Bowman’s membrane and the basal epithelium (Figure 1). Subbasal nerves form a gentle spiral-like clockwise assemblage of long, curvilinear nerve fibres that converge on an imaginary center, or vortex, located inferior and slightly nasal to the corneal apex. This assembly is believed to be influenced by the electromagnetic fields of the eye [17][30]. They then form intraepithelial terminals that are distributed abundantly throughout the epithelium.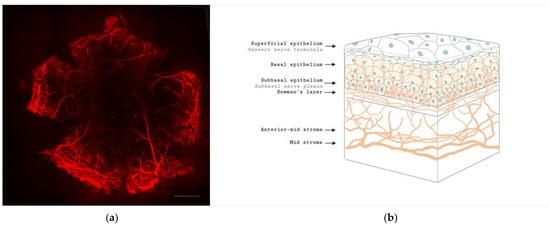
Figure 1. Anatomy of corneal nerves. (a) Whole mount staining with anti-class β III tubulin of mice cornea showing the distributions of corneal nerve. Scale bar: 500 μm. (b) Cross section of corneal nerves. (b) is created by Biorender.
