Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | J. Kloprogge | + 7679 word(s) | 7679 | 2022-02-18 08:42:14 | | | |
| 2 | Amina Yu | -7 word(s) | 7672 | 2022-02-25 08:00:44 | | | | |
| 3 | Amina Yu | -16 word(s) | 7663 | 2022-02-25 08:09:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kloprogge, J. Clays and the Origin of Life. Encyclopedia. Available online: https://encyclopedia.pub/entry/19903 (accessed on 05 March 2026).
Kloprogge J. Clays and the Origin of Life. Encyclopedia. Available at: https://encyclopedia.pub/entry/19903. Accessed March 05, 2026.
Kloprogge, J.. "Clays and the Origin of Life" Encyclopedia, https://encyclopedia.pub/entry/19903 (accessed March 05, 2026).
Kloprogge, J. (2022, February 25). Clays and the Origin of Life. In Encyclopedia. https://encyclopedia.pub/entry/19903
Kloprogge, J.. "Clays and the Origin of Life." Encyclopedia. Web. 25 February, 2022.
Copy Citation
Clays are able to replicate and drive the evolution of metabolism; they have the catalytic ability to synthesize monomers (amino acids, nucleotides and so on) and polymerize them, resulting in RNA–peptide worlds in which RNA replicates (genes) and, in cooperation with coded peptides, drives the evolution of the cell.
clay
catalysis
Mars
Earth
montmorillonite
nontronite
saponite
origin of life
smectite
1. Clay Minerals on Early Earth
There are very few rocks still present on the Earth’s surface from its early days. Pre-early Archean (before 3.5 billion years ago) crust is thought to have been predominantly composed of magmatic rocks in the form of basalt and komatiite lavas [1]. During the Hadean and early Archean, the geothermal gradient was probably much higher than it is now, and convection cells in the mantle were much smaller and more rapid [2]. Hence, the surface of early Earth most likely consisted of solidified lava containing cooled solid blocks comprising peridotite (olivine-rich, high Fe and Mg content)-derived rocks. Consequently, the komatiite–basalt crust was recycled faster than the current ocean floor. Hence, despite the possible presence of some granitoids older than 4 billion years, the Earth’s crust during that period consisted primarily of komatiitic–basalts that created the ocean floor and emerging plateaus.
The original chemical composition of these peridotite and komatiite rocks favored the formation of Fe-Mg clay minerals (saponite, hectorite, and nontronite) instead of Al-rich clay minerals (montmorillonite and beidellite) (Table 1). Fe-Mg clay minerals were produced within chemical microsystems due to seawater weathering or hydrothermal alteration, mostly by post-magmatic processes. Under current atmospheric conditions, magmatic minerals (olivine and pyroxene, which are generally high in Mg and Fe) and their subsequent metamorphic reaction products (serpentine) will readily react when in contact with meteoritic water, and they are then initially altered to trioctahedral phyllosilicates such as talc, kerolite, or stevensite–saponite. When weathering increases in intensity, these secondary minerals will become destabilized, resulting in the formation of dioctahedral Fe-rich clay minerals (mainly nontronite). Because of the lack of oxygen in the Hadean and early Archean atmosphere, iron ions would be mostly in the ferrous state. However, nontronite was discovered in significant quantities on the surface of Mars.
Table 1. Clay minerals mentioned, clay mineral type, and ideal chemical composition.
| Clay Mineral | Type | Chemical Composition |
|---|---|---|
| Allophane | Short-range-order clay | (Al2O3)(SiO2)1.3–2·2.5–3H2O |
| Kaolinite | 1:1 clays, dioctahedral | Al2Si2O5(OH)4 |
| Lizardite | 1:1 serpentine, trioctahedral | Mg3Si2O5(OH)4 |
| Antigorite | 1:1 serpentine, trioctahedral | Mg3Si2O5(OH)4 |
| Chrysotile | 1:1 serpentine, trioctahedral | Mg3Si2O5(OH)4 |
| Talc | 2:1 clay, trioctahedral | Mg3Si4O10(OH)2 |
| Montmorillonite | 2:1 clays, dioctahedral | (M+y.nH2O)(Al3+2-yMg2+y)Si4+4O10(OH)2 |
| Beidellite | 2:1 clays, dioctahedral | (M+x.nH2O)Al3+2(Si4+4-xAl3+x)O10(OH)2 |
| Nontronite | 2:1 clays, dioctahedral | (M+x.nH2O)Fe3+2(Si4+4-xAl3+x)O10(OH)2 |
| Hectorite | 2:1 clays, trioctahedral | (M+y.nH2O)(Mg2+3-yLi+y)(Si4+4O10(OH)2 |
| Saponite | 2:1 clays, trioctahedral | (M+x.nH2O)Mg2+3(Si4+4-xAl3+x)O10(OH)2 |
| Sauconite | 2:1 clays, trioctahedral | (M+x.nH2O)Zn2+3(Si4+4-xAl3+x)O10(OH)2 |
| Stevensite | 2:1 clays, trioctahedral | (Ca,Na)xMg3-x(Si4O10)(OH)2 |
| Vermiculite | 2:1 clays, trioctahedral | Mg0.7(Mg,Fe,Al)6(Si,Al)8O20(OH)4.8H2O |
| Corrensite | 1:1 regular interstratified 1 | (Mg,Fe)9((Si,Al)8O20)(OH)10·nH2O |
| Chlorite | 2:1 clays, interlayer octahedral sheet | ([Mg2+Fe2+]5Al3+)(Si4+3Al3)O10(OH)8 |
M+ is an exchangeable interlayer cation, such as K+ or Na+. 1 Corrensite is a 1:1 regular interstratification of a trioctahedral chlorite with either a trioctahedral vermiculite (low layer charge corrensite) or a trioctahedral smectite (high layer charge corrensite).
During the Hadean, oceans were much larger than they are now and covered most of the Earth’s surface [3]. Consequently, seawater weathering of the cooled and brecciated margins of basalt and komatiite pillow lavas or flows most likely drove major clay-forming processes. Although seawater had a different composition compared to current seawater as well as different conditions (for example, increased temperatures, slightly more acidic (pH 5–6), and dissolved iron concentration close to 100 ppm) [4], it is likely that the mineral reactions were not that different from current reactions because the reactions were buffered by the chemical/mineralogical composition of the rock. Arndt et al. [5][6] indicated that komatiite lava surfaces were generally vitreous and brecciated. Interactions with seawater throughout the quenching stage possibly resulted in the formation of a palagonite-like crust (mixture of altered glass with embryos of clay minerals) in which Fe-Mg clay minerals were formed. During the Hadean and early Archean, this type of weathering at low temperature did not take place for periods long enough to allow the formation of clay minerals in similar amounts compared to other alteration or post-magmatic processes. In fact, seawater was repeatedly heated to boiling as a result of large meteorite impacts and rapidly heated rocks while it circulated through fracture networks produced by the impact shocks [7].
2. Clay Minerals Found in Our Solar System
The occurrence of clay minerals is not restricted to the own planet but are likewise present on the Martian surface and in/on meteorites, asteroids, and comets.
2.1. Mars
The discovery and characterization of clay minerals on Mars have mainly been based on orbital very-near-IR (VNIR) spectra between approximately 0.4 and 5 mm. Thermal infrared (TIR) spectra obtained in the mid-IR (MIR) range between approximately 200 and 2000 cm−1 (approximately 5–50 mm) have similarly provided orbital data about the mineralogy of Mars, including clay minerals. The Mars Science Laboratory (MSL) rover launched by NASA in 2011, landing on Mars in 2012, employs an XRD instrument known as CheMin (short for “Chemistry and Mineralogy”) to detect clay minerals present on the Martian surface at its landing site within the Gale Crater [8], and a number of different Martian missions have obtained surface chemistry information, which was then utilized for the deduction of the possible mineralogical composition [9][10][11]. The most important data for clay minerals on Mars were provided by the OMEGA [12] and CRISM [13] orbital imaging spectrometers, which detected sunlight reflected back from the surface of Mars in the VNIR spectral range. The Fe/Mg-smectites on Mars are mostly detected in rocks about 4 billion years old. Viking’s elemental analyses of surface material led to the hypothesis that clay minerals probably existed on the surface of Mars. Modeling of the major element results seemed to be in agreement with the presence of about 60–80 wt.% smectite [9][10]. It was not until 2004, when the Observatoire pour la Minéralogie, l’Eau, les Glaces et l’Activité (OMEGA) VNIR imaging spectrometer on Mars Express began observing the surface of Mars at approximately 1–3 km resolution, that clay minerals were conclusively detected [14]. Once the Compact Reconnaissance Imaging Spectrometer for Mars (CRISM) instrument on the Mars Reconnaissance Orbiter started measuring targeted high-resolution (18 m/pixel) hyperspectral imagery in 2006, areas with various sized clay mineral-containing outcrops were found almost everywhere where ancient rocks were exposed on the Martian surface [15][16].
The initial evaluation of clay minerals observed on Mars using OMEGA data suggesting that the clay minerals occurred mostly in the old Noachian terrains seems to be correct for most of the planet’s surface [17]. Younger clay-rich deposits do exist in some isolated areas, such as Noctis Labyrinthus, e.g., [18], Coprates Chasma, e.g., [19], and some impact craters. Initial studies of clay minerals containing outcrops in Mawrth Vallis showed that Al-rich clay minerals were consistently found in strata younger than those with Fe/Mg-rich smectite [20]. A later investigation determined that this is true for most of the surface of Mars where clay minerals are exposed [21]. Identification of the clay minerals through analytical instruments onboard the Curiosity rover in the Gale crater provided evidence for the formation of smectites in Hesperian rocks in environments characterized by a low water-to-rock ratio (see, e.g., [22][23][24][25]). Beyond the preserved diversity of clay minerals (smectites comprising nontronite, saponite, beidellite and montmorillonite, vermiculites, corrensites, chlorites, K/Al-rich micas, mixed-layer Fe2+-smectite/mica, kaolinite, mixed-layered kaolinite–smectite, allophane, talc, and serpentines), planetwide mineralogical trends occur. Most of Mars’ alteration (more than 80% of the deposits) seems to have resulted in the formation of Fe/Mg-rich clay minerals with a high smectitic contribution [26][27][28]. The domination of Fe/Mg-rich clay minerals over other clay minerals on Mars is in agreement with water-restricted alteration of the original basaltic and ultramafic rocks, though the related geological environment and associated alteration reactions continue to be a mystery in some instances. A major part of the alteration reactions probably took place in the Martian subsurface, although it is evident that surface alteration reactions did take place all over ancient Mars.
2.2. Meteorites
A meteorite is defined as a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, originating from space and surviving the fall through the atmosphere to impact the surface of a planet or moon. As such, meteorites deliver extraterrestrial clay minerals from space to Earth. Meteorites are classified based on their petrology and origin, with certain meteorites originating from the Moon or Mars, while numerous other meteorites originate from the asteroid belt, found mainly in between the orbits of Jupiter and Mars, or other origins. Most meteorites are of the stony type (chondrites and achondrites), whereas a smaller number belong to the iron or stony-iron types [29][30][31]. Lunar and Martian meteorites belong to the achondrite category.
Early observations of clay minerals and other altered matter present in carbonaceous chondrites have been published for the Allende [32], Plainview [33], and Mokoia [34] meteorites, although whether these clay minerals were formed by terrestrial alteration after arriving on the Earth’s surface or were of extraterrestrial origin was a matter of intense discussion among scientists. Carbonaceous chondrites belonging to the Ivuna and Orgueil types have clay minerals that are thoroughly blended with carbon-rich matter, interpreted as a preterrestrial alteration through reactions with aqueous solutions [35]. The C2 Tagish Lake meteorite contains a combination of clay minerals, carbon, carbonate minerals, and sulfide minerals that are likewise believed to be of preterrestrial origin because of the very brief contact with the Earth’s environment of this meteorite [36]. Most of the Martian meteorites fall into one of three categories: shergottites, nakhlites, and chassignites, with the names derived from the locations where these meteorites were found [37]. The question of whether the clay minerals observed in these particular meteorites were produced through terrestrial weathering instead of preterrestrial reactions has been a matter of intense debate between scientists for many years and was only commonly recognized after large amounts of clay minerals were found on the surface of Mars. Ongoing studies of clay minerals in these meteorites resulted in the identification of mainly Fe-rich smectite [38]. Regrettably, the clay minerals found in meteorites are generally present in very small amounts, on the scale of nanometers or smaller, therefore making identification and characterization extremely difficult. The nakhlite meteorite group has the highest concentration of clay minerals, and therefore, these meteorites have been and are being studied much more thoroughly than others. The most common clay minerals observed consist of high-Fe3+/2+-saponite as well as serpentine [38]. Modeling of the geochemical environments necessary for the crystallization of the detected carbonate minerals and Fe/Mg-smectite in nakhlite-type meteorites suggests that there had to be hydrothermal fluid rich in CO2 at a temperature between 150 and 200 °C with a pH of approximately 6–8 and a water/rock ratio of approximately 300 [39].
2.3. Asteroids and Comets
Information about the hydrated mineral composition of asteroids is essential for understanding the origin of water on Earth, understanding the meteorite record, and solving problems related to the processes that occurred in the beginning of the history of our solar system. Jones et al. [40] studied a series of low-albedo objects, mainly belonging to the C class and its subclasses (using the Tholen asteroid taxonomy [41]) and concluded that the percentage of hydrated asteroids declined with the increase in the semimajor axis from the center of the asteroid belt in an outward direction. They thought that the asteroids initially consisted of a blend of ice together with anhydrous silicates and that the hydrated minerals were produced by aqueous reactions instead of in the initial nebula. In their opinion, in the middle of the belt, hydrated asteroids’ temperatures were sufficiently high to melt the internal ice and produce phyllosilicates, whereas at no time did the outer-belt asteroids reach temperatures sufficiently high to melt ice. The C, B, G, and F asteroids were suggested to encompass an alteration series [42] . These four subclasses each have various amounts of hydrated minerals, ranging from the common hydrated G asteroids to the nearly anhydrous F asteroids. This suggests an alteration series, from the G asteroids, with temperature increases to where melted ice could result in widespread aqueous alteration, to the F asteroids, with temperature increases so high that hydrated minerals broke down [43].
The asteroid Ceres (classified as a dwarf planet since 2006), detected for the first time on 1 January 1801 by Giuseppe Piazzi using the Palermo Astronomical Observatory in Sicily (Italy), is the biggest object in the asteroid belt (940 km diameter) and consists mainly of silicate minerals [44][45]. Spectral features in the near 3 mm telescopic data first raised the idea that clay minerals existed on Ceres [46]. The presence of ammonium-containing clay minerals was based on telescopic measurements of spectral bands around 3.07 mm [47][48]. These data were most consistent with NH4-saponite. Further investigation of better telescopic data of Ceres additionally showed the existence of carbonate as well as Fe-smectite [49]. A relatively recent review by Berg et al. [50] of telescopic spectral data of asteroids and NH4-containing minerals indicated that, besides Ceres, 10-Hygiea and 324-Bamberga also showed spectral characteristics around 3.05–3.07 mm, attributed to ammonium-clay minerals. A number of additional spectral bands are typically observed for NH4-exchanged clay minerals, though these particular bands are more challenging to observe in telescopic spectra of asteroids. Consequently, the present-day interpretation of the Ceres data validates the existence of ammonium-containing clay minerals, although the exact nature of the clay minerals remains to be identified.
The Spitzer Space Telescope has measured TIR spectra (5–40 mm) of a number of Jupiter-family comets, among which is 9B-Tempel 1 [51]. The Spitzer Telescope spectra were measurements of dust particles ejected from the coma of 9B-Tempel 1 prior to and directly following the impact of the Deep Impact mission (launched by NASA in 2005) on its nucleus on 4 July 2005. Spectral features related to clay minerals, carbonate minerals, water ice, and organic compounds were observed in the Spitzer spectral data of the dust particles directly following the impact [52]. Fine particles of crystalline as well as amorphous olivine and pyroxenes were detected in the dust particles around 9B-Tempel 1 prior to and immediately following the impact and have been modeled in combination with the clay minerals, carbonate minerals, water ice, and organic compounds [53]. Lisse et al. [52] determined that nontronite corresponded best to the clay mineral spectral features and estimated a concentration of approximately 5–10% in the dust particles. Next, Lisse et al. [54] assessed the Spitzer Telescope spectral data for comet 9B-Tempel 1 relative to the International Space Observatory (ISO) spectral data of comet Hale–Bopp after its 1995–1996 apparition caused significant dust outflow [55] and an ISO spectrum of HD100546, a young star frequently used for comparison to comets [56]. Spectral analyses of these three bodies suggested the presence of similar components, including nontronite, for comet Hale–Bopp [54].
3. The Clay Hypothesis and the Origin of Life, a Biochemist’s View
The true complexity of the problems concerning the origin and early evolution of life could not be appreciated until the 1960s, during which was acquired knowledge of the genetic code, the Rosetta stone of molecular biology. It had become clear that replication and mutation were properties of nucleic acids, while catalysis and other functions of the cell were mediated by proteins. This situation could not be primitive; it was this separation between genotype (nucleic acids) and phenotype (proteins) that suggested that simpler systems must have preceded the elegant system of molecular biology. The search for simpler systems leading to the independently reproducible subsystem of the present system was usually thought of in terms of nucleic acids or proteins, but an alternative suggestion based on inorganic crystal growth mechanisms was made by Cairns-Smith [57]. Analogies between the behavior of crystals and organisms have a long history. Troland, for example, observed that replication resembled the laying down of one crystal layer from solution onto a pre-existing layer. However, Cairns-Smith went on to make such ideas much more specific, even proposing that the crystals of choice for the first genetic material might be clay minerals.
Clays, because of their enormous surface area and common occurrence (approximately 50% of sedimentary rocks), had already been suggested as sites where concentration and catalysis could take place. In Bernal’s view, clays would be important in the origin of life, but their role was more passive, as they did not replicate but only catalyzed reactions [58]. Schrödinger [59] had speculated on the nature of the modern genetic material before the role of DNA was understood. He suggested that genes would turn out to be “aperiodic crystals”. As Schrodinger put it, “compared with the aperiodic crystal, they [homogeneous crystals] are rather plain and dull. The difference in structure is one of the same kinds as that between an ordinary wallpaper in which the same pattern is repeated again and again in regular periodicity and a masterpiece of embroidery, say a Raphael tapestry, which shows no dull repetition but an elaborate, coherent, meaningful design by the great master”. In a sense, the clay theory proposed the evolution of a two-dimensional chemical tapestry by natural selection. Cairns-Smith [57] stressed that clays were by no means simple homogeneous crystals but full of irregularities of various sorts. Thus, information might be encoded in the substitutions of one ion (for example, magnesium) by another ion (for example, iron) or by dislocations. By analogy with DNA, the distribution of ions in the clay structure might encode information that could replicate, given the right conditions of crystal growth, where, for example, new layers form on pre-existing layers; clays such as montmorillonite are unlike three-dimensional crystals in that any such replicated layer could easily separate from the nucleating layer, allowing new adsorptive and catalytic surfaces to be immediately functional. The two-dimensional character of clay minerals allows them to interact with the aqueous environment more fully than three-dimensional crystals, where the interior is inaccessible.
Here was a suggestion, given in some detail, that made clays not merely catalytic but able to replicate, mutate, and evolve—in other words, the first organisms. However, this generated a new problem. How could a system of replicating clays evolve into the modern cell with its genetic code? This problem was taken up by Hartman, who suggested that the whole of central biochemistry evolved, in conjunction with replicating clays, from (mainly) carbon dioxide, nitrogen, and water [60]. The evolution of metabolism in these proposals contradicted Horowitz’s idea that metabolism had evolved from the outside inwards [61]. Granick’s proposal that “Biosynthesis recapitulates Biopoesis” was revived [62]. Biosynthetic pathways were seen to have evolved from carbon dioxide fixation through the citric acid cycle, culminating in amino acids and nucleotides. The evolution of metabolism was from the inside outwards. Hartman considered iron-rich clays as particular candidates for primitive systems [63]. Hartman further went on to propose an evolutionary pathway leading to the genetic code [64][65]. According to this speculation, the first polypeptides were structural and only later evolved their full catalytic potentiality. In replicating clay systems, complex organizations would evolve by adding to simpler systems, and thus, the evolutionary path could be reconstructed. Thus, it was formed the ideas that generated the Glasgow Workshop: Just how central might clays have been to the origin of life on the Earth?
3.1. Clay Replication
In 1981, a paper was published by Weiss [66] that put the Clay hypothesis front and center among considerations on the problem of the origin of life. With phyllosilicates as models, the notion of replication. For example, the spontaneous self-multiplication of an information carrier, can be proven to be a common property of some macromolecular systems. Mistakes in replication and feedback along with environmental effects may result in mutants with higher or lower replication rates, thereby facilitating evolution. Taking these findings into consideration, the question of whether or not chemical evolution resulted directly in the nucleic acid/protein system, for example, the genetic basis shared among all living systems recognized thus far, has to be answered. It seems possible that as an initial step, a much simpler replicating system had developed: such a system may subsequently have experienced the evolution of replicating systems, producing the final nucleic acid/protein system. The principle of replication and self-multiplication is not restricted to the nucleic acid/protein system; it is a more general property of distinct macromolecular systems. More primitive forms of life or types of protolife, therefore, must also be discussed in the context of the origin of life. Perhaps the question of how nucleic acids and proteins were formed and aptly selected in the course of chemical evolution has been incorrectly phrased. Both might have developed in the course of the evolution of replicating systems. The highly expanding clay minerals are an excellent replicating system model; in the course of an immense number of replications, they might have experienced evolution and selection, forced on the system by the environment, in just the manner expected for the most primitive forms of protolife. The paper had many graphs and diagrams and made the case for the Clay hypothesis. It was well written and was very convincing, but no methods were described.
When the Armin Weiss paper was published, John Lewis and Hyman Hartman immediately thought that a Gordon Conference on the Origin of Life should be organized, so the Gordon Conference on the Origin of Life was begun in 1982 and has flourished since then. Lewis managed the planetary section, and Hartman coordinated the rest, concentrating on Clays and the Origin of Life with an emphasis on the Weiss paper. Hartman invited clay chemists that he knew and hoped that they would critically consider his claims. There was an added bonus for him in that he met Graham Cairns-Smith for the first time at this Gordon Conference. The conference was a great success, as the clay chemists were impressed with Armin Weiss, so Hartman proposed to meet in Glasgow at the University where Cairns-Smith was a lecturer. Having received funding from NASA, he met with other attendees in Glasgow for what was a set of talks that were to be written up for a book that would be published by Cambridge University Press. There were 26 speakers. This resulted in the book: Clay Minerals and the Origin of Life, edited by Cairns-Smith and Hartman [67].
The Glasgow meeting was held in 1983. It was thought that all of the chapters would be written by 1984. There were 25 authors of the book, and it was published in 1986, three years after the meeting. Why did this happen? It happened because there was a problem, and that was that the 26th speaker was Armin Weiss. The whole book was designed to culminate with Armin Weiss’ contribution to the book, this time providing the experimental details so that the experiments could be repeated in other laboratories. For two years, the editors asked for his chapter, and each time, another disaster occurred that prevented him from finishing his chapter. Finally, someone went to Munich and checked the references in his 1981 paper and found that they were irrelevant. Arrhenius et al. [68] were forced to send a letter to Angewendte Chemie indicating that there were troubling questions concerning Armin Weiss’s paper. In a paper published in 1981 in Angewandte Chemie, Professor Armin Weiss described observations with potential importance for the origin and early evolution of life [68]. It has been widely cited since it claimed experimental proof of clay mineral replication, mutational variability in replicating clay crystals, and specific catalytic effects. The results have been invoked in support of the idea that an inorganic evolution through natural selection preceded the evolution of organic life. The information presented in the 1981 Weiss paper is altogether inadequate for others to repeat the key experiments that are said to be “proof of replication”. A single reference in this section reads: “Unpublished results: published in part in G. Mai, Dissertation, Universitat München 1969; P. Brunner, ibid. 1978; S. Fritz, ibid. 1978”. Arrhenius et al. [68] were unable to find the first and third of these theses, while the second had no information that they deemed to have specific relevance to clay replication, nor were repeated attempts to obtain the experimental protocol from Professor Armin Weiss successful. Armin Weiss, like King Ludwig, had built castles that were based on his imagination, but Weiss’ castles were in his head and on paper. This was to be, in the context of a scientific project, the worst possible scenario: specifically, an experimental paper makes a central claim and then does not publish the methods in detail so that others can repeat the experiment. Hartman was furious that he had invested time and NASA money in what had turned out to be a fraud. The damage was done. Clays were no longer at the center of the discussion. The recovery would be long and painful.
3.2. Clay Interactions with Peptides
Hartman met Gerry Soffen in Cambridge, MA, who, at that time, was a project scientist for NASA’s Viking program of Mars landers, the first successful mission to operate unmanned experiments on the Martian surface. As such, he supervised all scientific experiments performed by both landers, managing more than 70 scientists around the USA. Hartman told him about the Clay hypothesis, and he suggested collaborating with a group working at NASA Ames led by Jim Lawless (smectites) and Sherwood Chang (kaolinites). They were working on clays, especially on their interaction with peptides. Hartman joined Jim Lawless’ group and spent three summers there (1978, 1979, and 1980). He spent three summers adding coenzymes, especially pyridoxal phosphate (vitamin B6), to clays. He began with the serine + pyridoxal phosphate reaction. The enzyme is called serine dehydratase (Scheme 1).
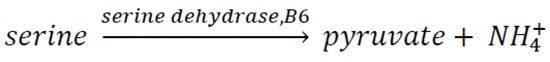
Scheme 1. Reaction of serine to form pyruvate using a coenzyme and clay as catalysts.
The enzyme is composed of a (protein) apoenzyme + a coenzyme (pyridoxal phosphate). Could the clay substitute for the protein (apoenzyme) (Scheme 2)?
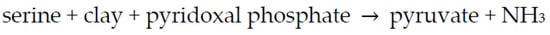
Scheme 2. Reaction of serine to form pyruvate using clay and coenzyme as catalysts.
The clay used was Wyoming bentonite—a montmorillonitic clay that was developed from the alteration of volcanic ash in seawater. The pyridoxal phosphate was added to the Mt in a buffered solution, and the reaction was monitored at various temperatures. The experiment worked beautifully—the Mt did substitute for the protein. Then, Hartman ran a control, which entailed the removal of the Mt and addition of the supernatant to serine and pyridoxal phosphate, and it worked as well. He learned that E.E. Snell, a biochemist, had found that serine underwent this reaction using pyridoxal phosphate and metal ions such as Cu2+ and Al3+. Serine underwent rapid transamination by pyridoxal phosphate in an Al3+- or Cu2+-catalyzed reaction. There was no need for clay. The bentonite was not a pure compound, and there were ions in the supernatant not attached to the clay. While Hartman was conducting these experiments, Max Mortland visited his laboratory. He was intrigued by these experiments. Mortland decided to focus on enzyme transaminase with the apoenzyme as a protein and pyridoxal phosphate as a coenzyme. Hartman decided not to publish, as Snell had already published his results [69].
Mortland [70] showed that glutamic acid was selectively deaminated through a mixture of pyridoxal phosphate (PLP) and Cu2+-smectite. Ammonia was formed together with α-ketoglutaric acid (Scheme 3).
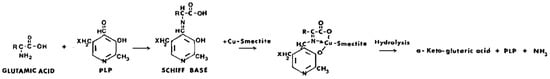
Scheme 3. Reaction of glutamic acid and pyridoxal phosphate to form α-ketoglutaric acid with Cu2+-smectite as the catalyst.
This reaction was far more active with Cu2+-smectite compared to when the Cu2+ cation was simply in solution. Likewise, Cu2+-kaolinite, vermiculite, and faujasites X and Y showed some activity, though at a significantly lower rate, indicating that their activity was probably confined to external surfaces only, whereas that of the smectites involved catalysis in the interlayer spaces. Following the addition of a strong ligand (o-phenanthroline) to the reaction, the reaction rate dropped, clearly indicating that coordination of the reacting species with Cu2+ on the smectite was essential. The suggested reaction pathway consists first of the formation of a Schiff base between the amino acid and pyridoxal phosphate, followed by complexation with Cu2+ at the smectite surface. Next, hydrolysis of the complex to NH3 and the α-keto acid occurs, or transamination takes place to form pyridoxamine phosphate, which is subsequently deaminated by Cu2+-smectite. The pyridoxal phosphate-Cu2+-smectite behaves like a pseudo enzyme, with the clay structure replacing the apoenzyme. Mortland thanked Drs. H. Hartman of the Massachusetts Institute of Technology and J. G. Lawless of the Ames Research Center (NASA), Moffett Field, CA, for sharing their results on pyridoxal phosphate–clay interactions with serine. The reaction mechanisms in both the glutamic and serine cases with pyridoxal phosphate and Cu2+ were identical. Mortland’s paper then stimulated others to explore the catalytic properties of clay minerals with coenzymes and metal ions.
The catalytic activity of smectites in numerous chemical reactions led various researchers to study their possible involvement in the origin of life [67][71][72]. Smectite-catalyzed amino acid polymerization has been shown. These initial results led researchers to think about clays playing the role of enzymes in biological systems. Several researchers, in particular, Mortland [70], Boyd and Mortland [73][74], Siffert and Naidja [75], and Naidja and Siffert [76], studied the catalytic activity of smectites in some simple biochemical reactions. The research by Naidja and Siffert [76] was aimed at learning more about the role of clay minerals as catalysts in certain biochemical reactions. They looked at the oxidative decarboxylation reaction of isocitric acid, which forms the third step of the Krebs cycle. The isocitric acid oxidative decarboxylation reaction was studied with and without homoionic Na+, Mn2+-, and Cu2+-Mt present. The catalytic activity of the Mt was a function of the nature of the interlayer exchangeable cation. Isocitric acid was converted to α-ketoglutaric acid in the presence of Na+-Mt, where Na+ does not produce a complex with the isocitrate anion (Scheme 4).
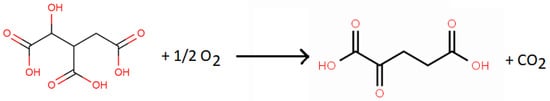
Scheme 15. Reaction of isocitric acid conversion to α-ketoglutaric acid with Na+-montmorillonite as the catalyst.
Instead, with Na+-Mt, the isocitrate anion was oxidized and converted to the α-ketoglutarate anion. The interlayer exchangeable Na+ ions first permitted the intercalation of isocitrate in the interlayer spaces, which did not result in the formation of complexes with isocitrate. Divalent cations (Mn2+ and Cu2+) were removed from the interlayer spaces, which resulted in the formation of “external complexes” with the isocitrate anions. However, the reaction rate was far lower than with the enzymatic system (isocitrate dehydrogenase enzyme and nicotinamide adenine dinucleotide phosphate coenzyme). The outstanding transition metal involved in the origin of life is iron, especially Fe2+ and Fe3+ in the iron clays found on Mars and carbonaceous chondrites. This was clear to Hartman when he convinced Richard Frankel at the Magnet Laboratory (MIT) to investigate the coenzyme flavomononucleotide (FMN) and Fe-clay.
Mortland et al. [77] observed that the adsorption isotherms and UV–visible and Mossbauer spectroscopic data suggested specific interactions between FMN and Fe3+-smectite. The highest adsorbed FMN amount was determined to be 0.3 mmole/g Fe3+-smectite, yielding a 1:1 molar proportion of Fe3+ to FMN. This pointed to the presence of a Fe3+–FMN complex on the smectite surface. Other homoionic smectites (Cu2+, Zn2+, and Ca2+) showed less adsorption and less obvious specific interaction. It is likely that the observed interaction takes place between the Fe3+ on the smectite and the phosphate group on the sugar moiety of FMN. The emphasis has been on transition-state metals rather than coenzymes. However, the behavior of the transition-metal ions in and on the clay lattice is significantly different from their behavior in solution.
3.3. Clay Minerals on Carbonaceous Chondrites
In 1980, Hartman went to MIT, where he joined John Lewis in the Earth Atmosphere and Planetary Sciences. He had received a grant from NASA to work in the field of Clays and the Origin of Life and began working with John Lewis on carbonaceous chondrites. Becker and Epstein [78] performed solvent extractions with CCl4 and CH3OH on the carbonaceous chondrites Murray, Murchison, Orgueil, and Renazzo. Around 2–10% of the total carbon in these meteorites could be extracted using normal methods, mostly in CH3OH. The extracts from Renazzo exhibited isotopic ratios indicating that they consisted primarily of terrestrial organic matter, with smaller amounts from indigenous organics. The CH3OH-soluble organic matter from Murchison and both untreated and HF-treated Murray showed δ13C values between approximately +5 and +10‰ and δ13N values between approximately +90 and +100‰, both of which are substantially higher than those determined for the bulk meteorites. Likewise, the Orgueil CH3OH extract exhibited a δ15N value considerably higher than that of the residual organic matter. δD values between +300 and +500‰ were observed for the CH3OH-soluble organic matter. These results for C, H, and N isotopes make it extremely doubtful that the CH3OH-soluble components were derived from, or simply related to, the insoluble organic polymer present in these meteorites. Organic matter soluble in CCl4 contained almost no N and had lower δ13C and δD values compared to the CH3OH-soluble compounds. Either large isotopic fractionations for C and H occurred between different soluble organic compounds, or the less polar compounds were in part of terrestrial origin. They concluded that the soluble organic material (amino acids and so on) was probably introduced to the meteorites, along with high-δ13C carbonates, during a hydrothermal event that produced hydrous silicates in CI1 and CM2 meteorites. Hartman et al. [79] indicated that organic compounds found in carbonaceous chondrites could be divided into three different fractions. The first fraction, which is insoluble in chloroform and methanol, has a portion that is of interstellar origin. The remaining two fractions, which are chloroform-soluble hydrocarbons and methanol-soluble polar organics, were assumed to have been formed on a planetoid body. They suggested that the polar organic compounds, for example, amino acids, were formed near the surface of the chondrite through the radiolysis of hydrocarbons and ammonium carbonate in a liquid water environment. Certain hydrocarbons could have been produced through a Fischer–Tropsch mechanism inside the chondrite body. The ferrous ion was thought to function as a protection against reverse reactions. The concurrent formation of Fe-rich clays and polar organics may be an indication of events associated with the origin of life on Earth. During the outgassing of the early Earth, events similar to those observed in the carbonaceous chondrites must also have taken place. In particular, in recent years, a new model for the origin of life has been proposed. It emphasizes the role of liquid water, Fe-rich clays, and ultraviolet light in the production of biologically significant molecules. Thus, although the radiation involved is not ultraviolet, carbonaceous chondrites may provide invaluable indications of the early steps that led to life on pre-Archean Earth.
In the summers that Hartman spent at NASA Ames, discussions were centered on how Viking was a failed mission, so he told the exobiology division what he thought about Mars and the Viking Mission, which was that they were looking only for organic molecules and completely ignoring the clays on Mars. They had set the laboratories of Sherwood Chang and Jim Lawless to study clays and paid no attention to what was happening in those laboratories. His ideas were published as an abstract at a meeting on Exobiology and Future Mars Missions [80]. To detect life in the Martian soil, the two Viking landers contained tests that were developed to search for respiration and photosynthesis. These two experiments (labeled release, LR, and pyrolytic release, PR) searching for life in the Martian soils provided positive results. However, no organic molecules were found in the soils of Mars. The explanation provided was that the inorganic compounds present in the Martian soil caused these results. The inorganic composition of the Martian soil was best modeled with a mixture consisting of 60–80% clay, iron oxide, and quartz, together with soluble salts such as halite (NaCl). The minerals most effective in replicating the PR and LR tests were Fe-rich clays. One theory considers clays to be the first organisms able to replicate, mutate, and catalyze and, therefore, to evolve. Clays were formed as a result of the weathering of rocks by liquid water. The distribution of ions, for example, of Al, Mg, and Fe, was thought to act in a manner similar to the sequence of bases in DNA. The information was stored in the distribution of these ions in the octahedral and tetrahedral sites in the clay layers, and they might, similar to RNA and DNA, replicate. When the clays replicated, each clay layer would act as a template for the next layer. The ion substitutions in one clay layer would result in a complementary or similar pattern in the next clay layer formed on its surface. It was hypothesized that on the surface of replicating Fe-rich clays, CO2 might react under light to form organic acids, e.g., formic or oxalic acid. If Mars had liquid water during a warm period in its past, clay formation would have been abundant, something that has since been proven to be the case. These clays would have been able to replicate and evolve until the liquid water disappeared as a result of the cooling of Mars. His suggestion was that they should study the Fe-rich clays of Mars. Since that time, Fe-rich clays have become central to the missions to Mars and to the origin of life on Mars.
3.4. Clay Surface Analysis with Atomic Force Microscopy
In 1988, Hartman moved back to the University of California, Berkeley, where he met Garrison Sposito in the Soil Science Department. They began to study smectites using an atomic force microscope (AFM). The only path that he could envision at the time was to explore new experimental techniques such as Mossbauer spectroscopy and atomic force microscopy. For this purpose, Hartman et al. [81] purified Mt samples of Crook County and Silver Hill Illite and prepared the Na forms, which were subsequently studied under 80% relative humidity with an atomic force microscope. Direct imaging distinctly showed the hexagonal rings of basal oxygen atoms of the SiO4 tetrahedra in the tetrahedral sheet in Mt. AFM might provide useful information at the molecular-scale resolution of clay mineral surfaces such as Mt containing adsorbed organic molecules. This period was followed by several years in which he was not involved in any research related to clay minerals and the origin of life.
3.5. Low-Temperature Clay Synthesis and Replication
Hartman returned to the clay world and decided to replicate clay minerals. It began when he spent the summer of 2005 with Dennis Eberl, who was with the USGS, Boulder, Colorado. They were motivated to explore the possibility that oxalic acid could catalyze the formation of clays from a gel. This was again inspired by the work of Becker and Epstein [78], who showed the coupling of clay formation on carbonaceous chondrites with dicarboxylic acids, amino acids, and other water-soluble organic chemicals. This experiment was run, and the results were analyzed by a group of clay chemists led by Hojatollah Vali (McGill University). The research was carried out by Dirk Schumann, a graduate student, which resulted in his PhD thesis and a paper in Astrobiology [82]. The possible role of clay minerals in the abiotic origin of life is the topic of ongoing debate that started several decades ago. The main focus is on the clay minerals detected in a class of meteorites called carbonaceous chondrites. These clay minerals were formed through aqueous alteration of anhydrous minerals, for example, olivine and orthopyroxene, that are frequently found in the chondrules. Furthermore, a strong correlation exists between the existence of these clay minerals and the occurrence of polar organic molecules. Laboratory experiments have proven that at low temperature and ambient pressure, polar organic molecules, for example, the oxalate ion observed in meteorites, are able to catalyze the formation of clay minerals. In this particular investigation, it was proven that oxalate was a strong catalyst in the formation of saponite, an Al- and Mg-rich trioctahedral clay mineral, from a silicate gel at a temperature of only 60 °C and ambient pressure. High-resolution transmission electron microscopy (TEM) of the synthetic saponite intercalated with octadecylammonium cations showed the presence of 2:1 layer structures with variable negative layer charge. The formation of these differently charged 2:1 layers within the saponite particles most likely took place independently. If polar organic molecules, such as oxalate, are able to catalyze the crystallization of clay minerals, which then can promote the formation of clay microenvironments and offer a large number of adsorption sites on the clay surfaces for other organic molecules existing in the solution, the interaction between the adsorbed molecules could result in polymerization, resulting in more complex organic molecules, such as RNA from nucleotides on early Earth. The major findings were twofold: (1) Oxalic acid was a catalyst of clay formation. This was suggested by B. Siffert in a chapter in Clay Minerals and the Origin of Life entitled “The role of Organic Complexing Agents” [83]. He stated, “A number of successful attempts to synthesize aluminum phyllosilicates at ambient temperature have been reported. In most cases organic aluminum complexes were used”. (2) The crystallization of these differently charged saponites most likely occurred independently. The fact that saponites with variable charge formed from the same gel has consequences for our interpretation of how life originated, as these 2:1 clay minerals most likely replicate via a process of template-catalyzed polymerization and transmit the charge distribution from one layer to the next layer. The reason that the second finding was so interesting was that Hartman had discussed his publication on the formation of clay minerals in soils with Isaac Barshad in the Soil Department at the University of California, Berkeley. He pointed out that his experience with clay formation in soils was that where conditions for clay formation were the same, the clays formed a diverse assemblage of different clays. The reason for his observation was that clay formation is catalyzed by seed clays [84].
The synthesis of clays in the years 2005–2006 took 3 months at 60 °C. This was not going to allow a lot of experiments to be performed, so Hartman searched for a simpler and shorter synthesis at temperatures lower than 100 °C. Then came the reason that he became the editor for Clays and the Origin of Life in the journal Life. He came across the paper by Kloprogge et al. [85] on the synthesis of smectite clay minerals, and on page 533, he read the following paragraph:
“Following a new approach, Vogels et al. (1995) synthesized saponites at 90 °C from a Si-Al gel and a solution containing urea and an M2+-nitrate [M2+ = Zn, Mg, Ni, Co] in only a few (5–20) h. Precipitation from homogeneous solution was induced by the slow hydrolysis of the urea, which resulted in a homogeneous release of hydroxyl ions. The octahedral divalent metal had a large influence on the characteristics of the saponite product, such as stacking order, surface area, and pore volume. With Mg the nucleation and growth were rather slow compared to saponites containing Co or Zn. After 20 h with Mg some gel was still present and even after 2 d almost no stacking was observed by transmission electron microscopy (TEM) in agreement with the absence of the (001) reflection in the XRD pattern. The results with different divalent cations indicated that stacking increased in the order Ni = Mg < Co < Zn. Both the surface area and pore volume increased as Zn < Co < Ni < Mg. The A1 distribution over the tetrahedral, octahedral, and interlayer sites was influenced by increasing the initial Si/A1 ratio in the starting gel from 5.7 to 39. Adjusting both the octahedral-sheet and tetrahedral-sheet composition by the choice of divalent metals or combinations of metals and the Si/A1 ratio offers the possibility to control properties like surface area, pore volume, and acidity” [86]. Hartman immediately began to synthesize Zn-clays using the method of Vogels et al. [86] in Roger Summon’s laboratory at MIT, and it worked. Hartman modified it by adding oxalic acid and NaOH, and this worked as well. He then contacted Marcelo Guzman at the University of Kentucky, and they began a collaboration with his graduate student Ruixin Zhou. The synthetic Zn-clays were characterized by Chris Matocha and Hojatollah Vali and his group (McGill University). The role of primordial metabolic networks, for example, the reverse tricarboxylic acid (rTCA) cycle, and the coevolution of clay mineral catalysts in the origin of life is still not properly understood. Although prebiotic reactions from the rTCA cycle have been achieved through photochemistry on semiconductor minerals [87], the formation of clay minerals has been shown to be catalyzed by oxalate at low temperature and ambient pressure [82]. Zhou et al. [88] reported on the succinate-catalyzed crystallization of sauconite as a model for clay minerals using a photoproduced intermediate from central metabolism as an example. In addition, they showed that seeding induced nucleation at low temperatures, speeding up the crystallization process. Their results indicate that the coevolution of clay minerals and early metabolites on the Earth’s surface might have been accelerated by sunlight-induced photochemistry, which played a major role in the intricate interactions between rock surfaces and life at a geological timescale. The most important finding is that seeding increased crystallization. The catalytic power of the synthesized clay was validated by replicating the synthesis at 70 °C after adding a single (macroscopic) sauconite particle obtained from the 90 °C synthesized product to the starting gel. The fact that a single sauconite particle acted as a seed crystal to produce a larger amount of sauconite provides an excellent example of the self-catalytic power of clay minerals with respect to their nucleation and crystallization. The surface of the seed particle is thought to enable heterogeneous nucleation at lower temperature via a reduction in the activation energy for crystallization. The detected acceleration in crystallization by seeding does not merely depend on random events but is the result of clay surface interactions with chemical species present in the starting gel at 70 °C. The seed particle interaction with soluble free and complexed ions moving freely in the gel creates intermolecular forces that are required to produce the crystal lattice. The addition of a seed particle to the gel offers a pathway to direct a process that is then independent of random interactions.
References
- O’Neil, J.; Carlson, R.W.; Francis, D.; Stevenson, R.K. Neodynium-142 evidence for Hadean mafic crust. Science 2008, 321, 1828–1831.
- Davies, G.F. On the emergence of plate tectonics. Geology 1992, 20, 963–966.
- Arndt, N.T. Why was flood volcanism on submerged continental platforms so common in the Precambrian? Precambrian Res. 1999, 97, 155–164.
- Macleod, G.; McKeown, C.; Hall, A.J.; Russell, M.J. Hydrothermal and oceanic pH conditions of possible relevance to the origin of life. Orig. Life Evol. Biosph. 1994, 24, 19–41.
- Arndt, N.; Lesher, C.M.; Barnes, S.J. Komatiite; Cambridge University Press: Cambridge, UK, 2008; p. 467.
- Arndt, N.T.; Naldrett, A.J.; Pyke, D.R. Komatiitic and Iron-rich tholeiitic lavas of Munro Township, Northeast Ontario. J. Petrol. 1977, 18, 319–369.
- Cockell, C.S. The origin and emergence of life under impact bombardment. Philos. Trans. R. Soc. B 2006, 361, 1845–1856.
- Blake, D.F.; Morris, R.V.; Kocurek, G.; Morrison, S.M.; Downs, R.T.; Bish, D.; Ming, D.W.; Edgett, K.S.; Rubin, D.; Goetz, W.; et al. Curiosity at Gale Crater, Mars: Characterization and Analysis of the Rocknest Sand Shadow. Science 2013, 341.
- Baird, A.K.; Castro, A.J.; Clark, B.C.; III, T.P.; Rose, H., Jr.; Keil, K.; Gooding, J.L. The Viking X ray fluorescence experiment: Sampling strategies and laboratory simulations. J. Geophys. Res. 1977, 82, 4595–4624.
- Toulmin, P., III; Baird, A.K.; Clark, B.C.; Keil, K.; Rose, H.J., Jr.; Christian, R.P.; Evans, P.H.; Kelliher, W.C. Geochemical and mineralogical interpretation of the Viking inorganic chemical results. J. Geophys. Res. (1896–1977) 1977, 82, 4625–4634.
- McSween, H.Y.J., Jr.; Taylor, G.J.; Wyat, M.B. Elemental composition of the Martian crust. Science 2009, 324, 736–739.
- Bibring, J.P.; Langevin, Y.; Gendrin, A.; Gondet, B.; Poulet, F.; Berthé, M.; Soufflot, A.; Arvidson, R.; Mangold, N.; Mustard, J.; et al. Mars surface diversity as revealed by the OMEGA/Mars Express observations. Science 2005, 307, 1576–1581.
- Murchie, S.L.; Seelos, F.P.; Hash, C.D.; Humm, D.C.; Malaret, E.; McGovern, J.A.; Choo, T.H.; Seelos, K.D.; Buczkowski, D.L.; Morgan, M.F.; et al. Compact Reconnaissance Imaging Spectrometer for Mars investigation and data set from the Mars Reconnaissance Orbiter’s primary science phase. J. Geophys. Res. Planets 2009, 114, E00D07.
- Poulet, F.; Bibring, J.-P.; Mustard, J.F.; Gendrin, A.; Mangold, N.; Langevin, Y.; Arvidson, R.E.; Gondet, B.; Gomez, C.; Omega Team. Phyllosilicates on Mars and implications for early Martian climate. Nature 2005, 438, 623–627.
- Mustard, J.F.; Murchie, S.L.; Pelkey, S.M.; Ehlmann, B.L.; Milliken, R.E.; Grant, J.A.; Bibring, J.-P.; Poulet, F.; Bishop, J.L.; Noe Dobrea, E.Z.; et al. Hydrated silicate minerals on mars observed by the mars reconnaissance orbiter CRISM instrument. Nature 2008, 454, 305–309.
- Murchie, S.L.; Mustard, J.F.; Ehlmann, B.L.; Milliken, R.E.; Bishop, J.L.; McKeown, N.K.; Noe Dobrea, E.Z.; Seelos, F.P.; Buczkowski, D.L.; Wiseman, S.M.; et al. A synthesis of Martian aqueous mineralogy after 1 Mars year of observations from the Mars Reconnaissance Orbiter. J. Geophys. Res. Planets 2009, 114.
- Bibring, J.-P.; Langevin, Y.; Mustard, J.F.; Poulet, F.; Arvidson, R.; Gendrin, A.; Gondet, B.; Mangold, N.; Pinet, P.; Forget, F. Global mineralogical and aqueous Mars history derived from OMEGA/Mars Express data. Science 2006, 312, 400–404.
- Weitz, C.M.; Bishop, J.L.; Thollot, P.; Mangold, N.; Roach, L.H. Diverse mineralogies in two troughs of Noctis Labyrinthus, Mars. Geology 2011, 39, 899–902.
- Weitz, C.M.; Bishop, J.L.; Baker, L.L.; Berman, D.C. Fresh exposures of hydrous Fe-bearing amorphous silicates on Mars. Geophys. Res. Lett. 2014, 41, 8744–8751.
- Bishop, J.L.; Noe Dobrea, E.Z.; McKeown, N.K.; Parente, M.; Ehlmann, B.L.; Michalski, J.R.; Milliken, R.E.; Poulet, F.; Swayze, G.A.; Mustard, J.F.; et al. Phyllosilicate diversity and past aqueous activity revealed at Mawrth Vallis, Mars. Science 2008, 321, 830–833.
- Carter, J.; Loizeau, D.; Mangold, N.; Poulet, F.; Bibring, J.-P. Widespread surface weathering on early Mars: A case for a warmer and wetter climate. Icarus 2015, 248, 373–382.
- McLennan, S.M.; Anderson, R.B.; Bell, J.F.; Bridges, J.C.; Calef, F.; Campbell, J.L.; Clark, B.C.; Clegg, S.; Conrad, P.; Cousin, A.; et al. Elemental Geochemistry of Sedimentary Rocks at Yellowknife Bay, Gale Crater, Mars. Science 2014, 343.
- Grotzinger, J.P.; Gupta, S.; Malin, M.C.; Rubin, D.M.; Schieber, J.; Siebach, K.; Sumner, D.Y.; Stack, K.M.; Vasavada, A.R.; Arvidson, R.E.; et al. Deposition, exhumation, and paleoclimate of an ancient lake deposit, Gale crater, Mars. Science 2015, 350.
- Grotzinger, J.P.; Sumner, D.Y.; Kah, L.C.; Stack, K.; Gupta, S.; Edgar, L.; Rubin, D.; Lewis, K.; Schieber, J.; Mangold, N.; et al. A habitable Fluvio-Lacustrine environment at Yellowknife Bay, Gale Crater, Mars. Science 2014, 343.
- Bristow, T.F.; Bish, D.L.; Vaniman, D.T.; Morris, R.V.; Blake, D.F.; Grotzinger, J.P.; Rampe, E.B.; Crisp, J.A.; Achilles, C.N.; Ming, D.W.; et al. The origin and implications of clay minerals from Yellowknife Bay, Gale crater, Mars. Am. Mineral. 2015, 100, 824–836.
- Carter, J.; Poulet, F.; Bibring, J.P.; Mangold, N.; Murchie, S. Hydrous minerals on Mars as seen by the CRISM and OMEGA imaging spectrometers: Updated global view. J. Geophys. Res. Planets 2013, 118, 831–858.
- Ehlmann, B.L.; Mustard, J.F.; Murchie, S.L.; Bibring, J.P.; Meunier, A.; Fraeman, A.A.; Langevin, Y. Subsurface water and clay mineral formation during the early history of Mars. Nature 2011, 479, 53–60.
- Michalski, J.R.; Cuadros, J.; Bishop, J.L.; Darby Dyar, M.; Dekov, V.; Fiore, S. Constraints on the crystal-chemistry of Fe/Mg-rich smectitic clays on Mars and links to global alteration trends. Earth Planet. Sci. Lett. 2015, 427, 215–225.
- Wasson, J.T. Meteorites: Their Record of Early Solar-System History; Freeman: New York, NY, USA, 1985; p. 267.
- Lauretta, D.S.; McSween, H.Y., Jr. (Eds.) Meteorites and the Early Solar System II; The University of Arizona Press: Tucson, AZ, USA, 2006; p. 942.
- Papike, J.J. (Ed.) Planetary Materials; Mineralogical Society of America: Washington, DC, USA, 1989; Volume 36, p. 1059.
- Dominik, B.; Jessberger, E.K.; Staudacher, T.; Nagel, K.; El Goresy, A. A new type of white inclusion in allende—petrography, mineral chemistry, 40Ar–39Ar ages and genetic implications. In Proceedings of the 9th Lunar Planet Science Conference, Houston, TX, USA, 13–17 March 1978; pp. 1249–1266.
- Nozette, S.; Wilkening, L.L. Evidence for aqueous alteration in a carbonaceous xenolith from the Plainview (H5) chondrite. Geochim. Cosmochim. Acta 1982, 46, 557–563.
- Cohen, R.E.; Kornacki, A.S.; Wood, J.A. Mineralogy and petrology of chondrules and inclusions in the Mokoia CV3 chondrite. Geochim. Cosmochim. Acta 1983, 47, 1739–1757.
- Morlok, A.; Bischoff, A.; Stephan, T.; Floss, C.; Zinner, E.; Jessberger, E.K. Brecciation and chemical heterogeneities of CI chondrites. Geochim. Cosmochim. Acta 2006, 70, 5371–5394.
- Brown, P.G.; Hildebrand, A.R.; Zolensky, M.E.; Grady, M.; Clayton, R.N.; Mayeda, T.K.; Tagliaferri, E.; Spalding, R.; Macrae, N.D.; Hoffman, E.L.; et al. The fall, recovery, orbit, and composition of the Tagish Lake Meteorite: A new type of carbonaceous chondrite. Science 2000, 290, 320–325.
- McSween, H.Y.J., Jr.; Treiman, A.H. Martian meteorites. In Planet. Mater; Papike, J.J., Ed.; Mineralogical Society of America: Washington, DC, USA, 1998; Volume 36, pp. 6:1–6:53.
- Treiman, A.H.; Barrett, R.A.; Gooding, J.L. Preterrestrial aqueous alteration of the Lafayette (SNC) meteorite. Meteoritics 1993, 28, 86–97.
- Bridges, J.C.; Schwenzer, S.P. The nakhlite hydrothermal brine on Mars. Earth Planet. Sci. Lett. 2012, 359–360, 117–123.
- Jones, T.D.; Lebofsky, L.A.; Lewis, J.S.; Marley, M.S. The composition and origin of the C, P, and D asteroids: Water as a tracer of thermal evolution in the outer belt. Icarus 1990, 88, 172–192.
- Tholen, D.J.; Barucci, M.A. Asteroid taxonomy. In Asteroids II; Binzel, R.P., Gehrels, T., Shapley Matthews, M., Eds.; University of Arizona Press: Tucson, AZ, USA, 1989; pp. 298–315.
- Bell, J.F.; Davis, D.R.; Hartmann, W.K.; Gaffey, M.J. The big picture. In Asteroids II; Binzel, R.P., Gehrels, T., Shapley Matthews, M., Eds.; University of Arizona Press: Tucson, AZ, USA, 1989; pp. 921–945.
- Rivkin, A.S.; Howell, E.S.; Vilas, F.; Lebofsky, L.A. Hydrated Minerals on Asteroids: The Astronomical Record. In Asteroids III; Bottke, W.F., Jr., Cellino, A., Paolicchi, P., Binzel, R.P., Eds.; University of Arizona Press: Tucson, AZ, USA, 2002; pp. 235–253.
- McCord, T.; Gaffey, M.J. Asteroids: Surface composition from reflectance spectroscopy. Science 1974, 186, 352–355.
- McCord, T.B.; Sotin, C. Ceres: Evolution and current state. J. Geophys. Res. Planets 2005, 110, E05009.
- Lebofsky, L.A.; Feierberg, M.A.; Tokunaga, A.T.; Larson, H.P.; Johnson, J.R. The 1.7- to 4.2-micron spectrum of asteroid 1 Ceres—evidence for structural water in clay minerals. Icarus 1981, 48, 453–459.
- King, T.V.V.; Clark, R.N.; Calvin, W.M.; Sherman, D.M.; Brown, R.H. Evidence for ammonium-bearing minerals on Ceres. Science 1992, 255, 1551–1553.
- Rivkin, A.S.; Davies, J.K.; Johnson, J.R.; Ellison, S.L.; Trilling, D.E.; Brown, R.H.; Lebofsky, L.A. Hydrogen concentrations on C-class asteroids derived from remote sensing. Meteor. Planet. Sci. 2003, 38, 1383–1398.
- Rivkin, A.S.; Volquardsen, E.L.; Clark, B.E. The surface composition of Ceres: Discovery of carbonates and iron-rich clays. Icarus 2006, 185, 563–567.
- Berg, B.L.; Cloutis, E.A.; Beck, P.; Vernazza, P.; Bishop, J.L.; Driss, T.; Reddy, V.; Applin, D.; Mann, P. Reflectance spectroscopy (0.35–25 mm) of ammonium-bearing minerals and comparison to Ceres family asteroids. Icarus 2016, 265, 218–237.
- Werner, M.W.; Roellig, T.L.; Low, F.J.; Rieke, G.H.; Rieke, M.; Hoffmann, W.F.; Young, E.; Houck, J.R.; Brandl, B.; Fazio, G.G.; et al. The Spitzer Space Telescope mission. Astrophys. J. Suppl. S. 2004, 154, 1–9.
- Lisse, C.M.; Vancleve, J.; Adams, A.C.; A’Hearn, M.F.; Fernández, Y.R.; Farnham, T.L.; Armus, L.; Grillmair, C.J.; Ingalls, J.; Belton, M.J.S.; et al. Spitzer spectral observations of the deep impact ejecta. Science 2006, 313, 635–640.
- Kelley, M.S.; Wooden, D.H. The composition of dust in Jupiter-family comets inferred from infrared spectroscopy. Planet. Space Sci. 2009, 57, 1133–1145.
- Lisse, C.M.; Kraemer, K.E.; Nuth III, J.A.; Li, A.; Joswiak, D. Comparison of the composition of the Tempel 1 ejecta to the dust in Comet C/Hale–Bopp 1995 O1 and YSO HD 100546. Icarus 2007, 187, 69–86.
- Williams, D.M.; Mason, C.G.; Gehrz, R.D.; Jones, T.J.; Charles, E.W.; Harker, D.E.; Hanner, M.S.; Wooden, D.H.; Witteborn, F.C.; Butner, H.M. Measurement of Submicron Grains in the Coma of Comet Hale-Bopp C/1995 O1 during 1997 February 15–20 UT. Astrophys. J. 1997, 489, L91–L94.
- Waelkens, C.; Malfait, K.; Waters, L.B.F.M. Comet Hale-Bopp, circumstellar dust, and the interstellar medium. Earth Moon Planets 1997, 79, 265–274.
- Cairns-Smith, A.G. The origin of life and the nature of the primitive gene. J. Theor. Biol. 1966, 10, 53–88.
- Bernal, J.D. The physical basis of life. Proc. Phys. Soc. A 1949, 62, 537–558.
- Schrödinger, E. What Is Life?: The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1944; p. 91.
- Hartman, H. Speculations on the origin and evolution of metabolism. J. Mol. Evol. 1975, 4, 359–370.
- Horowitz, N.H. On the Evolution of Biochemical Syntheses. Proc. Nat. Acad. Sci. USA 1945, 31, 153–157.
- Granick, S. Speculations on the origins and evolution of photosynthesis. Ann. N. Y. Acad. Sci. 1957, 69, 292–308.
- Hartman, H. Photosynthesis and the origin of life. Orig. Life Evol. Biosph. 1998, 28, 515–521.
- Hartman, H. Speculations on the evolution of the genetic code. Orig. Life 1975, 6, 423–427.
- Hartman, H.; Smith, T.F. Origin of the Genetic Code Is Found at the Transition between a Thioester World of Peptides and the Phosphoester World of Polynucleotides. Life 2019, 9, 69.
- Weiss, A. Replication and evolution in inorganic systems. Angew. Chem. Int. Ed. 1981, 20, 850–860.
- Cairns-Smith, A.G.; Hartman, H. (Eds.) Clays as catalysts. In Clay Minerals And The Origin Of Life; Cambridge University Press: Cambridge, UK, 1986; pp. 130–138.
- Arrhenius, G.; Cairns-Smith, A.G.; Hartman, H.; Miller, S.L.; Orgel, L.E. Remarks on the Review Article “Replication and Evolution in Inorganic Systems” by Armin Weiss. Angew. Chem. Int. Ed. 1986, 98, 654.
- Metzler, D.E.; Snell, E.E. Deamination of serine. I. Catalytic deamination of serine and cysteine by pyridoxal and metal salts. J. Biol. Chem. 1952, 198, 353–361.
- Mortland, M.M. Deamination of glutamic acid by pyridoxal phosphate-Cu2+-smectite catalysts. J. Mol. Catal. 1984, 27, 143–155.
- Cairns-Smith, A.G. Genes made of clay. New Sci. 1974, 64, 274–276.
- Cairns-Smith, A.G. The first organisms. Sci. Am. 1985, 252, 74–82.
- Boyd, S.A.; Mortland, M.M. Manipulating the activity of immobilized enzymes with different organo-smectite complexes. Experientia 1985, 12, 1564–1566.
- Boyd, S.A.; Mortland, M.M. Selective effects of smectite-organic complexes on the activities of immobilized enzymes. J. Mol. Catal. 1986, 34, 1–8.
- Siffert, B.; Naidja, A. Décarboxylation catalytique de l’acide oxaloacétique en présence de montmorillonite. Clay Miner. 1987, 22, 435–446.
- Naidja, A.; Siffert, B. Oxidative decarboxylation of isocitric acid in the presence of montmorillonite. Clay Miner. 1990, 25, 27–37.
- Mortland, M.M.; Lawless, J.G.; Hartman, H.; Frankel, R. Smectite interactions with flavomononucleotide. Clays Clay Miner. 1984, 32, 279–282.
- Becker, R.H.; Epstein, S. Carbon, hydrogen and nitrogen isotopes in solvent-extractable organic matter from carbonaceous chondrites. Geochim. Cosmochim. Acta 1982, 46, 97–103.
- Hartman, H.; Sweeney, M.A.; Kropp, M.A.; Lewis, J.S. Carbonaceous chondrites and the origin of life. Orig. Life Evol. Biosph. 1993, 23, 221–227.
- Hartman, H. Mars, Clays and the origins of life. In Proceedings of the Exobiology and Future Mars Missions: A NASA Symposium Held in Sunnyvale, Sunnyvale, CA, USA, 23–25 March 1988; pp. 30–31.
- Hartman, H.; Sposito, G.; Yang, A.; Manne, S.; Gould, S.A.C.; Hansma, P.K. Molecular-scale imaging of clay mineral surfaces with the atomic microscope. Clays Clay Miner. 1990, 38, 337–342.
- Schumann, D.; Hartman, H.; Eberl, D.D.; Sears, S.K.; Hesse, R.; Vali, H. Formation of Replicating Saponite from a Gel in the Presence of Oxalate: Implications for the Formation of Clay Minerals in Carbonaceous Chondrites and the Origin of Life. Astrobiology 2012, 12, 549–561.
- Siffert, B. The role of organic complexing agents. In Clay Minerals and the Origin of Life; Cairns-Smith, A.G., Hartman, H., Eds.; Cambridge University Press: Cambridge, UK, 1986; pp. 75–78.
- Barshad, I. Soil Development. In Chemistry of the Soil; Bear, F.E., Ed.; Reinhold Publishing Corp.: New York, NY, USA, 1955; pp. 1–52.
- Kloprogge, J.T.; Komarneni, S.; Amonette, J.E. Synthesis of smectite clay minerals: A critical review. Clays Clay Miner. 1999, 47, 529–554.
- Vogels, R.J.M.J.; Kerkhoffs, M.J.H.V.; Geus, J.W. Non-hydrothermal synthesis, characterisation and catalytic properties of saponite clays. Stud. Surf. Sci. Catal. 1995, 91, 1153–1161.
- Guzman, M.I. Abiotic Photosynthesis: From Prebiotic Chemistry to Metabolism. In Origins of Life: The Primal Self-Organization; Egel, R., Lankenau, D.-H., Mulkidjanian, A.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 85–105.
- Zhou, R.; Basu, K.; Hartman, H.; Matocha, C.J.; Sears, S.K.; Vali, H.; Guzman, M.I. Catalyzed Synthesis of Zinc Clays by Prebiotic Central Metabolites. Sci. Rep. 2017, 7, 533.
More
Information
Subjects:
Mineralogy; Evolutionary Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
6.3K
Entry Collection:
Organic Synthesis
Revisions:
3 times
(View History)
Update Date:
25 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





