
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Toshihiro Kita | + 8685 word(s) | 8685 | 2022-03-01 05:19:48 | | | |
| 2 | Rita Xu | -6074 word(s) | 2611 | 2022-03-04 09:19:25 | | | | |
| 3 | Rita Xu | Meta information modification | 2611 | 2022-03-07 05:15:47 | | |
Video Upload Options
The COVID-19 pandemic is still in progress, and a significant number of patients have presented with severe illness. Recently introduced vaccines, antiviral medicines, and antibody formulations can suppress COVID-19 symptoms and decrease the number of patients exhibiting severe disease. However, complete avoidance of severe COVID-19 has not been achieved and there are insufficient methods to treat it. Adrenomedullin (AM) is an endogenous peptide that maintains vascular tone and endothelial barrier function. The AM plasma level is markedly increased during severe inflammatory disorders, such as sepsis, pneumonia, and COVID-19, and associated with its prognosis. Exogenous AM administration reduced inflammation and related organ damage in rodent models. The results strongly suggest that AM could be an alternative therapy for COVID-19. Researchers are currently conducting an investigator-initiated phase 2a trial for moderate to severe COVID-19 using AM.
1. Introduction
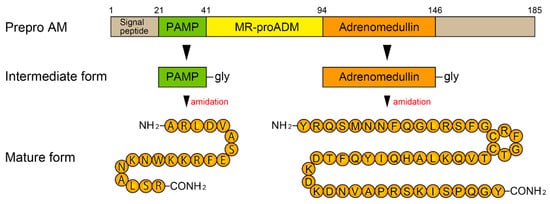
2. Biosynthesis of AM and Its Receptors
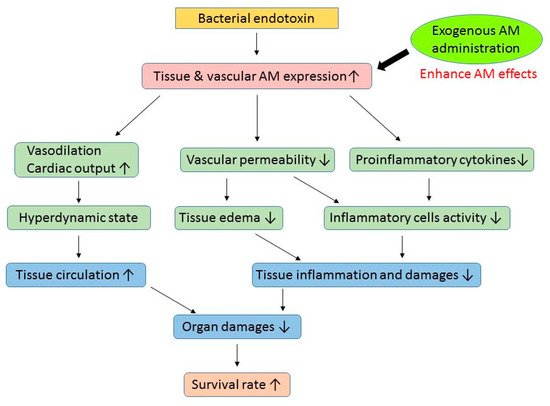
3. AM and Sepsis
| Genetic Intervention | |||
| Animal | Procedure | Results | Reference |
| Mouse | AM-deficient (+/−) + LPS-endotoxemia |
compared to WT mice · ↑ mortality · ↑ liver dysfunction |
[58] |
| Mouse | AM-deficient (+/−) + LPS-endotoxemia |
compared to WT mice · ↑ TNF-α, IL-1β · ↑ liver dysfunction |
[59] |
| Mouse | AM transgenic + LPS-endotoxemia |
compared to WT mice · ↓ BP decline · ↓ organ damage · ↑ survival rate |
[60] |
| Exogenous Adrenomedullin Administration | |||
| Animal | Procedure | Effects | Reference |
| Mouse | Pneumococcal pneumonia + Mechanical ventilation |
· ↓ VILI (pulmonary permeability↓) · ↓ liver and gut injury |
[25] |
| Rat | BDL + CLP (obstructive jaundice + polymicrobial sepsis) |
· ↓ tissue injury and inflammatory responses · ↑ survival rate |
[61] |
| Rat | Staphylococcus aureus α-toxin induced septic shock | · ↓ translocation of dextran from the gut into the systemic circulation | [62] |
| Rat | Cecal ligation and puncture (CLP) | · ↓ tissue injury · ↓ proinflammatory cytokine levels · ↓ intestinal-barrier dysfunction · ↑ survival rate |
[63] |
| Sheep | Endotoxin (LPS) infusion | · ↑ cardiac index · ↓ mean pulmonary artery pressure |
[64] |
| Rat | Endotoxin (LPS) injection | · ↑ PPER-γ level · ↓ TNF-α |
[65] |
| Rat | Intestinal ischemia/reperfusion | · ↓ lung injury · ↓ proinflammatory cytokines |
[66] |
| Rat | Staphylococcus aureus α-toxin induced septic shock | · ↓ vascular hyperpermeability · ↑ survival rate |
[67] |
| Rat | Intestinal ischemia/reperfusion | · ↓ inflammatory cytokines · ↓ tissue injury · ↑ survival rate |
[68] |
AM: adrenomedullin, LPS: lipopolysaccharide, WT: wild type. TNF: tumor necrosis factor, IF: interferon, BP: blood pressure. VILI: ventilator induced lung injury, BDL: common bile duct ligation. PPER: peroxisome proliferator-activated receptor.
4. Adrecizumab and Sepsis
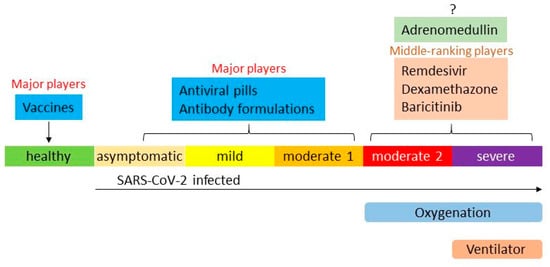
5. Overview of Therapies for COVID-19
References
- COVID-19 Dashboard by the Center for Systems Science and Engineering at Johns Hopkins University. Available online: https://coronavirus.jhu.edu/map.html (accessed on 5 January 2022).
- Mahmud, M.S.; Kamrujjaman, M.; Adan, M.M.Y.; Hossain, M.A.; Rahman, M.M.; Islam, M.S.; Mohebujjaman, M.; Molla, M.M. Vaccine Efficacy and SARS-CoV-2 Control in California and U.S. During the Session 2020–2026: A Modeling Study. Infect. Dis. Modell. 2022, 7, 62–81.
- Bernal, A.J.; da Silva, M.M.G.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Reyes, V.D.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2021. Online ahead of print.
- Mahase, E. COVID-19: Pfizer’s Paxlovid Is 89% Effective in Patients At Risk of Serious Illness, Company Reports. BMJ 2021, 375, n2713.
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus Etesevimab in Mild or Moderate COVID-19. N. Engl. J. Med. 2021, 385, 1382–1392.
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 229–237.
- Crichton, M.L.; Goeminne, P.C.; Tuand, K.; Vandendriessche, T.; Tonia, T.; Roche, N.; Chalmers, J.D.; European Respiratory Society. COVID-19 Task Force. The Impact of Therapeutics on Mortality in Hospitalised Patients with COVID-19: Systematic Review and Meta-Analyses Informing the European Respiratory Society Living Guideline. Eur. Respir. Rev. 2021, 30, 210171.
- ACTIV-3/TICO LY-CoV555 Study Group; Lundgren, J.D.; Grund, B.; Barkauskas, C.E.; Holland, T.L.; Gottlieb, R.L.; Sandkovsky, U.; Brown, S.M.; Knowlton, K.U.; Self, W.H.; et al. A Neutralizing Monoclonal Antibody for Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 905–914.
- Rahim, F.; Amin, S.; Noor, M.; Bahadur, S.; Gul, H.; Mahmood, A.; Usman, M.; Khan, M.A.; Ullah, R.; Shahab, K. Mortality of Patients with Severe COVID-19 in the Intensive Care Unit: An Observational Study from a Major COVID-19 Receiving Hospital. Cureus 2020, 12, e10906.
- Roedl, K.; Jarczak, D.; Thasler, L.; Bachmann, M.; Schulte, F.; Bein, B.; Weber, C.F.; Schäfer, U.; Veit, C.; Hauber, H.P.; et al. Mechanical Ventilation and Mortality Among 223 Critically Ill Patients with Coronavirus disease 2019: A Multicentric Study in Germany. Aust. Crit. Care. 2021, 34, 167–175.
- Christ-Crain, M.; Morgenthaler, N.G.; Struck, J.; Harbarth, S.; Bergmann, A.; Müller, B. Mid-Regional Pro-Adrenomedullin as a Prognostic Marker in Sepsis: An Observational Study. Crit. Care 2005, 9, R816–R824.
- Valenzuela Sanchez, F.; Valenzuela Méndez, B.; Bohollo de Austria, R.; Rodríguez Gutierrez, J.F.; Jaen Franco, M.; García, G.; Jareño Chaumel, A. Diagnostic and prognostic usefulness of mid-regional pro-adrenomedullin levels in patients with severe sepsis. Intensive Care Med. Exp. 2015, 3 (Suppl. 1), A306.
- Enguix-Armada, A.; Escobar-Conesa, R.; García-De La Torre, A.G.; De La Torre-Prados, M.V. Usefulness of Several Biomarkers in the Management of Septic Patients: C-Reactive Protein, Procalcitonin, Presepsin and Mid-Regional Pro-Adrenomedullin. Clin. Chem. Lab. Med. 2016, 54, 163–168.
- Andaluz-Ojeda, D.; Nguyen, H.B.; Meunier-Beillard, N.; Cicuéndez, R.; Quenot, J.P.; Calvo, D.; Dargent, A.; Zarca, E.; Andrés, C.; Nogales, L.; et al. Superior Accuracy of Mid-Regional Proadrenomedullin for Mortality Prediction in Sepsis with Varying Levels of Illness Severity. Ann. Intensive Care 2017, 7, 15.
- Charles, P.E.; Péju, E.; Dantec, A.; Bruyère, R.; Meunier-Beillard, N.; Dargent, A.; Prin, S.; Wilson, D.; Quenot, J.P. Mr-Proadm Elevation upon Icu Admission Predicts the Outcome of Septic Patients and Is Correlated with Upcoming Fluid Overload. Shock 2017, 48, 418–426.
- Elke, G.; Bloos, F.; Wilson, D.C.; Brunkhorst, F.M.; Briegel, J.; Reinhart, K.; Loeffler, M.; Kluge, S.; Nierhaus, A.; Jaschinski, U.; et al. The Use of Mid-Regional Proadrenomedullin to Identify Disease Severity and Treatment Response to Sepsis—A Secondary Analysis of a Large Randomised Controlled Trial. Crit. Care 2018, 22, 79.
- Spoto, S.; Fogolari, M.; De Florio, L.; Minieri, M.; Vicino, G.; Legramante, J.; Lia, M.S.; Terrinoni, A.; Caputo, D.; Costantino, S.; et al. Procalcitonin and MR-proAdrenomedullin Combination in the Etiological Diagnosis and Prognosis of Sepsis and Septic Shock. Microb. Pathog. 2019, 137, 103763.
- Spoto, S.; Nobile, E.; Carnà, E.P.R.; Fogolari, M.; Caputo, D.; De Florio, L.; Valeriani, E.; Benvenuto, D.; Costantino, S.; Ciccozzi, M.; et al. Best Diagnostic Accuracy of Sepsis Combining SIRS Criteria or qSOFA Score with Procalcitonin and Mid-Regional Pro-Adrenomedullin Outside ICU. Sci. Rep. 2020, 10, 16605.
- Marino, R.; Struck, J.; Maisel, A.S.; Magrini, L.; Bergmann, A.; Di Somma, S.; Adrenomedullin, P. Plasma Adrenomedullin Is Associated with Short-Term Mortality and Vasopressor Requirement in Patients Admitted with Sepsis. Crit. Care 2014, 18, R34.
- Chen, Y.X.; Li, C.S. Prognostic Value of Adrenomedullin in Septic Patients in the ED. Am. J. Emerg. Med. 2013, 31, 1017–1021.
- Guignant, C.; Voirin, N.; Venet, F.; Poitevin, F.; Malcus, C.; Bohé, J.; Lepape, A.; Monneret, G. Assessment of Provasopressin and Pro-Adrenomedullin as Predictors of 28-Day Mortality in Septic Shock Patients. Intensive Care Med. 2009, 35, 1859–1867.
- Caironi, P.; Latini, R.; Struck, J.; Hartmann, O.; Bergmann, A.; Maggio, G.; Cavana, M.; Tognoni, G.; Pesenti, A.; Gattinoni, L.; et al. Circulating Biologically Active Adrenomedullin (Bio-ADM) Predicts Hemodynamic Support Requirement and Mortality During Sepsis. Chest 2017, 152, 312–320.
- Mebazaa, A.; Geven, C.; Hollinger, A.; Wittebole, X.; Chousterman, B.G.; Blet, A.; Gayat, E.; Hartmann, O.; Scigalla, P.; Struck, J.; et al. Circulating Adrenomedullin Estimates Survival and Reversibility of Organ Failure in Sepsis: The Prospective Observational Multinational Adrenomedullin and Outcome in Sepsis and Septic Shock-1 (AdrenOSS-1) Study. Crit. Care 2018, 22, 354.
- Geven, C.; Kox, M.; Pickkers, P. Adrenomedullin and Adrenomedullin-Targeted Therapy as Treatment Strategies Relevant for Sepsis. Front. Immunol. 2018, 9, 292.
- Müller-Redetzky, H.C.; Will, D.; Hellwig, K.; Kummer, W.; Tschernig, T.; Pfeil, U.; Paddenberg, R.; Menger, M.D.; Kershaw, O.; Gruber, A.D.; et al. Mechanical Ventilation Drives Pneumococcal Pneumonia into Lung Injury and Sepsis in Mice: Protection by Adrenomedullin. Crit. Care 2014, 18, R73.
- van Oers, J.A.H.; Kluiters, Y.; Bons, J.A.P.; de Jongh, M.; Pouwels, S.; Ramnarain, D.; de Lange, D.W.; de Grooth, H.J.; Girbes, A.R.J. Endothelium-Associated Biomarkers Mid-Regional Proadrenomedullin and C-Terminal proendothelin-1 Have Good Ability to Predict 28-Day Mortality in Critically Ill Patients with SARS-CoV-2 Pneumonia: A Prospective Cohort Study. J. Crit. Care 2021, 66, 173–180.
- García de Guadiana-Romualdo, L.; Martínez Martínez, M.; Rodríguez Mulero, M.D.; Esteban-Torrella, P.; Hernández Olivo, M.; Alcaraz García, M.J.; Campos-Rodríguez, V.; Sancho-Rodríguez, N.; Galindo Martínez, M.; Alcaraz, A.; et al. Circulating MR-ProADM Levels, as an Indicator of Endothelial Dysfunction, for Early Risk Stratification of Mid-Term Mortality in COVID-19 Patients. Int. J. Infect. Dis. 2021, 111, 211–218.
- Zaninotto, M.; Mion, M.M.; Marchioro, L.; Padoan, A.; Plebani, M. Endothelial Dysfunction and Mid-Regional Proadrenomedullin: What Role in SARS-CoV-2 Infected Patients? Clin. Chim. Acta 2021, 523, 185–190.
- Lo Sasso, B.; Gambino, C.M.; Scichilone, N.; Giglio, R.V.; Bivona, G.; Scazzone, C.; Muratore, R.; Milano, S.; Barbagallo, M.; Agnello, L.; et al. Clinical Utility of Midregional Proadrenomedullin in Patients with COVID-19. Lab. Med. 2021, 52, 493–498.
- Roedl, K.; Jarczak, D.; Fischer, M.; Haddad, M.; Boenisch, O.; de Heer, G.; Burdelski, C.; Frings, D.; Sensen, B.; Karakas, M.; et al. MR-proAdrenomedullin as a Predictor of Renal Replacement Therapy in a Cohort of Critically Ill Patients with COVID-19. Biomarkers 2021, 26, 417–424.
- García de Guadiana-Romualdo, L.; Calvo Nieves, M.D.; Rodríguez Mulero, M.D.; Calcerrada Alises, I.; Hernández Olivo, M.; Trapiello Fernández, W.; González Morales, M.; Bolado Jiménez, C.; Albaladejo-Otón, M.D.; Fernández Ovalle, H.; et al. MR-ProADM as Marker of Endotheliitis Predicts COVID-19 Severity. Eur. J. Clin. Investig. 2021, 51, e13511.
- Spoto, S.; Agrò, F.E.; Sambuco, F.; Travaglino, F.; Valeriani, E.; Fogolari, M.; Mangiacapra, F.; Costantino, S.; Ciccozzi, M.; Angeletti, S. High Value of Mid-Regional Proadrenomedullin in COVID-19: A Marker of Widespread Endothelial Damage, Disease Severity, and Mortality. J. Med. Virol. 2021, 93, 2820–2827.
- Gregoriano, C.; Koch, D.; Kutz, A.; Haubitz, S.; Conen, A.; Bernasconi, L.; Hammerer-Lercher, A.; Saeed, K.; Mueller, B.; Schuetz, P. The Vasoactive Peptide MR-Pro-Adrenomedullin in COVID-19 Patients: An Observational Study. Clin. Chem. Lab. Med. 2021, 59, 995–1004.
- Sozio, E.; Tascini, C.; Fabris, M.; D’Aurizio, F.; De Carlo, C.; Graziano, E.; Bassi, F.; Sbrana, F.; Ripoli, A.; Pagotto, A.; et al. MR-ProADM as Prognostic Factor of Outcome in COVID-19 Patients. Sci. Rep. 2021, 11, 5121.
- Montrucchio, G.; Sales, G.; Rumbolo, F.; Palmesino, F.; Fanelli, V.; Urbino, R.; Filippini, C.; Mengozzi, G.; Brazzi, L. Effectiveness of Mid-Regional Pro-Adrenomedullin (MR-ProADM) as Prognostic Marker in COVID-19 Critically Ill Patients: An Observational Prospective Study. PLoS ONE 2021, 16, e0246771.
- Benedetti, I.; Spinelli, D.; Callegari, T.; Bonometti, R.; Molinaro, E.; Novara, E.; Cassinari, M.; Frino, C.; Guaschino, R.; Boverio, R.; et al. High Levels of Mid-Regional Proadrenomedullin in ARDS COVID-19 Patients: The Experience of a Single, Italian Center. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1743–1751.
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A Novel Hypotensive Peptide Isolated from Human Pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560.
- Wimalawansa, S.J. Amylin, Calcitonin Gene-Related Peptide, Calcitonin, and Adrenomedullin: A Peptide Superfamily. Crit. Rev. Neurobiol. 1997, 11, 167–239.
- Takei, Y.; Inoue, K.; Ogoshi, M.; Kawahara, T.; Bannai, H.; Miyano, S. Identification of Novel Adrenomedullin in Mammals: A Potent Cardiovascular and Renal Regulator. FEBS Lett. 2004, 556, 53–58.
- Kitamura, K.; Kato, J.; Kawamoto, M.; Tanaka, M.; Chino, N.; Kangawa, K.; Eto, T. The Intermediate Form of Glycine-Extended Adrenomedullin Is the Major Circulating Molecular Form in Human Plasma. Biochem. Biophys. Res. Commun. 1998, 244, 551–555.
- Struck, J.; Tao, C.; Morgenthaler, N.G.; Bergmann, A. Identification of an Adrenomedullin Precursor Fragment in Plasma of Sepsis Patients. Peptides 2004, 25, 1369–1372.
- Fischer, J.P.; Els-Heindl, S.; Beck-Sickinger, A.G. Adrenomedullin—Current Perspective on a Peptide Hormone with Significant Therapeutic Potential. Peptides 2020, 131, 170347.
- Shindo, T.; Kurihara, Y.; Nishimatsu, H.; Moriyama, N.; Kakoki, M.; Wang, Y.; Imai, Y.; Ebihara, A.; Kuwaki, T.; Ju, K.H.; et al. Vascular Abnormalities and Elevated Blood Pressure in Mice Lacking Adrenomedullin Gene. Circulation 2001, 104, 1964–1971.
- Caron, K.M.; Smithies, O. Extreme Hydrops fetalis and Cardiovascular Abnormalities in Mice Lacking a Functional Adrenomedullin Gene. Proc. Natl. Acad. Sci. USA 2001, 98, 615–619.
- Dackor, R.T.; Fritz-Six, K.; Dunworth, W.P.; Gibbons, C.L.; Smithies, O.; Caron, K.M. Hydrops fetalis, Cardiovascular Defects, and Embryonic Lethality in Mice Lacking the Calcitonin Receptor-Like Receptor Gene. Mol. Cell. Biol. 2006, 26, 2511–2518.
- Shindo, T.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Shimoyama, N.; Iinuma, N.; Arai, T.; Miyagawa, S. Regulation of Adrenomedullin and Its Family Peptide by RAMP System—Lessons from Genetically Engineered Mice. Curr. Protein Pept. Sci. 2013, 14, 347–357.
- Yamauchi, A.; Sakurai, T.; Kamiyoshi, A.; Ichikawa-Shindo, Y.; Kawate, H.; Igarashi, K.; Toriyama, Y.; Tanaka, M.; Liu, T.; Xian, X.; et al. Functional Differentiation of RAMP2 and RAMP3 in Their Regulation of the Vascular System. J. Mol. Cell. Cardiol. 2014, 77, 73–85.
- Li, P.; Wang, C.; Pang, S. The Diagnostic Accuracy of Mid-Regional Pro-Adrenomedullin for Sepsis: A Systematic Review and Meta-Analysis. Minerva Anestesiol. 2021, 87, 1117–1127.
- Bełtowski, J.; Jamroz, A. Adrenomedullin--what do we know 10 years since its discovery? Pol. J. Pharmacol. 2004, 56, 5–27.
- Eto, T.; Kato, J.; Kitamura, K. Regulation of Production and Secretion of Adrenomedullin in the Cardiovascular System. Regul. Pept. 2003, 112, 61–69.
- Nagaya, N.; Goto, Y.; Satoh, T.; Sumida, H.; Kojima, S.; Miyatake, K.; Kangawa, K. Intravenous adrenomedullin in myocardial function and energy metabolism in patients after myocardial infarction. J. Cardiovasc. Pharmacol. 2002, 39, 754–760.
- Terata, K.; Miura, H.; Liu, Y.; Loberiza, F.; Gutterman, D.D. Human coronary arteriolar dilation to adrenomedullin: Role of nitric oxide and K(+) channels. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2620–H2626.
- Iring, A.; Jin, Y.J.; Albarrán-Juárez, J.; Siragusa, M.; Wang, S.; Dancs, P.T.; Nakayama, A.; Tonack, S.; Chen, M.; Künne, C.; et al. Shear Stress-Induced Endothelial Adrenomedullin Signaling Regulates Vascular Tone and Blood Pressure. J. Clin. Investig. 2019, 129, 2775–2791.
- Temmesfeld-Wollbrück, B.; Hocke, A.C.; Suttorp, N.; Hippenstiel, S. Adrenomedullin and Endothelial Barrier Function. Thromb. Haemost. 2007, 98, 944–951.
- Yang, S.; Zhou, M.; Chaudry, I.H.; Wang, P. Novel Approach to Prevent the Transition from the Hyperdynamic Phase to the Hypodynamic Phase of Sepsis: Role of Adrenomedullin and Adrenomedullin Binding protein-1. Ann. Surg. 2002, 236, 625–633.
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D.; ADQI XIV Workgroup. The Endothelium in Sepsis. Shock 2016, 45, 259–270.
- Müller-Redetzky, H.C.; Suttorp, N.; Witzenrath, M. Dynamics of Pulmonary Endothelial Barrier Function in Acute Inflammation: Mechanisms and Therapeutic Perspectives. Cell Tissue Res. 2014, 355, 657–673.
- Saito, R.; Shimosawa, T.; Ogihara, T.; Maruyama, N.; Fujita, T.; Okamura, N.; Nakahara, K. Function of Adrenomedullin in Inflammatory Response of Liver Against LPS-Induced Endotoxemia. APMIS 2012, 120, 706–711.
- Dackor, R.; Caron, K. Mice Heterozygous for Adrenomedullin Exhibit a More Extreme Inflammatory Response to Endotoxin-Induced Septic Shock. Peptides 2007, 28, 2164–2170.
- Shindo, T.; Kurihara, H.; Maemura, K.; Kurihara, Y.; Kuwaki, T.; Izumida, T.; Minamino, N.; Ju, K.H.; Morita, H.; Oh-Hashi, Y.; et al. Hypotension and Resistance to Lipopolysaccharide-Induced Shock in Transgenic Mice Overexpressing Adrenomedullin in Their Vasculature. Circulation 2000, 101, 2309–2316.
- Yang, J.; Wu, R.; Zhou, M.; Wang, P. Human Adrenomedullin and Its Binding Protein Ameliorate Sepsis-Induced Organ Injury and Mortality in Jaundiced Rats. Peptides 2010, 31, 872–877.
- Temmesfeld-Wollbrück, B.; Brell, B.; zu Dohna, C.; Dorenberg, M.; Hocke, A.C.; Martens, H.; Klar, J.; Suttorp, N.; Hippenstiel, S. Adrenomedullin Reduces Intestinal Epithelial Permeability In Vivo and In Vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G43–G51.
- Wu, R.; Higuchi, S.; Dong, W.; Ji, Y.; Zhou, M.; Marini, C.P.; Ravikumar, T.S.; Wang, P. Reversing Established Sepsis in Rats with Human Vasoactive Hormone Adrenomedullin and Its Binding Protein. Mol. Med. 2009, 15, 28–33.
- Ertmer, C.; Morelli, A.; Rehberg, S.; Lange, M.; Hucklenbruch, C.; Van Aken, H.; Booke, M.; Westphal, M. Exogenous Adrenomedullin Prevents and Reverses Hypodynamic Circulation and Pulmonary Hypertension in Ovine Endotoxaemia. Br. J. Anaesth. 2007, 99, 830–836.
- Miksa, M.; Wu, R.; Cui, X.; Dong, W.; Das, P.; Simms, H.H.; Ravikumar, T.S.; Wang, P. Vasoactive Hormone Adrenomedullin and Its Binding Protein: Anti-Inflammatory Effects by Up-Regulating Peroxisome Proliferator-Activated Receptor-Gamma. J. Immunol. 2007, 179, 6263–6272.
- Dwivedi, A.J.; Wu, R.; Nguyen, E.; Higuchi, S.; Wang, H.; Krishnasastry, K.; Marini, C.P.; Ravikumar, T.S.; Wang, P. Adrenomedullin and Adrenomedullin Binding protein-1 Prevent Acute Lung Injury After Gut Ischemia-Reperfusion. J. Am. Coll. Surg. 2007, 205, 284–293.
- Temmesfeld-Wollbrück, B.; Brell, B.; Dávid, I.; Dorenberg, M.; Adolphs, J.; Schmeck, B.; Suttorp, N.; Hippenstiel, S. Adrenomedullin Reduces Vascular Hyperpermeability and Improves Survival in Rat Septic Shock. Intensive Care Med. 2007, 33, 703–710.
- Carrizo, G.J.; Wu, R.; Cui, X.; Dwivedi, A.J.; Simms, H.H.; Wang, P. Adrenomedullin and Adrenomedullin-Binding protein-1 Downregulate Inflammatory Cytokines and Attenuate Tissue Injury After Gut Ischemia-Reperfusion. Surgery 2007, 141, 245–253.
- Zaks-Zilberman, M.; Salkowski, C.A.; Elsasser, T.; Cuttitta, F.; Vogel, S.N. Induction of Adrenomedullin mRNA and Protein by Lipopolysaccharide and Paclitaxel (Taxol) in Murine Macrophages. Infect. Immun. 1998, 66, 4669–4675.
- Geven, C.; van Lier, D.; Blet, A.; Peelen, R.; ten Elzen, B.; Mebazaa, A.; Kox, M.; Pickkers, P. Safety, Tolerability and Pharmacokinetics/-Dynamics of the Adrenomedullin Antibody Adrecizumab in a First-Inhuman Study and During Experimental Human Endotoxemia in Healthy Subjects. Br. J. Clin. Pharmacol. 2018, 84, 2129–2141.
- Geven, C.; Peters, E.; Schroedter, M.; Struck, J.; Bergmann, A.; McCook, O.; Radermacher, P.; Kox, M.; Pickkers, P. Effects of the Humanized Anti-Adrenomedullin Antibody Adrecizumab (HAM8101) on Vascular Barrier Function and Survival in Rodent Models of Systemic Inflammation and Sepsis. Shock 2018, 50, 648–654.
- Blet, A.; Deniau, B.; Geven, C.; Sadoune, M.; Caillard, A.; Kounde, P.R.; Polidano, E.; Pickkers, P.; Samuel, J.L.; Mebazaa, A. Adrecizumab, a Non-Neutralizing Anti-Adrenomedullin Antibody, Improves Haemodynamics and Attenuates Myocardial Oxidative Stress in Septic Rats. Intensive Care Med. Exp. 2019, 7, 25.
- Thiele, C.; Simon, T.P.; Szymanski, J.; Daniel, C.; Golias, C.; Hartmann, O.; Struck, J.; Martin, L.; Marx, G.; Schuerholz, T. Effects of the Non-Neutralizing Humanized Monoclonal Anti-Adrenomedullin Antibody Adrecizumab on Hemodynamic and Renal Injury in a Porcine Two-Hit Model. Shock 2020, 54, 810–818.
- Laterre, P.F.; Pickkers, P.; Marx, G.; Wittebole, X.; Meziani, F.; Dugernier, T.; Huberlant, V.; Schuerholz, T.; François, B.; Lascarrou, J.B.; et al. Safety and Tolerability of Non-Neutralizing Adrenomedullin Antibody Adrecizumab (HAM8101) in Septic Shock Patients: The AdrenOSS-2 phase 2a Biomarker-Guided Trial. Intensive Care Med. 2021, 47, 1284–1294.
- Assouline, B.; Faivre, A.; Verissimo, T.; Sangla, F.; Berchtold, L.; Giraud, R.; Bendjelid, K.; Sgardello, S.; Elia, N.; Pugin, J.; et al. Thiamine, Ascorbic Acid, and Hydrocortisone as a Metabolic Resuscitation Cocktail in Sepsis: A Meta-Analysis of Randomized Controlled Trials with Trial Sequential Analysis. Crit. Care Med. 2021, 49, 2112–2120.
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615.
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423.
- Noori, M.; Nejadghaderi, S.A.; Arshi, S.; Carson-Chahhoud, K.; Ansarin, K.; Kolahi, A.A.; Safiri, S. Potency of BNT162b2 and mRNA-1273 Vaccine-Induced Neutralizing Antibodies Against Severe Acute Respiratory Syndrome-CoV-2 Variants of Concern: A Systematic Review of In Vitro Studies. Rev. Med. Virol. 2021, e2277.
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.G.; Gray, G. Effectiveness of BNT162b2 Vaccine Against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496.
- Falcone, M.; Tiseo, G.; Valoriani, B.; Barbieri, C.; Occhineri, S.; Mazzetti, P.; Vatteroni, M.L.; Suardi, L.R.; Riccardi, N.; Pistello, M.; et al. Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern. Infect. Dis. Ther. 2021, 10, 2479–2488.
- Singh, A.K.; Singh, A.; Singh, R.; Misra, A. An Updated Practical Guideline on Use of Molnupiravir and Comparison with Agents Having Emergency Use Authorization for Treatment of COVID-19. Diabetes Metab. Syndr. 2022, 16, 102396.
- Agarwal, A.; Rochwerg, B.; Lamontagne, F.; Siemieniuk, R.A.; Agoritsas, T.; Askie, L.; Lytvyn, L.; Leo, Y.S.; Macdonald, H.; Zeng, L.; et al. A Living WHO Guideline on Drugs for COVID-19. BMJ 2020, 370, m3379.
- Ngamprasertchai, T.; Kajeekul, R.; Sivakorn, C.; Ruenroegnboon, N.; Luvira, V.; Siripoon, T.; Luangasanatip, N. Efficacy and Safety of Immunomodulators in Patients with COVID-19: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Infect. Dis. Ther. 2022, 11, 231–248.
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704.
- Abani, O.; Abbas, A.; Abbas, F.; Abbas, M.; Abbasi, S.; Abbass, H.; Abbott, A.; Abdallah, N.; Abdelaziz, A.; Abdelfattah, M.; et al. Tocilizumab in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2021, 397, 1637–1645.
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; Kartman, C.E.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Alatorre-Alexander, J.; de Cassia Pellegrini, R.; et al. Efficacy and Safety of Baricitinib for the Treatment of Hospitalised Adults with COVID-19 (COV-BARRIER): A Randomised, Double-Blind, Parallel-Group, Placebo-Controlled phase 3 Trial. Lancet Respir. Med. 2021, 9, 1407–1418.
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807.
- Gómez-Mesa, J.E.; Galindo-Coral, S.; Montes, M.C.; Muñoz Martin, A.J. Thrombosis and Coagulopathy in COVID-19. Curr. Probl. Cardiol. 2021, 46, 100742.
- Hadid, T.; Kafri, Z.; Al-Katib, A. Coagulation and Anticoagulation in COVID-19. Blood Rev. 2021, 47, 100761.
- Asakura, H.; Ogawa, H. COVID-19-associated Coagulopathy and Disseminated Intravascular Coagulation. Int. J. Hematol. 2021, 113, 45–57.
- Vincent, J.L.; Levi, M.; Hunt, B.J. Prevention and Management of Thrombosis in Hospitalised Patients with COVID-19 Pneumonia. Lancet Respir. Med. 2021, 21, S2213–S2600.




