
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Avishek Das | + 1334 word(s) | 1334 | 2022-02-25 07:24:52 | | | |
| 2 | Jessie Wu | Meta information modification | 1334 | 2022-03-01 04:32:35 | | |
Video Upload Options
There is already a societal awareness of the growing impact of nanoscience and nanotechnology, with nanomaterials (with at least one dimension less than 100 nm) now incorporated in items as diverse as mobile phones, clothes or dentifrices. In the healthcare area, nanoparticles of biocompatible materials have already been used for cancer treatment or bioimaging enhancement. Nanotechnology in dentistry, or nanodentistry, has already found some developments in dental nanomaterials for caries management, restorative dentistry and orthodontic adhesives.
1. Introduction
Nanoscience and nanotechnology, which deals with science and technology at the nanoscale, have developed in such a way that it has already been used in several areas of knowledge, with social applications and economic impact [1]. From the Internet of Nano Things [2] to health benefits [3], exploiting the nanoworld has made a big impact. This is no different in dentistry, although still in its infancy with respect to other areas of healthcare [4]. The authors of ref. [5] provided a good and updated account of nanomaterials in a diversity of dentistry applications. Among those, dental materials have received increasing attention [6], dental nanomaterials for caries management [7], restorative dentistry [8] and orthodontic adhesives [9]. Additionally, another importance to nanodentistry is silver nanoparticles, due to their antibactericidal properties [10].
2. Nanodentistry
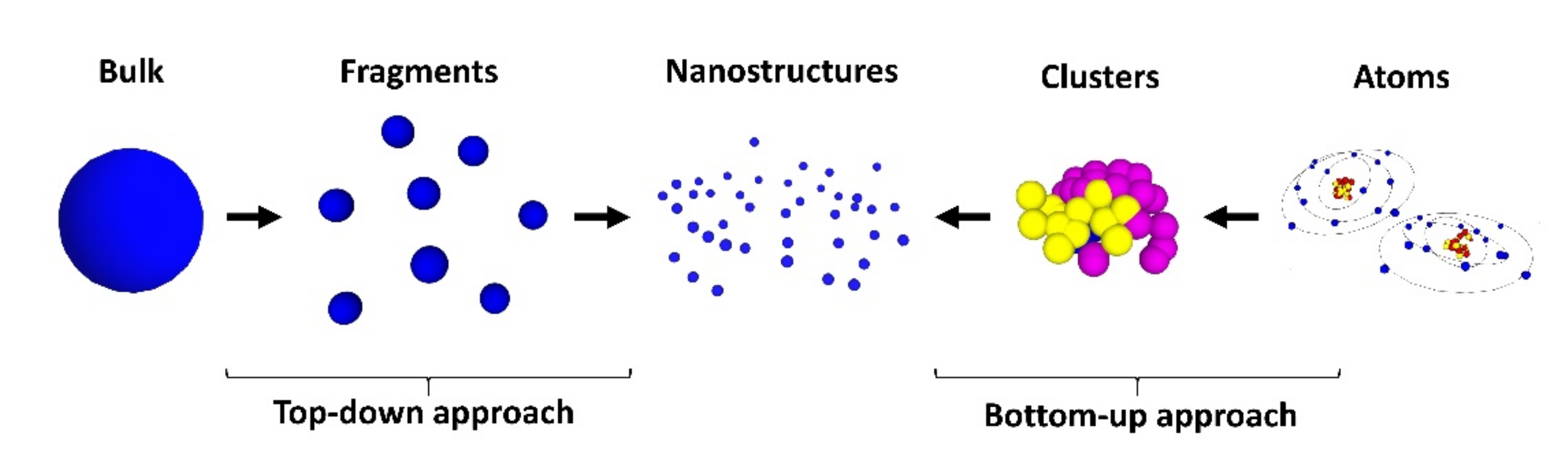
3. From Basics to Applications in Nanodentistry
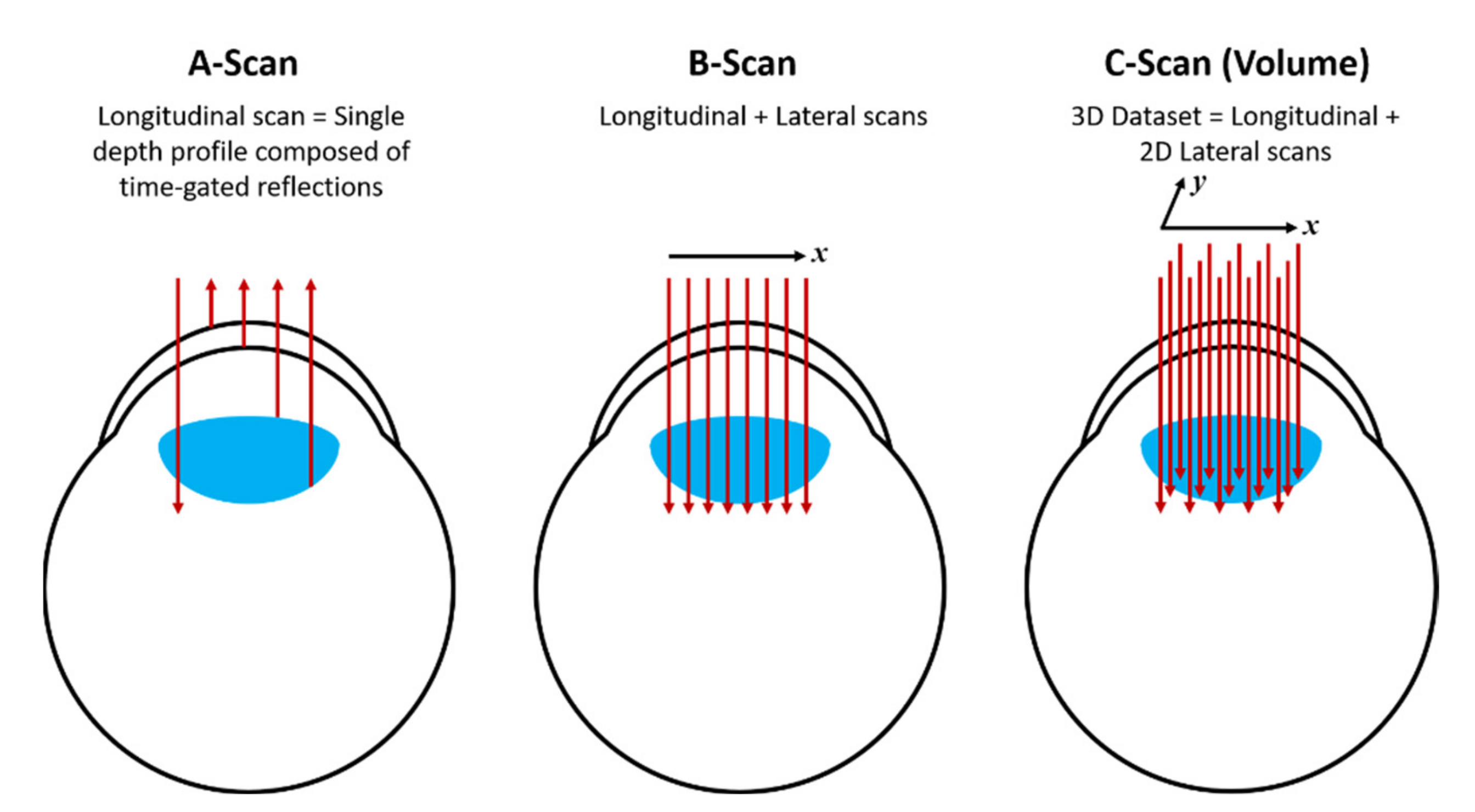
4. OCT in Nanodentistry: Image Enhancement with Gold Nanoparticles
References
- Yadav, S.K.; Khan, Z.A.; Mishra, B. Impact of Nanotechnology on Socio-Economic Aspects: An Overview. Rev. Nanosci. Nanotechnol. 2013, 2, 127–142.
- Miraz, M.H.; Ali, M.; Excell, P.S.; Picking, R. A Review on Internet of Things (IoT), Internet of Everything (IoE) and Internet of Nano Things (IoNT). In Proceedings of the 2015 Internet Technologies and Applications (ITA), Wrexham, UK, 8–11 September 2015; pp. 219–224.
- Salamanca-Buentello, F.; Daar, A.S. Nanotechnology, Equity and Global Health. Nat. Nanotechnol. 2021, 16, 358–361.
- Desai, K.; Somasundaram, J. Nanodentistry–An Overview. Eur. J. Mol. Clin. Med. 2020, 7, 2879–2887.
- Foong, L.K.; Foroughi, M.M.; Mirhosseini, A.F.; Safaei, M.; Jahani, S.; Mostafavi, M.; Ebrahimpoor, N.; Sharifi, M.; Varma, R.S.; Khatami, M. Applications of Nano-Materials in Diverse Dentistry Regimes. RSC Adv. 2020, 10, 15430–15460.
- Padovani, G.C.; Feitosa, V.P.; Sauro, S.; Tay, F.R.; Durán, G.; Paula, A.J.; Durán, N. Advances in Dental Materials through Nanotechnology: Facts, Perspectives and Toxicological Aspects. Trends Biotechnol. 2015, 33, 621–636.
- Melo, M.A.S.; Guedes, S.F.F.; Xu, H.H.K.; Rodrigues, L.K.A. Nanotechnology-Based Restorative Materials for Dental Caries Management. Trends Biotechnol. 2013, 31, 459–467.
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; Abu Reqaiba, A. Advances in Nanotechnology for Restorative Dentistry. Materials 2015, 8, 717–731.
- Yamagata, S.; Hamba, Y.; Nakanishi, K.; Abe, S.; Akasaka, T.; Ushijima, N.; Uo, M.; Iida, J.; Watari, F. Introduction of Rare-Earth-Element-Containing ZnO Nanoparticles into Orthodontic Adhesives. Nano Biomed. 2012, 4, 11–17.
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562.
- Hannig, M.; Hannig, C. Nanomaterials in Preventive Dentistry. Nat. Nanotechnol. 2010, 5, 565–569.
- Ellis, P.J. Nanodentistry: The Benefits of Nanotechnology in Dentistry and Its Impact on Oral Health. J. Student Sci. Technol. 2017, 10, 45–50.
- Sinha, N.; Kulshreshtha, N.M.; Dixit, M.; Jadhav, I.; Shrivastava, D.; Bisen, P.S. Nanodentistry: Novel Approaches. In Nanostructures for Oral Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 751–776.
- Adeola, H.A.; Sabiu, S.; Adekiya, T.A.; Aruleba, R.T.; Aruwa, C.E.; Oyinloye, B.E. Prospects of Nanodentistry for the Diagnosis and Treatment of Maxillofacial Pathologies and Cancers. Heliyon 2020, 6, e04890.
- Raura, N.; Garg, A.; Arora, A.; Roma, M. Nanoparticle Technology and Its Implications in Endodontics: A Review. Biomater. Res. 2020, 24, 21.
- Seth, N.I.; Khan, K.H. Dentistry at the Nano Level: The Advent of Nanodentistry. Int. Healthc. Res. J. 2017, 1, 3–9.
- Brun, A.; Moignot, N.; Colombier, M.-L.; Dursun, E. Emerging Nanotechnology in Non-Surgical Periodontal Therapy in Animal Models: A Systematic Review. Nanomaterials 2020, 10, 1414.
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181.
- Fujimoto, J.G.; Drexler, W. Introduction to OCT. In Optical Coherence Tomography; Springer International Publishing: Cham, Germany, 2015; pp. 3–64.
- Hemmer, E.; Benayas, A.; Légaré, F.; Vetrone, F. Exploiting the Biological Windows: Current Perspectives on Fluorescent Bioprobes Emitting above 1000 Nm. Nanoscale Horiz. 2016, 1, 168–184.
- Sahyoun, C.C.; Subhash, H.M.; Peru, D.; Ellwood, R.P.; Pierce, M.C. An Experimental Review of Optical Coherence Tomography Systems for Noninvasive Assessment of Hard Dental Tissues. Caries Res. 2020, 54, 43–54.
- Shimada, Y.; Sadr, A.; Sumi, Y.; Tagami, J. Application of Optical Coherence Tomography (OCT) for Diagnosis of Caries, Cracks, and Defects of Restorations. Curr. Oral Heal. Rep. 2015, 2, 73–80.
- Machoy, M.; Seeliger, J.; Szyszka-Sommerfeld, L.; Koprowski, R.; Gedrange, T.; Woźniak, K. The Use of Optical Coherence Tomography in Dental Diagnostics: A State-of-the-Art Review. J. Healthc. Eng. 2017, 2017, 1–31.
- Graça, N.D.; Silva, A.R.; Fernandes, L.O.; da Silva Pedrosa, M.; Guimarães, R.P.; Dos Santos, S.C.; Gomes, A.S.; da Silva, C.H. In Vivo Optical Coherence Tomographic Imaging to Monitor Gingival Recovery and the Adhesive Interface in Aesthetic Oral Rehabilitation: A Case Report. Imaging Sci. Dent. 2019, 49, 171.
- Fernandes, L.O.; Mota, C.C.; Oliveira, H.O.; Neves, J.K.; Santiago, L.M.; Gomes, A.S. Optical Coherence Tomography Follow-up of Patients Treated from Periodontal Disease. J. Biophotonics 2019, 12, e201800209.
- de Melo, L.S.A.; de Araujo, R.E.; Freitas, A.Z.; Zezell, D.; Vieira, N.D.; Girkin, J.; Hall, A.; Carvalho, M.T.; Gomes, A.S.L. Evaluation of Enamel Dental Restoration Interface by Optical Coherence Tomography. J. Biomed. Opt. 2005, 10, 064027.
- Suassuna, F.C.M.; Maia, A.M.A.; Melo, D.P.; Antonino, A.C.D.; Gomes, A.S.L.; Bento, P.M. Comparison of Microtomography and Optical Coherence Tomography on Apical Endodontic Filling Analysis. Dentomaxillofac. Radiol. 2018, 47, 20170174.
- de Oliveira, B.P.; Câmara, A.C.; Duarte, D.A.; Gomes, A.S.L.; Heck, R.J.; Antonino, A.C.D.; Aguiar, C.M. Detection of Apical Root Cracks Using Spectral Domain and Swept-Source Optical Coherence Tomography. J. Endod. 2017, 43, 1148–1151.
- Fernandes, L.O.; Mota, C.C.B.O.; de Melo, L.S.A.; da Costa Soares, M.U.S.; da Silva Feitosa, D.; Gomes, A.S.L. In Vivo Assessment of Periodontal Structures and Measurement of Gingival Sulcus with Optical Coherence Tomography: A Pilot Study. J. Biophotonics 2017, 10, 862–869.
- de Melo Monteiro, G.Q.; Montes, M.A.J.R.; Rolim, T.V.; de Oliveira Mota, C.C.B.; Kyotoku, B.D.B.C.; Gomes, A.S.L.; de Freitas, A.Z. Alternative Methods for Determining Shrinkage in Restorative Resin Composites. Dent. Mater. 2011, 27, e176–e185.
- Maia, A.M.A.; Karlsson, L.; Margulis, W.; Gomes, A.S.L. Evaluation of Two Imaging Techniques: Near-Infrared Transillumination and Dental Radiographs for the Detection of Early Approximal Enamel Caries. Dentomaxillofac. Radiol. 2011, 40, 429–433.
- Maia, A.M.A.; Fonsêca, D.D.D.; Kyotoku, B.B.C.; Gomes, A.S.L. Characterization of Enamel in Primary Teeth by Optical Coherence Tomography for Assessment of Dental Caries. Int. J. Paediatr. Dent. 2010, 20, 158–164.
- Braz, A.K.; Kyotoku, B.B.; Braz, R.; Gomes, A.S. Evaluation of Crack Propagation in Dental Composites by Optical Coherence Tomography. Dent. Mater. 2009, 25, 74–79.
- Fonsêca, D.D.D.; Kyotoku, B.B.C.; Maia, A.M.A.; Gomes, A.S.L. In Vitro Imaging of Remaining Dentin and Pulp Chamber by Optical Coherence Tomography: Comparison between 850 and 1280 Nm. J. Biomed. Opt. 2009, 14, 024009.
- Alexandrov, S.; Subhash, H.; Leahy, M. Nanosensitive Optical Coherence Tomography for the Study of Changes in Static and Dynamic Structures. Quantum Electron. 2014, 44, 657–663.
- Alexandrov, S.A.; Subhash, H.M.; Zam, A.; Leahy, M. Nano-Sensitive Optical Coherence Tomography. Nanoscale 2014, 6, 3545–3549.
- Lal, C.; Alexandrov, S.; Rani, S.; Zhou, Y.; Ritter, T.; Leahy, M. Nanosensitive Optical Coherence Tomography to Assess Wound Healing within the Cornea. Biomed. Opt. Express 2020, 11, 3407.
- Braz, A.; de Araujo, R.; Ohulchanskyy, T.; Shukla, S.; Bergey, E.; Gomes, A.; Prasad, P. In Situ Gold Nanoparticles Formation: Contrast Agent for Dental Optical Coherence Tomography. J. Biomed. Opt. 2012, 17, 066003.





