
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ilya Gordeev | + 1314 word(s) | 1314 | 2022-02-18 07:47:56 | | | |
| 2 | Yvaine Wei | Meta information modification | 1314 | 2022-03-03 05:28:48 | | |
Video Upload Options
Anarhichotrema Shimazu, 1973 is a monotypic digenean genus, with the type- and only species, Anarhichotrema ochotense Shimazu, 1973, known to infect North Pacific fishes. This genus was originally described as a member of the Lissorchiidae (Monorchioidea) and later moved to the Zoogonidae (Microphalloidea).
1. Introduction
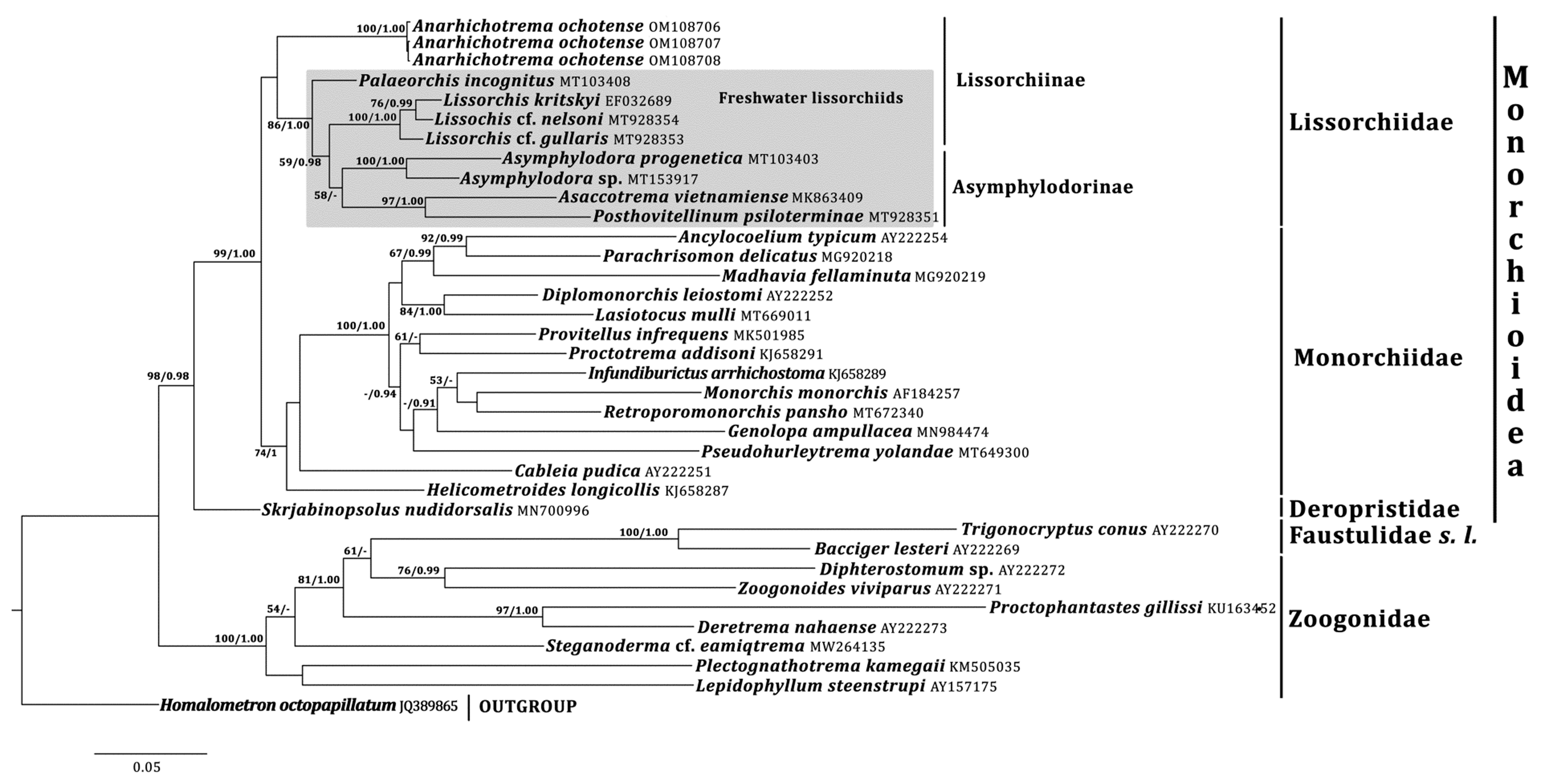
2. Phylogenetic Relationships

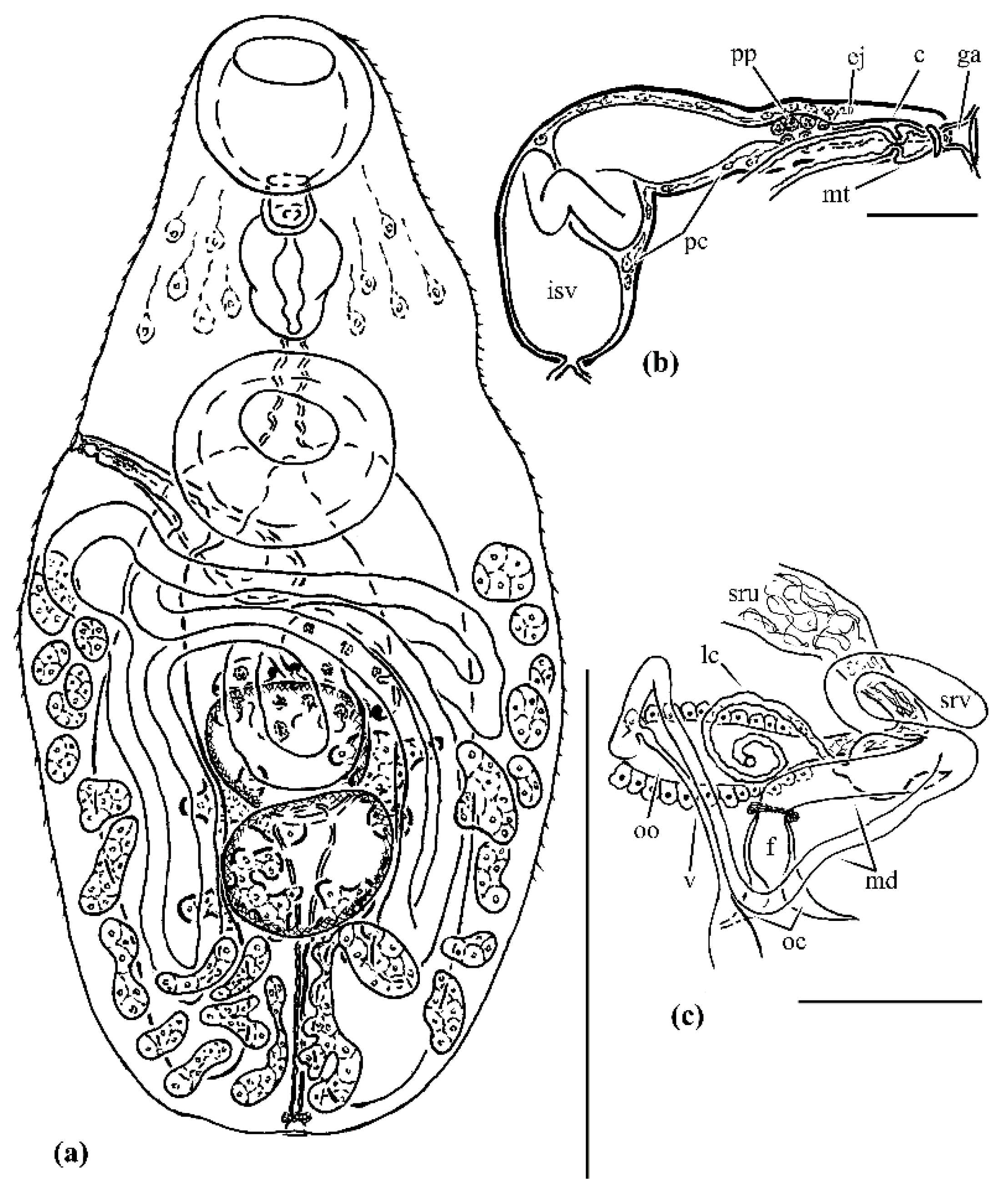
3. Systematics
4. The Results of Phylogenetic Analysis
The specimens of A. ochotense described are similar to those from Shimazu [1] in most of the morphological features related to the shape and location of the organs. Only three features appear to be different, based on the drawing in Shimazu [1]: the relative position of the testes (overlapping vs. separate), the position of the anterior border of the ventrolateral fields of the vitelline follicles (at the level of or slightly anteriorly to the posterior edge of the ventral sucker vs. distinctly posterior to the ventral sucker), and the arrangement of the cirrus sac (overlapped by the ventral sucker vs. lying outside the projection of the sucker). However, Machida [2] demonstrated that these features can be variable.
At the same time, the trematode specimens were noticeably smaller than those studied by T. Shimazu and M. Machida. This difference concerned all morphometric characteristics except egg length. The small size of the parasites could be associated with the crowding effect: A. ochotense infection intensity was 375 individuals as compared with six individuals in Shimazu [1] and five individuals in Machida [2]. The so-called crowding effect is a result of competition for host‘s finite resources by parasites. Typically, this effect increases with increasing numbers of parasites within a host and manifests in reduced body size and thus fitness [7][8][9].
Phylogenetic analyses indicate that Anarhichotrema is a member of the Lissorchiidae [1].The family Lissorchiidae is currently divided into two subfamilies, the Lissorchiinae Magath, 1917 and the Asymphylodorinae Szidat, 1943 [5]. The division is based on the number of testes: two in the former and one in the latter subfamily [5]. Phylogenetic data support the unification of the lissorchiine and the asymphylodorine digeneans into the same family but do not support the monophyly of the Lissorchiinae ([10][11][12][13], Present Study). Notably, the position of two lissorchiine genera, Lissorchis and Palaeorchis Szidat, 1943, is unstable in the phylograms of different authors [11][12], Present Study. Anarhichotrema, in contrast to the other lissorchiid genera, has a marine life history and the filamented eggs compare with [5][10][12]. Taking into account the ecological and morphological features of Anarhichotrema as well as the phylogenetic data (the basal position of Anarhichotrema and the paraphyly of the Lissorchiinae), it is conjectured that a separate subfamily may have to be erected for this genus in the future and that all freshwater lissorchiids should probably be considered within one subfamily (Lissorchiinae). However, for now, Anarhichotrema to the Lissorchiinae is conditionally considered.
References
- Shimazu, T. Anarhichotrema ochotense gen. et sp. n., a new digenetic trematode from the Bering wolf-fish, Anarhichas orientalis, from the Okhotsk Sea (Trematoda: Lissorchiidae). Jpn. J. Parasitol. 1973, 22, 303–306.
- Machida, M. A new lissorchiid trematode from zoarcid fish in the Sea of Japan. Mem. Natl. Sci. Mus. Tokyo 1985, 18, 117–120.
- Bray, R.A. Family Zoogonidae Odhner, 1902. In Keys to the Trematoda; Bray, R.A., Gibson, D.I., Jones, A., Eds.; CAB International and Natural History Museum: Cambridge, MA, USA, 2008; Volume 3, pp. 605–629.
- Kuramochi, T. Digenean trematodes of deep-sea fishes from the Sea of Japan. In Deep-Sea Fauna of the Sea of Japan; Fujita, T., Ed.; National Museum of Nature and Science: Tokyo, Japan, 2014; pp. 29–37.
- Bray, R.A. Family Lissorchiidae Magath, 1917. In Keys to the Trematoda; Bray, R.A., Gibson, D.I., Jones, A., Eds.; CAB International and Natural History Museum: Cambridge, MA, USA, 2008; Volume 3, pp. 417–436.
- Sokolov, S.; Shchenkov, S.; Gordeev, I.; Ryazanova, T. Description of a metacercaria of a zoogonid trematode Steganoderma cf. eamiqtrema Blend and Racz, 2020 (Microphalloidea: Zoogonidae), with notes on the phylogenetic position of the genus Steganoderma Stafford, 1904, and resurrection of the subfamily Lecithostaphylinae Odhner, 1911. Parasitol. Res. 2021, 120, 1669–1676.
- Stillson, L.L.; Platt, T.R. The crowding effect and morphometric variability in Echinostoma caproni (Digenea: Echinostomatidae) from ICR mice. J. Parasitol. 2007, 93, 242–246.
- Read, C.P. The “crowding effect” in tapeworm infections. J. Parasitol. 1951, 37, 174–178.
- Fong, C.; Moron, N.; Kuris, A. Two’s a crowd? Crowding effect in a parasitic castrator drives differences in reproductive resource allocation in single vs double infections. Parasitology 2017, 144, 662–668.
- Sokolov, S.G.; Gordeev, I.I. Asaccotrema vietnamiense n. gen.; n. sp. (Trematoda: Monorchioidea), a new aberrant representative of lissorchiid trematodes from the sidestripe rasbora, Rasbora paviana Tirant (Actinopterygii: Cyprinidae), Vietnam. Zootaxa 2019, 4674, 451–462.
- Petkevičiūtė, R.; Stanevičiūtė, G.; Stunžėnas, V. Exploring species diversity of lissorchiid trematodes (Digenea: Lissorchiidae) associated with the gravel snail, Lithoglyphus naticoides, in European freshwaters. J. Helminthol. 2020, 94, e152.
- Truong, T.N.; Warren, M.B.; Ksepka, S.P.; Curran, S.S.; Bullard, S.A. Posthovitellinum psiloterminae n. gen., n. sp.(Digenea: Lissorchiidae) infecting the intestine of Cyclocheilos enoplos (Cypriniformes: Cyprinidae) in the Mekong River, Vietnam. J. Parasitol. 2021, 107, 431–445.
- Besprozvannykh, V.V.; Ermolenko, A.V.; Atopkin, D.M. The life cycle of Asymphylodora perccotti sp. n. (Trematoda: Lissorchiidae) in the Russian Southern Far East. Parasitol. Int. 2012, 61, 235–241.




