
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Khaled M. Aboshanab | + 8538 word(s) | 8538 | 2022-03-01 06:54:27 | | | |
| 2 | Beatrix Zheng | -4414 word(s) | 4124 | 2022-03-07 09:49:39 | | |
Video Upload Options
Staphylococcus aureus is a fatal Gram-positive pathogen threatening numerous cases of hospital-admitted patients worldwide. The emerging resistance of the pathogen to several antimicrobial agents has pressurized research to propose new strategies for combating antimicrobial resistance. Novel strategies include targeting the virulence factors of S. aureus. One of the most prominent virulence factors of S. aureus is its eponymous antioxidant pigment staphyloxanthin (STX), which is an auspicious target for anti-virulence therapy.
1. Naturally Occurring STX Inhibitors
1.1. Flavonoids
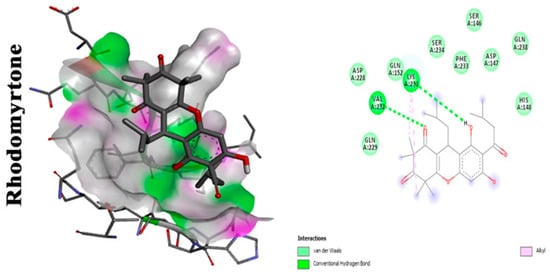
1.2. Rhodomyrtone
1.3. Marine Bioresource: Chitosan
1.4. Pogostemon heyneanus and Cinnamomum tamala Essential Oils
1.5. 2-Hydroxy-4-Methoxybenzaldehyde (HMB)
1.6. Myrtenol
1.7. Euphorbia tirucalli Latex
1.8. Schinus terebinthifolia Leaf Lectin
1.9. Callistemon citrinus Skeels
1.10. The Essential Oil of Eugenia brejoensis L. (Myrtaceae)
1.11. Ginkgo biloba Exocarp Extract
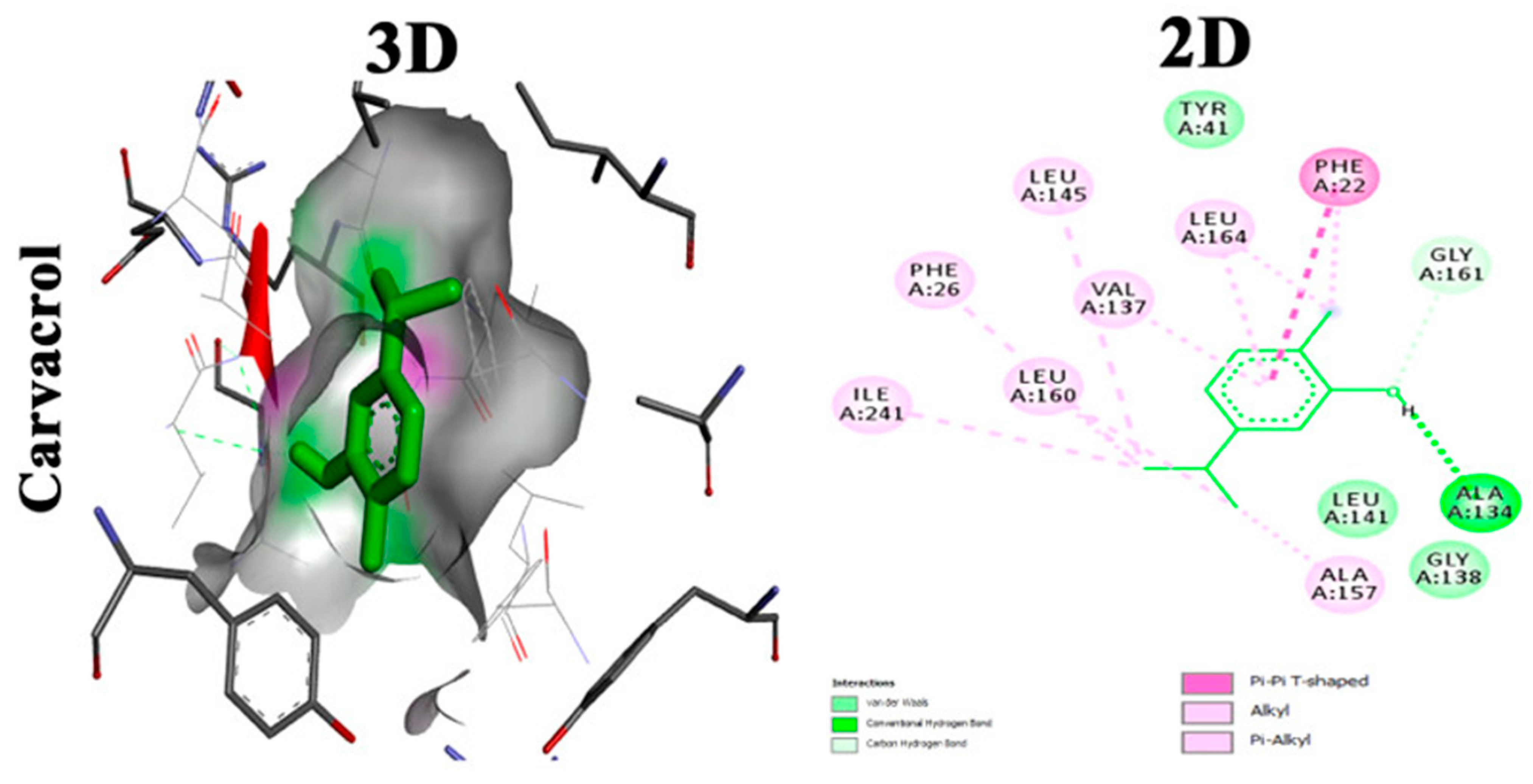
1.12. Carvacrol
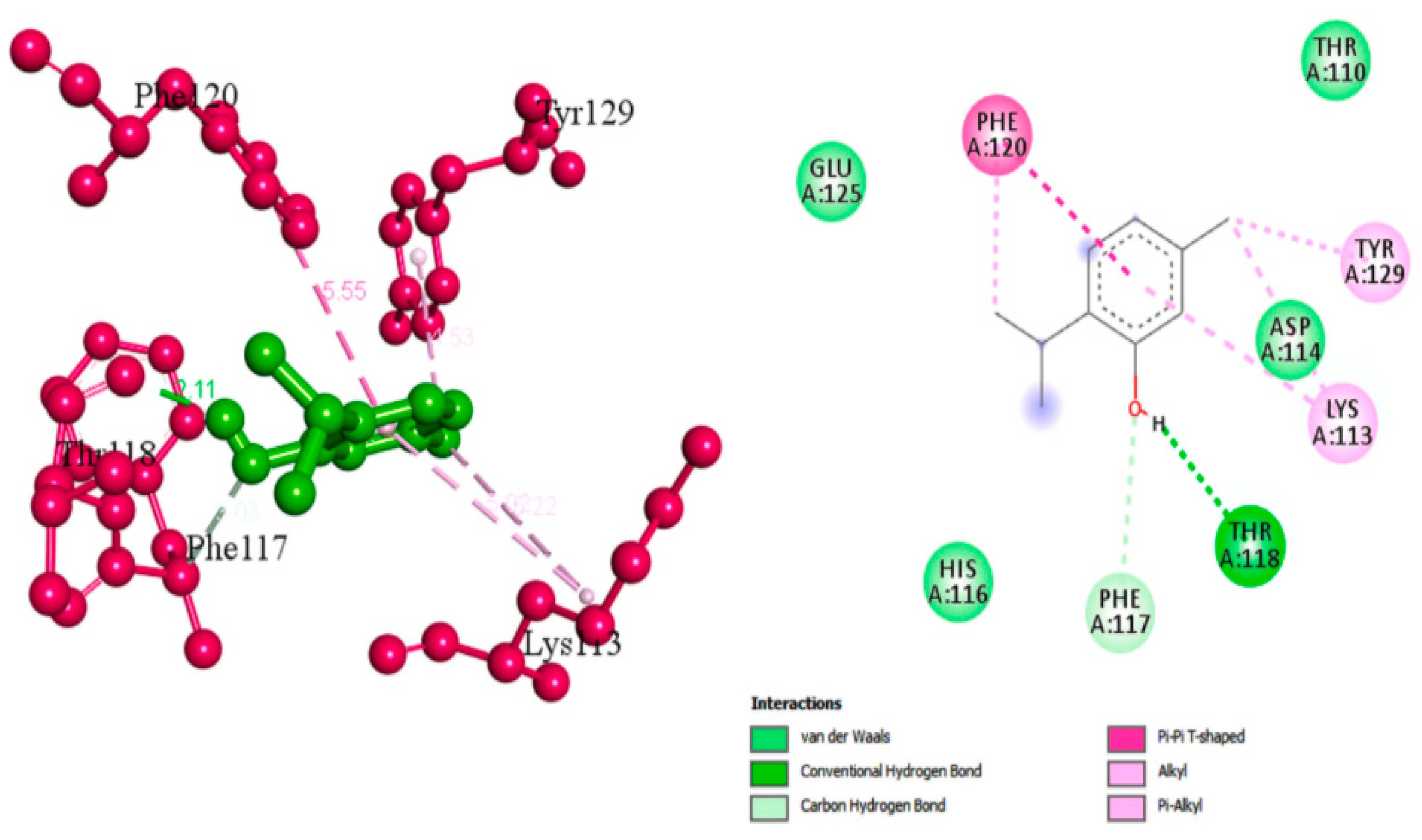
1.13. Thymol
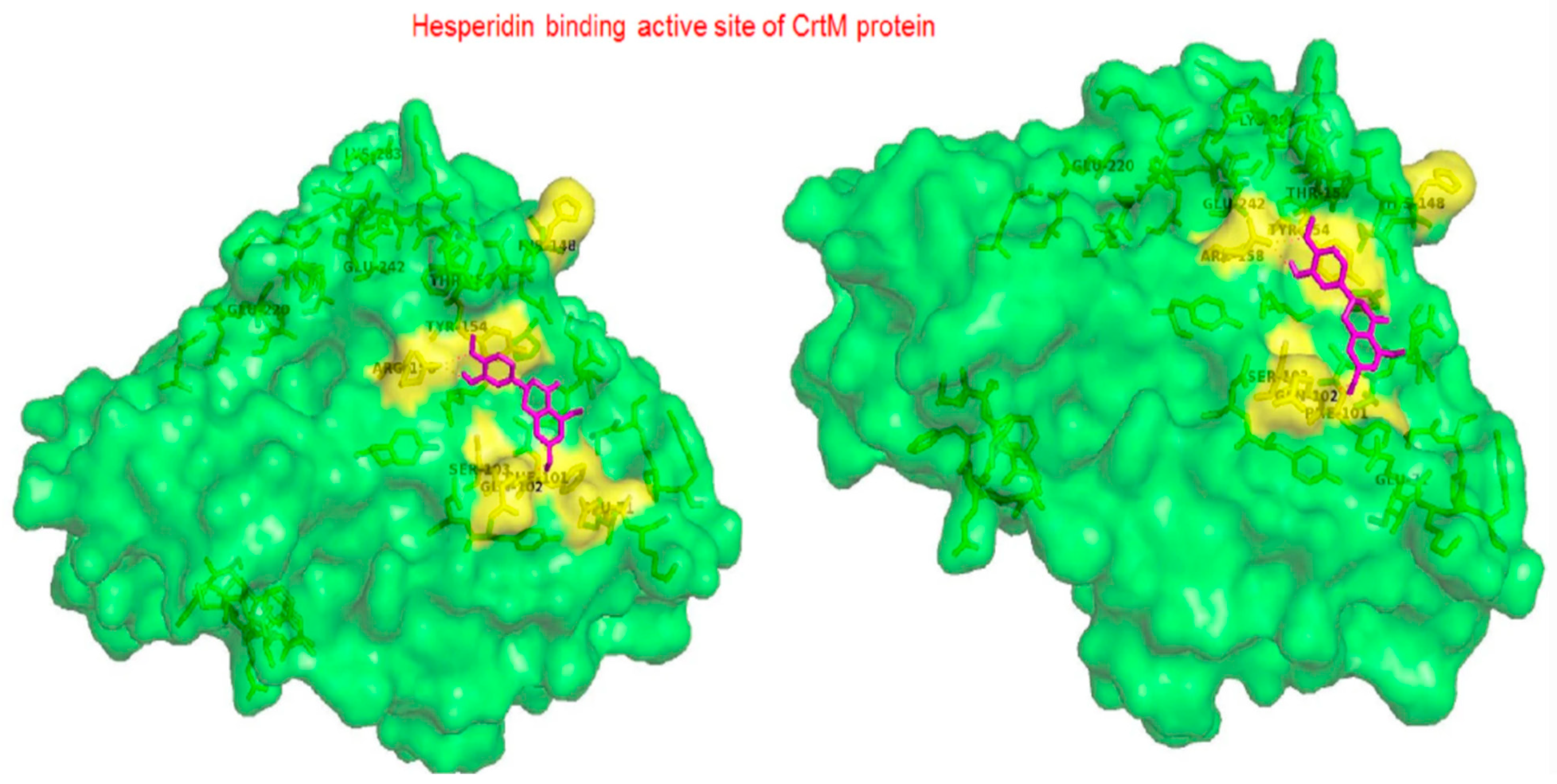
1.14. Hesperidin
2. Chemically Synthesized Inhibitors
2.1. Indole and Halogenated Indoles
2.2. Tetrangomycin Derivatives
2.3. Repurposing FDA-Approved Drugs
2.3.1. Cholesterol-Lowering Agents
In accordance with Oldfield, the catalysis of two farnesyl diphosphate (FPP) molecules into presqualene diphosphate by CrtM is considered the first basic step in the biosynthesis of staphyloxanthin by S. aureus. The resemblance in structure between CrtM and human squalene synthase (SQS), responsible for cholesterol biosynthesis in humans, aided the repurposing of some cholesterol-lowering agents into STX blockers (Figure 5). The utilization of cholesterol inhibitors as anti-virulence drugs has caused a significant inhibition to S. aureus virulence through directly inhibiting the carotenoid pigment production, rendering the treated strains vulnerable to the oxidative stress of human innate immunity and hence rapid clearance of the microorganism [38].
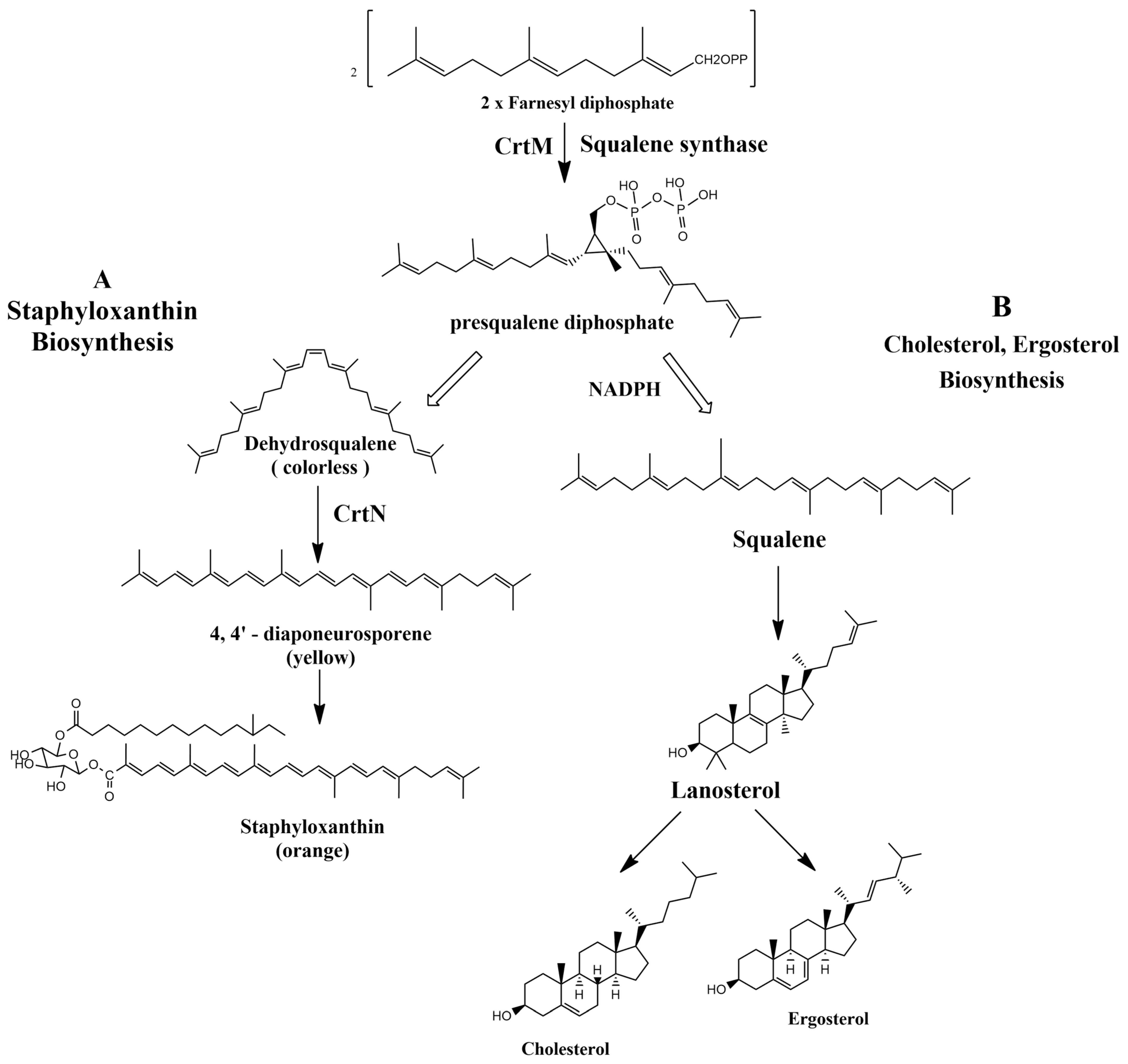
Figure 5. (A) Staphyloxanthin biosynthesis pathway in S. aureus. (B) Cholesterol and ergosterol biosynthesis pathway.
In 2008, a study conducted by Liu et al. suggested evident structural resemblance between S. aureus CrtM and human SQS. In attribution to the deduction, the analogy between the biosynthetic pathway of cholesterol in humans and that of STX production in S. aureus could be further studied. One cholesterol-lowering agent has been previously tested as a successful STX inhibitor that rendered S. aureus susceptible to oxidative stress of the human neutrophils in a mouse model after depigmenting the strain [39]. Later in 2009, Song et al. evaluated the possibility of inhibiting CrtM by potent phosphonosulfonates especially with halogen substitution and were able to prove their inhibitory effect on STX production with no effect against human squalene synthase [40].
Similarly, phosphonoacetamides were tested for STX inhibition in vitro and in a mouse infection model where a significant inhibition of disease progression was evident in the latter. X-ray crystallography revealed the most active compound to be N-3-(3-phenoxyphenyl) propylphosphonoacetamide [41].
With focus on cholesterol-lowering drugs, lapaquistat acetate and squalestatins are reported to inhibit SQS in humans and hence their cholesterol-lowering activity. Molecular docking analysis was performed to detect the mode of binding of lapaquistat acetate and squalestatin analogs to CrtM enzyme of S. aureus (Figure 6). Molecular docking confirmed the involvement of specific target sites on the CrtM enzyme when introduced to the respective SQS inhibitors. Among the most prominent target residues were His18, Arg45, Asp48, Asp52, Tyr129, Gln165, Asn168 and Asp172 [42].
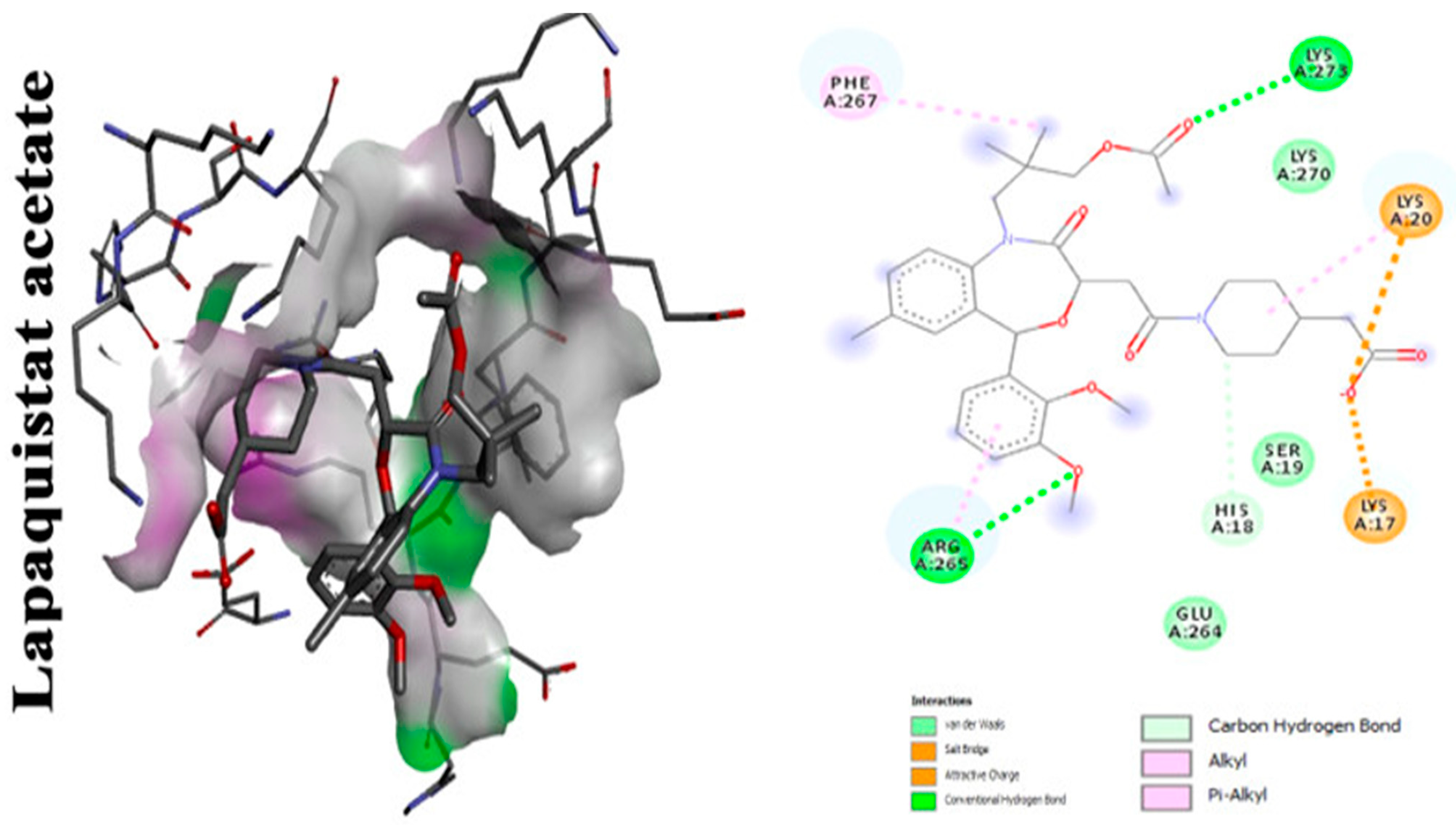
Figure 6. Molecular docking analysis showing 2D (on the right panel) and 3D (on the left panel) representation of interaction patterns of lapaquistat acetate with dehydrosqualene synthase receptor [6].
2.3.2. Glyceryl Trinitrate (GTN)
GTN is a well-known medication for the treatment of cardiovascular diseases. Not only does GTN provide renowned anti-angina effects, but in a recent study it was also evident that this drug is capable of STX inhibition, biofilm disruption and oxidative stress resistance in S. aureus strains. Regarding in silico studies, it is reported that GTN binds with high affinity to CrtM which explains its marked STX inhibition activity. Thus, GTN could be a promising antipathogenic candidate against S. aureus [43].
2.3.3. Diclofenac
Abbas et al. proposed that the renowned anti-inflammatory drug diclofenac possesses a notable anti-virulence effect against MRSA strains. The assumption was based on the discovery of the drug antipathogenic activity against Pseudomonas aeruginosa and Proteus mirabilis. In this work, diclofenac exerted an anti-STX production activity against MRSA clinical isolates at sub-MICs that reached 8–57.2% when compared to controls. Diclofenac treatment resulted also in decreased biofilm formation (22.67–70%) and noteworthy inhibition of hemolysin activity (5.4–66.34%). The phenotypic results were further confirmed by transcriptomic analysis using quantitative real time PCR that revealed marked downregulation of the previously tested virulence genes. Hence, diclofenac therapy along with other antimicrobials is recommended as an anti-virulence treatment against deleterious MRSA strains [44].
2.3.4. Domperidone
Domperidone, an FDA-approved antiemetic drug, was studied by El-Ganiny et al. to detect its potential anti-virulence activity against S. aureus. Significant inhibition of the carotenoid pigment of S. aureus was detected using sub-inhibitory concentrations of domperidone to reach 76.4–81.23% at 1/8 MIC (9.8 μg/mL) and 1/4 MIC (19.5 μg/mL), respectively. Furthermore, the inhibition of the biofilm formation using sub-inhibitory concentrations of domperidone reached 84.37% at 1/4 MIC and 80.16% at 1/8 MIC. Gene expression analysis using qRT-PCR further confirmed the phenotypic results revealing decreased expression levels of virulence genes such as CrtM, SigB, SarA, AgrA, hla, fnbA, and icaA by domperidone treatment [45].
2.3.5. Candesartan
Candesartan, a widely used drug in the treatment of high blood pressure, is now being re-studied for inherent anti-virulence characteristics against S. aureus. Candesartan’s ability to inhibit the antioxidant carotenoid pigment of S. aureus was evaluated using sub-inhibitory concentrations of the drug to yield pigment inhibition of 85.57% at 1/4 MIC (1.2 μg/mL) and 80.57% at 1/8 MIC (0.6 μg/mL), respectively. Furthermore, the inhibition of the biofilm formation using sub-inhibitory concentrations of domperidone reached 87.63% at 1/4 MIC and 71.5% at 1/8 MIC. Quantitative gene analysis revealed downregulation of virulence genes of S. aureus with the greatest inhibition activity against CrtM, sigB, sarA, agrA, hla and icaA genes [45].
2.3.6. Antifungal Agents
Feifei et al. reported that the antifungal naftifine exerted a potent STX inhibitory activity via competitive inhibition of CrtN enzyme when tested on MSSA cells. The drug was capable of inhibiting the carotenoid pigment without affecting the growth of MSSA cells in a dose-dependent manner (up to 0.2 mM ~64.8 μg/mL) [46]. Later in 2020, Jing et al. proposed the synergistic role of naftifine to photodynamic antimicrobial chemotherapy (PACT) against S. aureus. The aiding role of naftifine is believed to be due to its inhibitory activity to STX that scavenges the reactive oxygen species (ROS) generated by PACT. Hence, the notorious antifungal resulted in an enhanced PACT activity when incubated with S. aureus cells at a concentration of 10 μM [47].
A recent study carried by El-Ganiny et al. focused on miconazole, which was reported to exhibit anti-virulence effects when studied against S. aureus standard strain (well-characterized strain with defined susceptibility or resistance profiles to the antimicrobial agents tested). In that study, 1/4 MIC (18.75 μg/mL) and 1/8 MIC (9.4 μg/mL) of miconazole were used to inhibit multiple virulence characteristics of S. aureus. STX inhibition reached 76.43–83.93% upon treatment with the indicated sub-inhibitory concentrations of the drug. Furthermore, the inhibition of the biofilm formation using sub-inhibitory concentrations of domperidone reached 90% at 1/4 MIC and 86.84% at 1/8 MIC. Transcriptomic analysis using qT-PCR divulged the reduced expression of CrtM, SigB, SarA, AgrA, hla, FnbA, and IcaA [45].
2.4. Newly Discovered CrtN Inhibitors
2.4.1. 5 m Analog
Wang et al. have previously revealed that CrtN is a promising target for anti-virulence therapy. They have further disclosed the ability of the famous antifungal naftifine to drastically abolish the carotenoid pigment production in S. aureus species. The discovery of 5 m, a novel type of Benzofuran-derived CrtN inhibitor, has recently followed in the footsteps of the repurposing strategy of naftifine. As a result, the analogy has reflected a typical effect of the 5 m analog on S. aureus Newman and three other methicillin-resistant strains with low IC50 values ranging from 0.38–5.45 nM. The treated cells were rendered susceptible to immune clearance and their virulence was markedly weakened [48].
2.4.2. Compound NP16
A newly discovered compound termed NP16 has showed a potent activity as a CrtN inhibitor in S. aureus strains. Consequently, notable interruption to the golden carotenoid pigment biosynthesis was evident that further caused an increased vulnerability to oxidative stress and neutrophil killing in vivo [49].
2.4.3. 1,4-Benzodioxan-derivatives
In 2018, 38 1,4-benzodioxan-derived CrtN inhibitors were synthesized to combat the downsides of the leading compound 4a. Derivative 47 exhibited a remarkable CrtN inhibitory effect with higher potency than 4a (pigment inhibition in S. aureus Newman: IC50 = 270.4 ± 43.8 nM by compound 47 vs. IC50 = 1.9 nM by compound 4a) in addition to enhanced water solubility. The sensitization effect of derivative 47 on MRSA strains was reported to be quite significant and successfully facilitated immune clearance in vitro [50].
2.5. Others
Farnesol
Candida albicans and S. aureus are among the most commonly known opportunistic pathogens that usually co-exist in mixed biofilms [51]. Both pathogens are often isolated together from hospital-admitted patients [52]. In a recent study, the role of C. albicans-secreted quorum sensing (QS) molecule (farnesol) was assessed against S. aureus cells. The study mimicked a mixed biofilm of C. albicans with S. aureus cells by repetitive exposure of S. aureus to farnesol. The sensitized S. aureus cells revealed significant inhibition of STX. The findings of transcriptional analysis further displayed marked changes in the expression of global regulators involved in resistance to oxidative stress. Unfortunately, the activation of stress-response mechanisms in S. aureus boosted its tolerance to intracellular killing and ROS. The pigment inhibition effect was reasoned then by proposing a theoretical binding model that indicated the binding of farnesol to CrtM enzyme causing blockage of the biosynthetic pathway of STX due to its structural resemblance to the substrate of CrtM. Those findings illustrate the role of the fungal-secreted QS mediator that could successfully elicit oxidative stress on S. aureus through thiol-based redox system activation. Moreover, the results of the previous study reported that depigmentation mediated by STX inhibitors was considered a transient conditional state, as upon the gradual removal of farnesol, gradual recovery of the pigment was observed in comparison with the control cells [53].
References
- Lee, J.-H.; Park, J.-H.; Cho, M.H.; Lee, J.J.C.M. Flavone reduces the production of virulence factors, staphyloxanthin and α-hemolysin, in Staphylococcus aureus. Curr. Microbiol. 2012, 65, 726–732.
- Silva, L.N.; da Hora, G.; Soares, T.; Bojer, M.S.; Ingmer, H.; Macedo, A.J.; Trentin, D.S. Myricetin protects Galleria mellonella against Staphylococcus aureus infection and inhibits multiple virulence factors. Sci. Rep. 2017, 7, 2823.
- Galati, G.; O’brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303.
- Siriyong, T.; Ontong, J.C.; Leejae, S.; Suwalak, S.; Coote, P.J.; Voravuthikunchai, S.P. In vivo safety assessment of rhodomyrtone, a potent compound, from Rhodomyrtus tomentosa leaf extract. Toxicol. Rep. 2020, 7, 919–924.
- Leejae, S.; Hasap, L.; Voravuthikunchai, S.P. Inhibition of staphyloxanthin biosynthesis in Staphylococcus aureus by rhodomyrtone, a novel antibiotic candidate. J. Med. Microbiol. 2013, 62, 421–428.
- Selvaraj, A.; Valliammai, A.; Muthuramalingam, P.; Priya, A.; Suba, M.; Ramesh, M.; Karutha Pandian, S.J.A.O. Carvacrol targets SarA and CrtM of methicillin-resistant Staphylococcus aureus to mitigate biofilm formation and staphyloxanthin synthesis: An in vitro and in vivo approach. ACS Omega 2020, 5, 31100–31114.
- Younes, I.; Rinaudo, M.J.M.D. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174.
- Rubini, D.; Banu, S.F.; Hari, B.N.V.; Devi, D.R.; Gowrishankar, S.; Pandian, S.K.; Nithyanand, P.J.F.; Toxicology, C. Chitosan extracted from marine biowaste mitigates staphyloxanthin production and biofilms of Methicillin-resistant Staphylococcus aureus. Food Chem. Toxicol. 2018, 118, 733–744.
- Rubini, D.; Banu, S.F.; Nisha, P.; Murugan, R.; Thamotharan, S.; Percino, M.J.; Subramani, P.; Nithyanand, P.J.M.P. Essential oils from unexplored aromatic plants quench biofilm formation and virulence of Methicillin resistant Staphylococcus aureus. Microb. Pathog. 2018, 122, 162–173.
- Veer, V.; Singh, L.J.T.B. Field evaluation of repellency of a polyherbal essential oil against blackflies and its dermal toxicity using rat model. Trop. Biomed. 2012, 29, 391–397.
- Kannappan, A.; Srinivasan, R.; Nivetha, A.; Annapoorani, A.; Pandian, S.K.; Ravi, A.V. Anti-virulence potential of 2-hydroxy-4-methoxybenzaldehyde against methicillin-resistant Staphylococcus aureus and its clinical isolates. Appl. Microbiol. Biotechnol. 2019, 103, 6747–6758.
- Rathi, N.; Harwalkar, K.; Jayashree, V.; Sharma, A.; Rao, N.N. 2-hydroxy-4-methoxybenzaldehyde, an astounding food flavoring metabolite: A review. AJPCR 2017, 10, 105–110.
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Fernandez, M.J.F.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; et al. Scientific opinion on flavouring group evaluation 414 (FGE. 414): 2-hydroxy-4-methoxybenzaldehyde. EFSA 2021, 19, e06883.
- Bejeshk, M.; Fekri, M.S.; Najafipour, H.; Rostamzadeh, F.; Jafari, E.; Rajizadeh, M.; Masoumi-Ardakani, Y. Anti-inflammatory and anti-remodeling effects of myrtenol in the lungs of asthmatic rats: Histopathological and biochemical findings. Allergol. Immunopathol. 2019, 47, 185–193.
- Selvaraj, A.; Jayasree, T.; Valliammai, A.; Pandian, S. Myrtenol attenuates MRSA biofilm and virulence by suppressing sarA expression dynamism. Front. Microbiol. 2019, 10, 2027.
- Colasso, A.H.M.; Barros, T.F.; Figueiredo, I.F.; Carvalho, A.R., Jr.; Fernandes, E.S.; Uchoa, M.R.B.; da Silva, L.C.N. The latex of Euphorbia tirucalli inhibits staphyloxanthin production and protects Tenebrio molitor larvae against Staphylococcus aureus infection. Nat. Prod. Res. 2020, 34, 3536–3539.
- Mali, P.Y.; Panchal, S. Euphorbia tirucalli L.: Review on morphology, medicinal uses, phytochemistry and pharmacological activities. Asian Pac. J. Trop. Biomed. 2017, 7, 603–613.
- Lima, I.M.D.S.F.; Zagmignan, A.; Santos, D.M.; Maia, H.S.; Silva, L.D.S.; Cutrim, B.D.S.; Vieira, S.L.; Filho, C.M.B.; de Sousa, E.M.; Napoleão, T.H.; et al. Schinus terebinthifolia leaf lectin (SteLL) has anti-infective action and modulates the response of Staphylococcus aureus-infected macrophages. Sci. Rep. 2019, 9, 18159.
- Ramos, D.D.B.M.; Araújo, M.T.D.M.F.; de Lima Araújo, T.C.; dos Santos Neto, O.G.; de Silva, M.G.; Silva, Y.A.; Torres, D.J.L.; de Siqueira Patriota, L.L.; de Melo, C.M.L.; de Lorena, V.M.B.J.J.O.E. Evaluation of antitumor activity and toxicity of Schinus terebinthifolia leaf extract and lectin (SteLL) in sarcoma 180-bearing mice. J. Ethnopharmacol. 2019, 233, 148–157.
- Sutar, N.; Sutar, R.; Kumar, M. Callistemon citrinus (bottle brush) an important medicinal plant: A review of its traditional uses, phytoconstituents and pharmacological properties. Indian J. Pharm. Sci. 2014, 1, 68–77.
- Shehabeldine, A.M.; Ashour, R.M.; Okba, M.M.; Saber, F.R.J.J.O.E. Callistemon citrinus bioactive metabolites as new inhibitors of methicillin-resistant Staphylococcus aureus biofilm formation. J. Ethnopharmacol. 2020, 254, 112669.
- da Silva, A.G.; Alves, R.C.C.; Filho, C.M.B.; Bezerra-Silva, P.C.; Santos, L.M.M.D.; Foglio, M.A.; Navarro, D.M.D.A.F.; Silva, M.V.D.; Correia, M.T.D.S. Chemical composition and larvicidal activity of the essential oil from leaves of Eugenia brejoensis Mazine (Myrtaceae). J. Essent. Oil Bear. Plants 2015, 18, 1441–1447.
- Bezerra Filho, C.M.; da Silva, L.C.N.; da Silva, M.V.; Løbner-Olesen, A.; Struve, C.; Krogfelt, K.A.; Correia, M.T.D.S.; Vilela Oliva, M.L. Antimicrobial and Antivirulence Action of Eugenia brejoensis Essential Oil in vitro and in vivo Invertebrate Models. Front. Microbiol. 2020, 11, 424.
- Cao, C.; Su, Y.; Gao, Y.; Luo, C.; Yin, L.; Zhao, Y.; Chen, H.; Xu, A. Ginkgo biloba exocarp extract inhibits the metastasis of B16-F10 melanoma involving PI3K/akt/NF-κB/MMP-9 signaling pathway. Evid. Based Complement. Altern. Med. 2018, 2018, 4969028.
- Diamond, B.J.; Shiflett, S.C.; Feiwel, N.; Matheis, R.J.; Noskin, O.; Richards, J.A.; Schoenberger, N.E. Ginkgo biloba extract: Mechanisms and clinical indications. Arch. Phys. Med. Rehabil. 2000, 81, 668–678.
- Wang, B.; Wei, P.-W.; Wan, S.; Yao, Y.; Song, C.-R.; Song, P.-P.; Xu, G.-B.; Hu, Z.-Q.; Zeng, Z.; Wang, C.; et al. Ginkgo biloba exocarp extracts inhibit S. aureus and MRSA by disrupting biofilms and affecting gene expression. J. Ethnopharmacol. 2021, 271, 113895.
- Can Baser, K.J.C. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119.
- Ghorani, V.; Alavinezhad, A.; Rajabi, O.; Mohammadpour, A.H.; Boskabady, M.H. Safety and tolerability of carvacrol in healthy subjects: A phase I clinical study. Drug Chem. Toxicol. 2021, 44, 177–189.
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380.
- Valliammai, A.; Selvaraj, A.; Muthuramalingam, P.; Priya, A.; Ramesh, M.; Pandian, S.K. Staphyloxanthin inhibitory potential of thymol impairs antioxidant fitness, enhances neutrophil mediated killing and alters membrane fluidity of methicillin resistant Staphylococcus aureus. Biomed. Pharmacother. 2021, 141, 111933.
- Man, M.-Q.; Yang, B.; Elias, P.M. Benefits of hesperidin for cutaneous functions. Evid. Based Complement. Altern. Med. 2019, 2019, 2676307.
- Vijayakumar, K.; Muhilvannan, S.; Vignesh, M.A. Hesperidin inhibits biofilm formation, virulence and staphyloxanthin synthesis in methicillin resistant Staphylococcus aureus by targeting SarA and CrtM: An in vitro and in silico approach. World J. Microbiol. Biotechnol. 2022, 38, 44.
- Dhuguru, J.; Skouta, R.J.M. Role of indole scaffolds as pharmacophores in the development of anti-lung cancer agents. Molecules 2020, 25, 1615.
- Lee, J.-H.; Cho, H.S.; Kim, Y.; Kim, J.-A.; Banskota, S.; Cho, M.H.; Lee, J. Indole and 7-benzyloxyindole attenuate the virulence of Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2013, 97, 4543–4552.
- Lee, J.-H.; Kim, Y.-G.; Gwon, G.; Wood, T.K.; Lee, J. Halogenated indoles eradicate bacterial persister cells and biofilms. AMB Express 2016, 6, 123.
- Özakin, S.; Davis, R.W.; Umile, T.P.; Pirinccioglu, N.; Kizil, M.; Celik, G.; Sen, A.; Minbiole, K.P.; İnce, E. The isolation of tetrangomycin from terrestrial Streptomyces sp. CAH29: Evaluation of antioxidant, anticancer, and anti-MRSA activity. Med. Chem. Res. 2016, 25, 2872–2881.
- Ribeiro, L.; Fumagalli, F.; Mello, R.; Froes, T.; da Silva, M.; Gómez, S.V.; Barros, T.; Emery, F.; Castilho, M.J.M.P. Structure-activity relationships and mechanism of action of tetragomycin derivatives as inhibitors of Staphylococcus aureus staphyloxanthin biosynthesis. Microb. Pathog. 2020, 144, 104127.
- Oldfield, E. Targeting isoprenoid biosynthesis for drug discovery: Bench to bedside. Acc. Chem. Res 2010, 43, 1216–1226.
- Liu, C.-I.; Liu, G.Y.; Song, Y.; Yin, F.; Hensler, M.E.; Jeng, W.-Y.; Nizet, V.; Wang, A.H.-J.; Oldfield, E.J.S. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. J. Sci. 2008, 319, 1391–1394.
- Song, Y.; Lin, F.-Y.; Yin, F.; Hensler, M.; Poveda, C.A.R.; Mukkamala, D.; Cao, R.; Wang, H.; Morita, C.; Gonzalez-Pacanowska, D.; et al. Phosphonosulfonates are potent, selective inhibitors of dehydrosqualene synthase and staphyloxanthin biosynthesis in Staphylococcus aureus. J. Med. Chem. 2009, 52, 976–988.
- Song, Y.; Liu, C.-I.; Lin, F.-Y.; No, J.H.; Hensler, M.; Liu, Y.-L.; Jeng, W.-Y.; Low, J.; Liu, G.Y.; Nizet, V.; et al. Inhibition of staphyloxanthin virulence factor biosynthesis in Staphylococcus aureus: In vitro, in vivo, and crystallographic results. J. Med. Chem. 2009, 52, 3869–3880.
- Kahlon, A.K.; Roy, S.; Sharma, A. Dynamics. Molecular docking studies to map the binding site of squalene synthase inhibitors on dehydrosqualene synthase of Staphylococcus aureus. J. Biomol. Struct. Dyn. 2010, 28, 201–210.
- Abbas, H.A.; Elsherbini, A.M.; Shaldam, M. Glyceryl trinitrate blocks staphyloxanthin and biofilm formation in Staphylococcus aureus. Afr. Health Sci. 2019, 19, 1376–1384.
- Abbas, H.A.; Atallah, H.; El-Sayed, M.A.; El-Ganiny, A.M. Diclofenac mitigates virulence of multidrug-resistant Staphylococcus aureus. Arch. Microbiol. 2020, 202, 2751–2760.
- El-Ganiny, A.M.; Gad, A.I.; El-Sayed, M.A.; Shaldam, M.A.; Abbas, H.A. The promising anti-virulence activity of candesartan, domperidone, and miconazole on Staphylococcus aureus. Braz. J. Microbiol. 2021, 2021, 1–18.
- Chen, F.; Di, H.; Wang, Y.; Cao, Q.; Xu, B.; Zhang, X.; Yang, N.; Liu, G.; Yang, C.-G.; Xu, Y.; et al. Small-molecule targeting of a diapophytoene desaturase inhibits S. aureus virulence. Nat. Chem. Biol. 2016, 12, 174–179.
- Sun, J.; Zhang, Y.; Su, J.; Dai, T.; Chen, J.; Zhang, L.; Wang, H.; Liu, W.; Huang, M.; Chen, Z. Naftifine enhances photodynamic therapy against Staphylococcus aureus by inhibiting staphyloxanthin expression. Dye. Pigm. 2020, 179, 108392.
- Wang, Y.; Chen, F.; Di, H.; Xu, Y.; Xiao, Q.; Wang, X.; Wei, H.; Lu, Y.; Zhang, L.; Zhu, J.J.J.O.M.C. Discovery of potent benzofuran-derived diapophytoene desaturase (CrtN) inhibitors with enhanced oral bioavailability for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections. J. Med. Chem. 2016, 59, 3215–3230.
- Gao, P.; Davies, J.; Kao, R.Y.T. Dehydrosqualene desaturase as a novel target for anti-virulence therapy against Staphylococcus aureus. mBIO 2017, 8, e01217–e01224.
- Ni, S.; Li, B.; Chen, F.; Wei, H.; Mao, F.; Liu, Y.; Xu, Y.; Qiu, X.; Li, X.; Liu, J. Novel staphyloxanthin inhibitors with improved potency against multidrug resistant Staphylococcus aureus. ACS Med. Chem. Lett. 2018, 9, 233–237.
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Andes, D.; van Dijck, P.; Jabra-Rizk, M.A. Commensal protection of Staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. mBio 2016, 7, e01316–e01365.
- Morales, D.K.; Hogan, D.A.J.P.P. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 2010, 6, e1000886.
- Vila, T.; Kong, E.F.; Ibrahim, A.; Piepenbrink, K.; Shetty, A.C.; McCracken, C.; Bruno, V.; Jabra-Rizk, M.A. Candida albicans quorum-sensing molecule farnesol modulates staphyloxanthin production and activates the thiol-based oxidative-stress response in Staphylococcus aureus. Virulence 2019, 10, 625–642.




