
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rodney Hull | + 2229 word(s) | 2229 | 2022-02-11 10:42:20 | | | |
| 2 | Lindsay Dong | Meta information modification | 2229 | 2022-03-04 03:48:37 | | | | |
| 3 | Lindsay Dong | -4 word(s) | 2225 | 2022-03-16 10:53:11 | | |
Video Upload Options
MicroRNAs (miRNA) are small non-coding RNAs that are 20–23 nucleotides in length, functioning as regulators of oncogenes or tumor suppressor genes. They are molecular modulators that regulate gene expression by suppressing gene translation through gene silencing/degradation, or by promoting translation of messenger RNA (mRNA) into proteins. Circulating miRNAs have attracted attention as possible prognostic markers of cancer, which could aid in the early detection of the disease. Epithelial to mesenchymal transition (EMT) has been implicated in tumorigenic processes, primarily by promoting tumor invasiveness and metastatic activity; this is a process that could be manipulated to halt or prevent brain metastasis. Studies show that miRNAs influence the function of EMT in glioblastomas. Thus, miRNA-related EMT can be exploited as a potential therapeutic target in glioblastomas.
1. Introduction
2. Epithelial Mesenchymal Transition in Glioblastoma
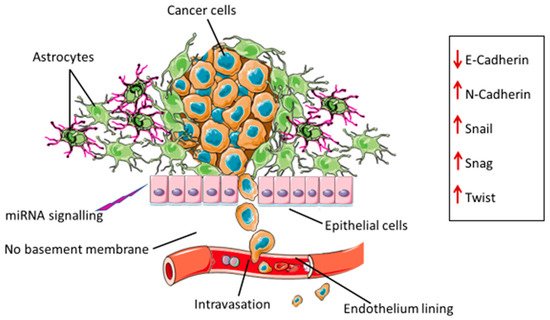
3. Drivers of EMT in Glioblastoma
3.1. Extracellular Vesicles (EVs)
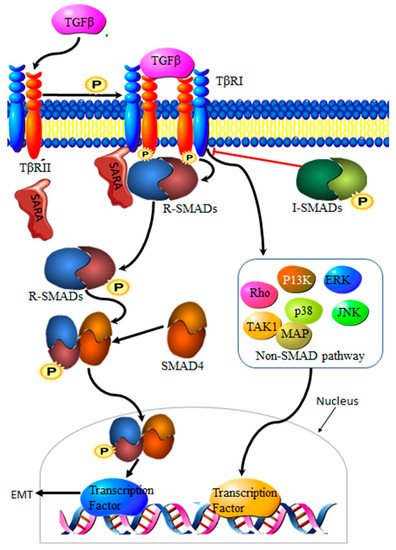
3.2. Transforming Growth Factor β Signaling Pathway
Transforming growth factor-β (TGF-β) is a family of multi-functional growth factors involved in cell proliferation, differentiation and apoptosis. During cancer development, TGF-β suppresses cancer growth via cell cycle arrest and apoptosis, but supports cancer progression at a later stage of the disease by promoting invasion, increasing migration capabilities and drug resistance [18]. The TGF-β complex consists of surface receptor type I (TβRI) and type II (TβRII) transmembrane serine/threonine kinases. Signaling is initiated by the binding of TGF-β to TβRII and TβRI receptors on the cell surface, resulting in a heterocomplex. Phosphorylation by TβRII activates TβRI, resulting in the recruitment and phosphorylation of Smad proteins 2 and 3, collectively known as R-Smads. Translocation to the nucleus requires the binding of R-Smads to Smad 4. The resultant R-Smads/Smad 4 complex will then induce the transcription of target proteins [19] (Figure 2). Aberrant TGF-β signaling pathways contribute to cancer development [20], making TGF-β an important driver of EMT in cancers.
3.3. Autophagy
3.4. MiRNAs
4. miRNA-EMT-Related Cancer Cell Invasion and Metastasis
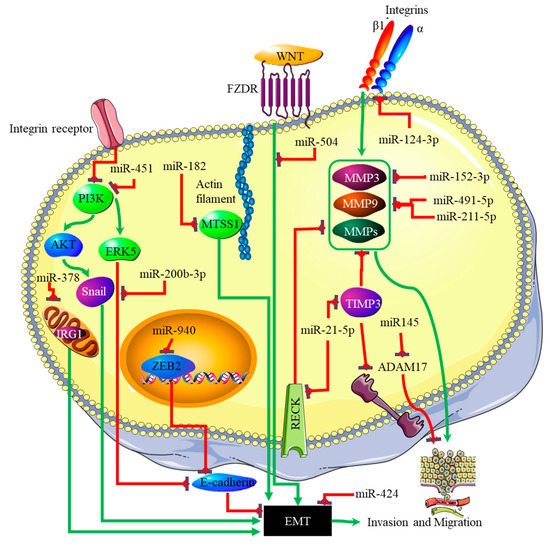
The link between miRNA expression signatures, EMT and, ultimately, cancer metastasis has long been established [29]. Several miRNA signatures are involved in EMT-associated mechanisms of invasion and metastasis in glioblastomas. Studies on miR-125a-5p found its expression to suppress the mesenchymal capabilities of glioblastoma cells, thus inhibiting EMT [30][31]. Recently, Nan et al. showed that miR-451 expression is able to suppress EMT and metastasis by blocking the PI3K/Akt/Snail signaling pathway through the activation of calcium-binding protein 39 (CAB39) in gliomas [32]. The expression of miR-451 was associated with the inhibition or reversal of the EMT processes in cancers [33]. Contrary to this finding, miR-451 expression plays different roles in gliomas, as it was associated with the proliferation of cancer cells by suppressing the CAB39/AMPK/mTOR pathway, and increased metastatic potential, which takes place via the activation of the Rac1/cofilin pathway [34]. The increased expression of miR-200b-3p prompted E-cadherin (essential for maintaining the structural integrity of epithelial cells) by the deactivation of extracellular signal-regulated kinase 5 (ERK5), resulting in reduced glioma cell proliferation and mesenchymal capabilities [35]. mR-424 showed promising results as a potential prognostic molecular marker and therapeutic target by blocking EMT processes and the resultant metastatic capabilities by targeting the kinesin-like protein KIF23 in human glioma [36]. The same effect was observed with miR-378, which inhibited EMT by targeting cis-aconitate decarboxylase (IRG1) in gliomas [37], miR-139-5p by targeting the notch1 oncogene in gliomas [38], miR-181a by targeting ZBTB33 expression in glioma cells [39], miR-623 by targeting TRIM-44 [40], miR-940 by targeting ZEB2 [41], and miR-7, which targeted T-Box 2 in glioblastoma [42]. In addition to these miRNAs, miR-182-targeted MTSS1 enhanced TGF-β1-related EMT in gliomas [43], and miR-504 inhibited EMT in glioblastomas by targeting the Wnt receptor FZD7/β-catenin pathway. The phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase (PTEN) tumor suppressor inhibits PI3K by dephosphorylating it. The expression of PTEN is inhibited through the action of three separate miRNAs. These are miR-17-5p [44], miR-23a-3p [45] and miR-26a-5p [46]. The p53 tumor suppressor is, itself, downregulated by miR-10b-5p. This miRNA also downregulates the tumor suppressor and cell cycle regulator p16 [47]. At the same time, the negative regulator of p53, MDM2, is upregulated in glioblastoma by miRNAs such as miR-32-5p, miR-25-3p and miR-17-3p [48]. The RB1 tumor suppressor is downregulated by the miRNA miR-28-5p. At the same time, the expression and activity of oncogenes that promote cell cycle progression are downregulated by various miRNAs in glioblastoma; these include miR-124-3p suppressing cyclin-dependent kinase (CKD) 4 [49], and miR-491-5p, miR-491-3p and miR-138-5p [50] suppressing CKD6. Finally, the proliferative activity of cyclin D is downregulated by miR-195-5p [51]. A decreased expression ratio of the miRNA miR-504/FZD7 was shown to be a potential molecular marker for identifying the mesenchymal subtype in glioblastoma [52]. The actions of the miRNAs discussed above are summarized in Figure 3.
5. miRNA-EMT-Related Angiogenesis
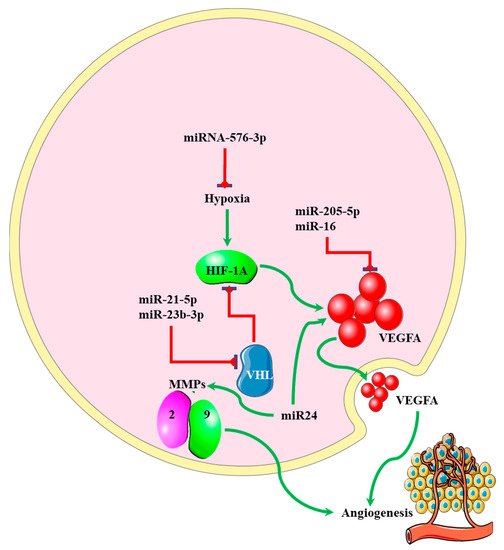
MicroRNAs regulating angiogenesis in cancers have been dubbed angiomiRNAs [53][54]. Several angiomiRNAs have been identified in glioblastomas [55], with some originating from glioblastoma-derived EVs [56]. However, studies indicating the relationship between angiomiRNAs and the EMT process in glioblastomas are limited. Dai et al. found that upregulated miR-24 expression induced the expression of the angiogenic markers VEGF and, TGF-β, and matrix metalloproteinases (MMP)-2 and -9, resulting in increased glioblastoma cell proliferation and development [57] (Figure 4). Increased expression of miR-16 in glioblastoma inhibited EMT processes by targeting polycomb complex protein BMI-1, and reducing the expression levels of the angiogenic markers VEGF-A and VEGF-C [58]. miR-576-3p was shown to inhibit EMT and the angiogenic properties of hypoxia-treated glioma cells by targeting HIF-1α [59] (Figure 4).
AngiomiRNA-EMT-Induced Drug Resistance
References
- Alexander, B.M.; Cloughesy, T.F. Adult Glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409.
- Ahmed, S.P.; Castresana, J.S.; Shahi, M.H. Glioblastoma and MiRNAs. Cancers 2021, 13, 1581.
- Zaravinos, A. The Regulatory Role of MicroRNAs in EMT and Cancer. J. Oncol. 2015, 2015, 865816.
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352.
- Zhang, Y.; Zeng, A.; Liu, S.; Li, R.; Wang, X.; Yan, W.; Li, H.; You, Y. Genome-wide identification of epithelial-mesenchymal transition-associated microRNAs reveals novel targets for glioblastoma therapy. Oncol. Lett. 2018, 15, 7625–7630.
- Drak Alsibai, K.; Meseure, D. Tumor microenvironment and noncoding RNAs as co-drivers of epithelial–mesenchymal transition and cancer metastasis. Dev. Dyn. 2018, 247, 405–431.
- Iwadate, Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol. Lett. 2016, 11, 1615–1620.
- Kahlert, U.D.; Nikkhah, G.; Maciaczyk, J. Epithelial-to-mesenchymal(-like) transition as a relevant molecular event in malignant gliomas. Cancer Lett. 2013, 331, 131–138.
- Miner, J.H.; Nguyen, N.M. Extracellular Matrix: Basement Membranes. In Encyclopedia of Respiratory Medicine, 2nd ed.; Janes, S.M., Ed.; Academic Press: Oxford, UK, 2022; pp. 130–136.
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2018, 4, 78–82.
- Jäkel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell Neurosci. 2017, 11.
- Iser, I.C.; Lenz, G.; Wink, M.R. EMT-like process in glioblastomas and reactive astrocytes. Neurochem. Int. 2019, 122, 139–143.
- Xavier, C.P.R.; Caires, H.R.; Barbosa, M.A.G.; Bergantim, R.; Guimarães, J.E.; Vasconcelos, M.H. The Role of Extracellular Vesicles in the Hallmarks of Cancer and Drug Resistance. Cells 2020, 9, 1141.
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638.
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e6.
- Kletukhina, S.; Neustroeva, O.; James, V.; Rizvanov, A.; Gomzikova, M. Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Epithelial–Mesenchymal Transition. Int. J. Mol. Sci. 2019, 20, 4813.
- Schweiger, M.W.; Li, M.; Giovanazzi, A.; Fleming, R.L.; Tabet, E.I.; Nakano, I.; Würdinger, T.; Chiocca, E.A.; Tian, T.; Tannous, B.A. Extracellular Vesicles Induce Mesenchymal Transition and Therapeutic Resistance in Glioblastomas through NF-κB/STAT3 Signaling. Adv. Biosyst. 2020, 4, e1900312.
- Gu, S.; Feng, X.H. TGF-β signaling in cancer. Acta Biochim. Biophys. Sin. 2018, 50, 941–949.
- Huang, F.; Chen, Y.-G. Regulation of TGF-β receptor activity. Cell Biosci. 2012, 2, 9.
- Pasche, B. Role of transforming growth factor beta in cancer. J. Cell Physiol. 2001, 186, 153–168.
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650.
- Su, X.; Yang, Y.; Guo, C.; Zhang, R.; Sun, S.; Wang, Y.; Qiao, Q.; Fu, Y.; Pang, Q. NOX4-Derived ROS Mediates TGF-β1-Induced Metabolic Reprogramming during Epithelial-Mesenchymal Transition through the PI3K/AKT/HIF-1α Pathway in Glioblastoma. Oxid. Med. Cell. Longev. 2021, 2021, 5549047.
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473.
- Polakis, P. Wnt signaling in cancer. Cold Spring Harb. Perspect. Biol. 2012, 4, a008052.
- Huang, B.S.; Luo, Q.Z.; Han, Y.; Huang, D.; Tang, Q.P.; Wu, L.X. MiR-223/PAX6 Axis Regulates Glioblastoma Stem Cell Proliferation and the Chemo Resistance to TMZ via Regulating PI3K/Akt Pathway. J. Cell Biochem. 2017, 118, 3452–3461.
- Crespo, S.; Kind, M.; Arcaro, A. The role of the PI3K/AKT/mTOR pathway in brain tumor metastasis. J. Cancer Metastasis Treat. 2016, 2, 80–89.
- Lv, B.; Yang, X.; Lv, S.; Wang, L.; Fan, K.; Shi, R.; Wang, F.; Song, H.; Ma, X.; Tan, X.; et al. CXCR4 Signaling Induced Epithelial-Mesenchymal Transition by PI3K/AKT and ERK Pathways in Glioblastoma. Mol. Neurobiol. 2015, 52, 1263–1268.
- Zhong, C.; Li, X.; Tao, B.; Peng, L.; Peng, T.; Yang, X.; Xia, X.; Chen, L. LIM and SH3 protein 1 induces glioma growth and invasion through PI3K/AKT signaling and epithelial-mesenchymal transition. Biomed. Pharmacother. 2019, 116, 109013.
- Zhang, J.; Ma, L. MicroRNA control of epithelial-mesenchymal transition and metastasis. Cancer Metastasis Rev. 2012, 31, 653–662.
- Zhu, X.D.; Gao, Z.J.; Zheng, G.D. miR-125a-5p inhibits cancer stem cells phenotype and epithelial to mesenchymal transition in glioblastoma. Rev. Assoc. Med. Bras. 2020, 66, 445–451.
- Sha, Y.; Lei, D.; He, L. Manipulating miR-125a-5p to regulate cancer stem cells phenotype and epithelial to mesenchymal transition in glioblastoma. Rev. Assoc. Med. Bras. 2020, 66, 706.
- Nan, Y.; Guo, L.; Lu, Y.; Guo, G.; Hong, R.; Zhao, L.; Wang, L.; Ren, B.; Yu, K.; Zhong, Y.; et al. miR-451 suppresses EMT and metastasis in glioma cells. Cell Cycle 2021, 20, 1270–1278.
- Bai, H.; Wu, S. miR-451: A Novel Biomarker and Potential Therapeutic Target for Cancer. OncoTargets Ther. 2019, 12, 11069–11082.
- Zhao, K.; Wang, L.; Li, T.; Zhu, M.; Zhang, C.; Chen, L.; Zhao, P.; Zhou, H.; Yu, S.; Yang, X. The role of miR-451 in the switching between proliferation and migration in malignant glioma cells: AMPK signaling, mTOR modulation and Rac1 activation required. Int. J. Oncol. 2017, 50, 1989–1999.
- Zou, M.; Zhu, W.; Wang, L.; Shi, L.; Gao, R.; Ou, Y.; Chen, X.; Wang, Z.; Jiang, A.; Liu, K.; et al. AEG-1/MTDH-activated autophagy enhances human malignant glioma susceptibility to TGF-β1-triggered epithelial-mesenchymal transition. Oncotarget 2016, 7, 13122–13138.
- Zhao, C.; Wang, X.B.; Zhang, Y.H.; Zhou, Y.M.; Yin, Q.; Yao, W.C. MicroRNA-424 inhibits cell migration, invasion and epithelial-mesenchymal transition in human glioma by targeting KIF23 and functions as a novel prognostic predictor. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6369–6378.
- Shi, H.Z.; Wang, D.; Sun, X.N.; Sheng, L. MicroRNA-378 acts as a prognosis marker and inhibits cell migration, invasion and epithelial-mesenchymal transition in human glioma by targeting IRG1. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3837–3846.
- Li, J.; Li, Q.; Lin, L.; Wang, R.; Chen, L.; Du, W.; Jiang, C.; Li, R. Targeting the Notch1 oncogene by miR-139-5p inhibits glioma metastasis and epithelial-mesenchymal transition (EMT). BMC Neurol. 2018, 18, 133.
- Wang, L.; Ma, J.; Wang, X.; Peng, F.; Chen, X.; Zheng, B.; Wang, C.; Dai, Z.; Ai, J.; Zhao, S. Kaiso (ZBTB33) Downregulation by Mirna-181a Inhibits Cell Proliferation, Invasion, and the Epithelial-Mesenchymal Transition in Glioma Cells. Cell Physiol. Biochem. 2018, 48, 947–958.
- Cui, D.; Wang, K.; Liu, Y.; Gao, J.; Cui, J. MicroRNA-623 Inhibits Epithelial-Mesenchymal Transition to Attenuate Glioma Proliferation by Targeting TRIM44. OncoTargets Ther. 2020, 13, 9291–9303.
- Xu, R.; Zhou, F.; Yu, T.; Xu, G.; Zhang, J.; Wang, Y.; Zhao, L.; Liu, N. MicroRNA-940 inhibits epithelial-mesenchymal transition of glioma cells via targeting ZEB2. Am. J. Transl. Res. 2019, 11, 7351–7363.
- Pan, C.-M.; Chan, K.-H.; Chen, C.-H.; Jan, C.-I.; Liu, M.-C.; Lin, C.-M.; Cho, D.-Y.; Tsai, W.-C.; Chu, Y.-T.; Cheng, C.-H.; et al. MicroRNA-7 targets T-Box 2 to inhibit epithelial-mesenchymal transition and invasiveness in glioblastoma multiforme. Cancer Lett. 2020, 493, 133–142.
- Li, Z.; Zhang, L.; Liu, Z.; Huang, T.; Wang, Y.; Ma, Y.; Fang, X.; He, Y.; Zhou, Y.; Huo, L.; et al. miRNA-182 regulated MTSS1 inhibits proliferation and invasion in Glioma Cells. J. Cancer 2020, 11, 5840–5851.
- Li, H.; Yang, B.B. Stress response of glioblastoma cells mediated by miR-17-5p targeting PTEN and the passenger strand miR-17-3p targeting MDM2. Oncotarget 2012, 3, 1653–1668.
- Tan, X.; Wang, S.; Zhu, L.; Wu, C.; Yin, B.; Zhao, J.; Yuan, J.; Qiang, B.; Peng, X. cAMP response element-binding protein promotes gliomagenesis by modulating the expression of oncogenic microRNA-23a. Proc. Natl. Acad. Sci. USA 2012, 109, 15805–15810.
- Huse, J.T.; Brennan, C.; Hambardzumyan, D.; Wee, B.; Pena, J.; Rouhanifard, S.H.; Sohn-Lee, C.; le Sage, C.; Agami, R.; Tuschl, T.; et al. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009, 23, 1327–1337.
- Lin, J.; Teo, S.; Lam, D.H.; Jeyaseelan, K.; Wang, S. MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis. 2012, 3, e398.
- Suh, S.-S.; Yoo, J.Y.; Nuovo, G.J.; Jeon, Y.-J.; Kim, S.; Lee, T.J.; Kim, T.; Bakàcs, A.; Alder, H.; Kaur, B.; et al. MicroRNAs/TP53 feedback circuitry in glioblastoma multiforme. Proc. Natl. Acad. Sci. USA 2012, 109, 5316–5321.
- Deng, X.; Ma, L.; Wu, M.; Zhang, G.; Jin, C.; Guo, Y.; Liu, R. miR-124 radiosensitizes human glioma cells by targeting CDK4. J. Neurooncol. 2013, 114, 263–274.
- Qiu, S.; Huang, D.; Yin, D.; Li, F.; Li, X.; Kung, H.F.; Peng, Y. Suppression of tumorigenicity by microRNA-138 through inhibition of EZH2-CDK4/6-pRb-E2F1 signal loop in glioblastoma multiforme. Biochim. Biophys. Acta 2013, 1832, 1697–1707.
- Hui, W.; Yuntao, L.; Lun, L.; WenSheng, L.; ChaoFeng, L.; HaiYong, H.; Yueyang, B. MicroRNA-195 inhibits the proliferation of human glioma cells by directly targeting cyclin D1 and cyclin E1. PLoS ONE 2013, 8, e54932.
- Liu, Q.; Guan, Y.; Li, Z.; Wang, Y.; Liu, Y.; Cui, R.; Wang, Y. miR-504 suppresses mesenchymal phenotype of glioblastoma by directly targeting the FZD7-mediated Wnt–β-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 358.
- Salinas-Vera, Y.M.; Marchat, L.A.; Gallardo-Rincón, D.; Ruiz-García, E.; Astudillo-De La Vega, H.; Echavarría-Zepeda, R.; López-Camarillo, C. AngiomiRs: MicroRNAs driving angiogenesis in cancer (Review). Int. J. Mol. Med. 2019, 43, 657–670.
- Wang, S.; Olson, E.N. AngiomiRs--key regulators of angiogenesis. Curr. Opin. Genet. Dev. 2009, 19, 205–211.
- Balandeh, E.; Mohammadshafie, K.; Mahmoudi, Y.; Hossein Pourhanifeh, M.; Rajabi, A.; Bahabadi, Z.R.; Mohammadi, A.H.; Rahimian, N.; Hamblin, M.R.; Mirzaei, H. Roles of Non-coding RNAs and Angiogenesis in Glioblastoma. Front. Cell Dev. Biol. 2021, 9, 2543.
- Lucero, R.; Zappulli, V.; Sammarco, A.; Murillo, O.D.; Cheah, P.S.; Srinivasan, S.; Tai, E.; Ting, D.T.; Wei, Z.; Roth, M.E.; et al. Glioma-Derived miRNA-Containing Extracellular Vesicles Induce Angiogenesis by Reprogramming Brain Endothelial Cells. Cell Rep. 2020, 30, 2065–2074.e4.
- Dai, D.; Huang, W.; Lu, Q.; Chen, H.; Liu, J.; Hong, B. miR-24 regulates angiogenesis in gliomas. Mol. Med. Rep. 2018, 18, 358–368.
- Chen, F.; Chen, L.; He, H.; Huang, W.; Zhang, R.; Li, P.; Meng, Y.; Jiang, X. Up-regulation of microRNA-16 in Glioblastoma Inhibits the Function of Endothelial Cells and Tumor Angiogenesis by Targeting Bmi-1. Anticancer Agents Med. Chem. 2016, 16, 609–620.
- Hu, Q.; Liu, F.; Yan, T.; Wu, M.; Ye, M.; Shi, G.; Lv, S.; Zhu, X. MicroRNA-576-3p inhibits the migration and proangiogenic abilities of hypoxia-treated glioma cells through hypoxia-inducible factor-1α. Int. J. Mol. Med. 2019, 43, 2387–2397.
- Naz, I.; Ramchandani, S.; Khan, M.R.; Yang, M.H.; Ahn, K.S. Anticancer Potential of Raddeanin A, a Natural Triterpenoid Isolated from Anemone raddeana Regel. Molecules 2020, 25, 1035.
- Wang, Q.; Mo, J.; Zhao, C.; Huang, K.; Feng, M.; He, W.; Wang, J.; Chen, S.; Xie, Z.a.; Ma, J.; et al. Raddeanin A suppresses breast cancer-associated osteolysis through inhibiting osteoclasts and breast cancer cells. Cell Death Dis. 2018, 9, 376.
- Peng, F.; Wang, X.; Shu, M.; Yang, M.; Wang, L.; Ouyang, Z.; Shen, C.; Hou, X.; Zhao, B.; Wang, X.; et al. Raddeanin a Suppresses Glioblastoma Growth by Inducing ROS Generation and Subsequent JNK Activation to Promote Cell Apoptosis. Cell Physiol. Biochem. 2018, 47, 1108–1121.
- Mathew, L.K.; Skuli, N.; Mucaj, V.; Lee, S.S.; Zinn, P.O.; Sathyan, P.; Imtiyaz, H.Z.; Zhang, Z.; Davuluri, R.V.; Rao, S.; et al. miR-218 opposes a critical RTK-HIF pathway in mesenchymal glioblastoma. Proc. Natl. Acad. Sci. USA 2014, 111, 291–296.




