
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lucas Henrique Rodrigues da Silva | + 2436 word(s) | 2436 | 2022-02-15 08:51:59 | | | |
| 2 | Yvaine Wei | Meta information modification | 2436 | 2022-02-25 02:48:41 | | |
Video Upload Options
Glioblastoma (GBM) is the primary cancer with the highest incidence within the central nervous system. Natural killer (NK) cells are innate lymphocytes that play an important role in immunosurveillance, acting alongside other immune cells in the response against various types of malignant tumors and the prevention of metastasis. NK cells are being exploited in many ways to treat cancer. The broad arsenal of NK-based therapies includes adoptive transfer of in vitro expanded and activated cells, genetically engineered cells to contain chimeric antigen receptors (CAR-NKs), in vivo stimulation of NK cells (by cytokine therapy, checkpoint blockade therapies, etc.), and tumor-specific antibody-guided NK cells, among others.
1. Introduction
Glioblastoma (GBM) considerably reduces patients’ quality of life [1]. Its current treatment—including maximal surgical resection, adjuvant radiotherapy, and chemotherapy with temozolomide—significantly improves patient survival [2]. However, this therapeutic regimen is still not enough, as half of the patients die within approximately 15 months after diagnosis [3], and less than 5% of them remain alive after 5 years [4]. Relapse occurs frequently, and death is inevitable.
A broad arsenal of treatments has been established to control or potentially cure neoplasms; more recently, immunotherapy has emerged as a new therapeutic pillar in oncology. Within this treatment modality, most strategies have been centered on either antibodies or T cells, but lately, new studies have sought to take advantage of the antitumor activity of natural killer (NK) cells.
2. Natural Killer Cells
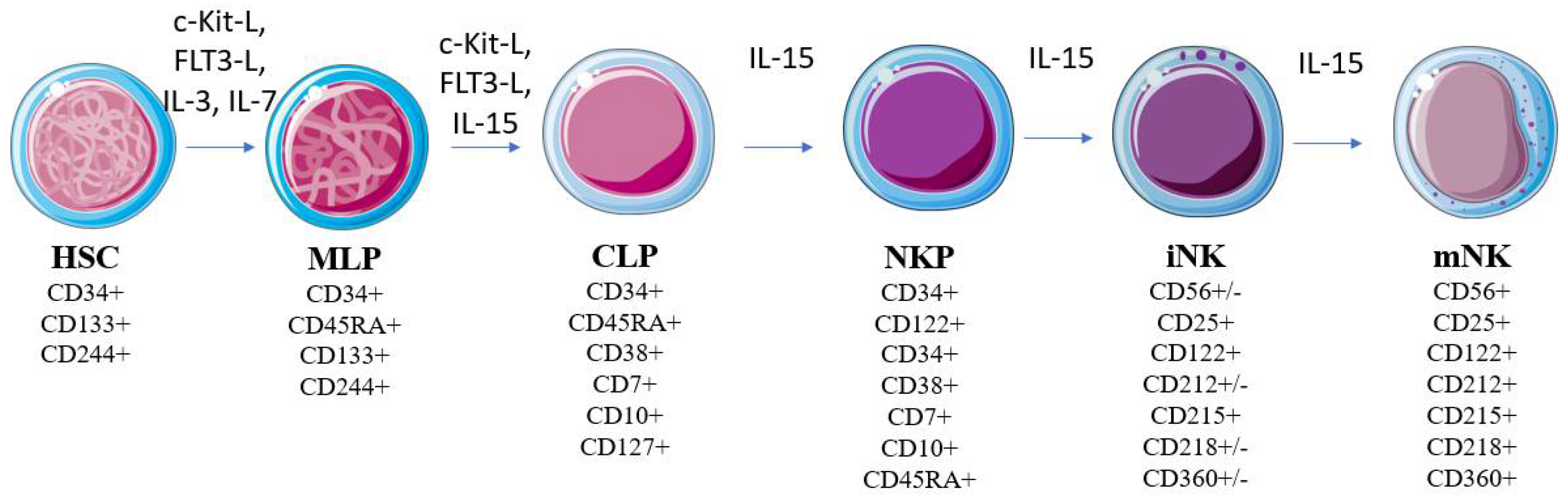
3. NK Cells in Immunosurveillance against Cancer
3.1. Tumor Cell Recognition
3.2. Antitumor Functions
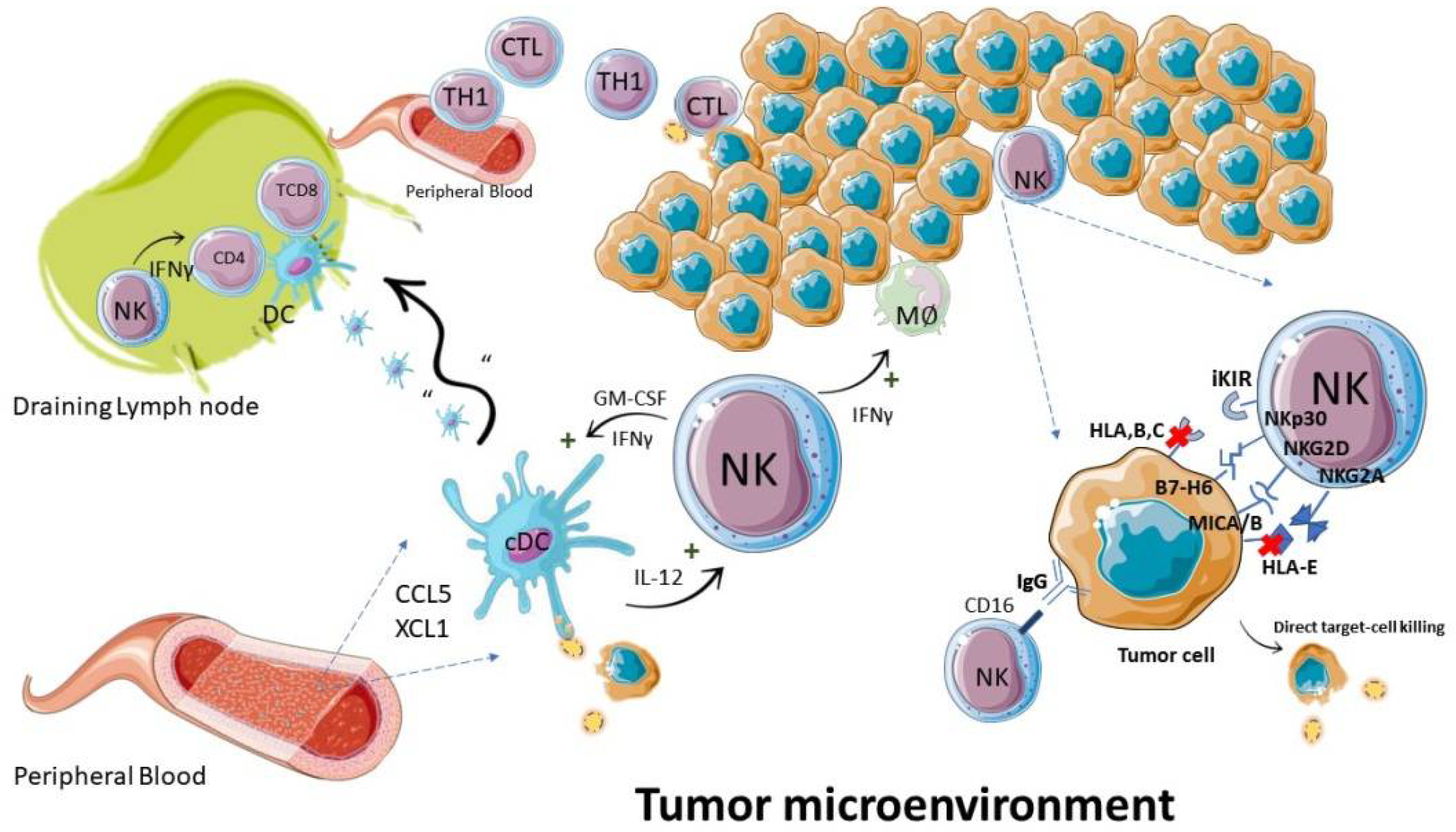
4. NK Cells in GBM
5. NK-Cell-Based Immunotherapy
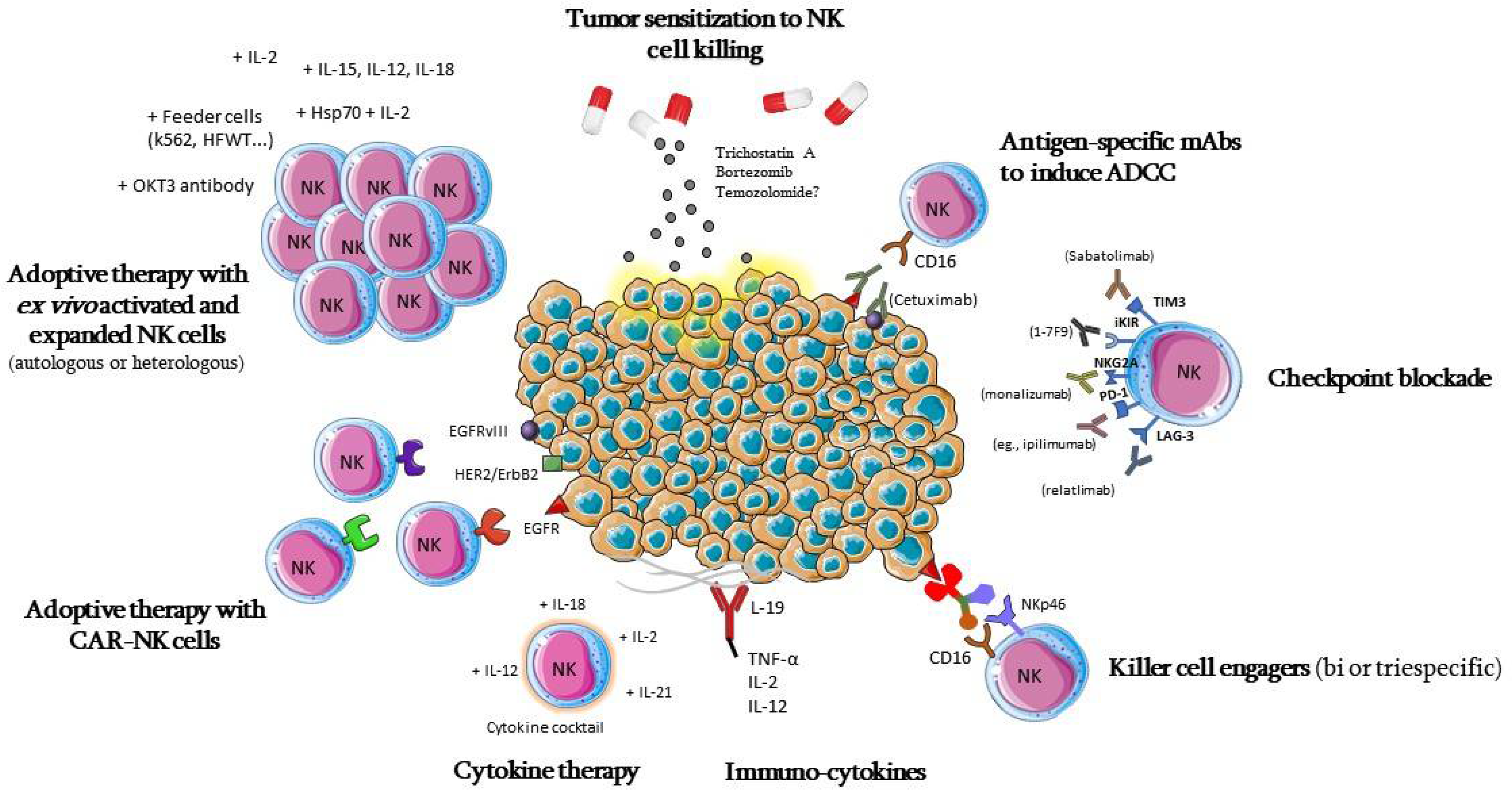
5.1. Adoptive Cell Therapy
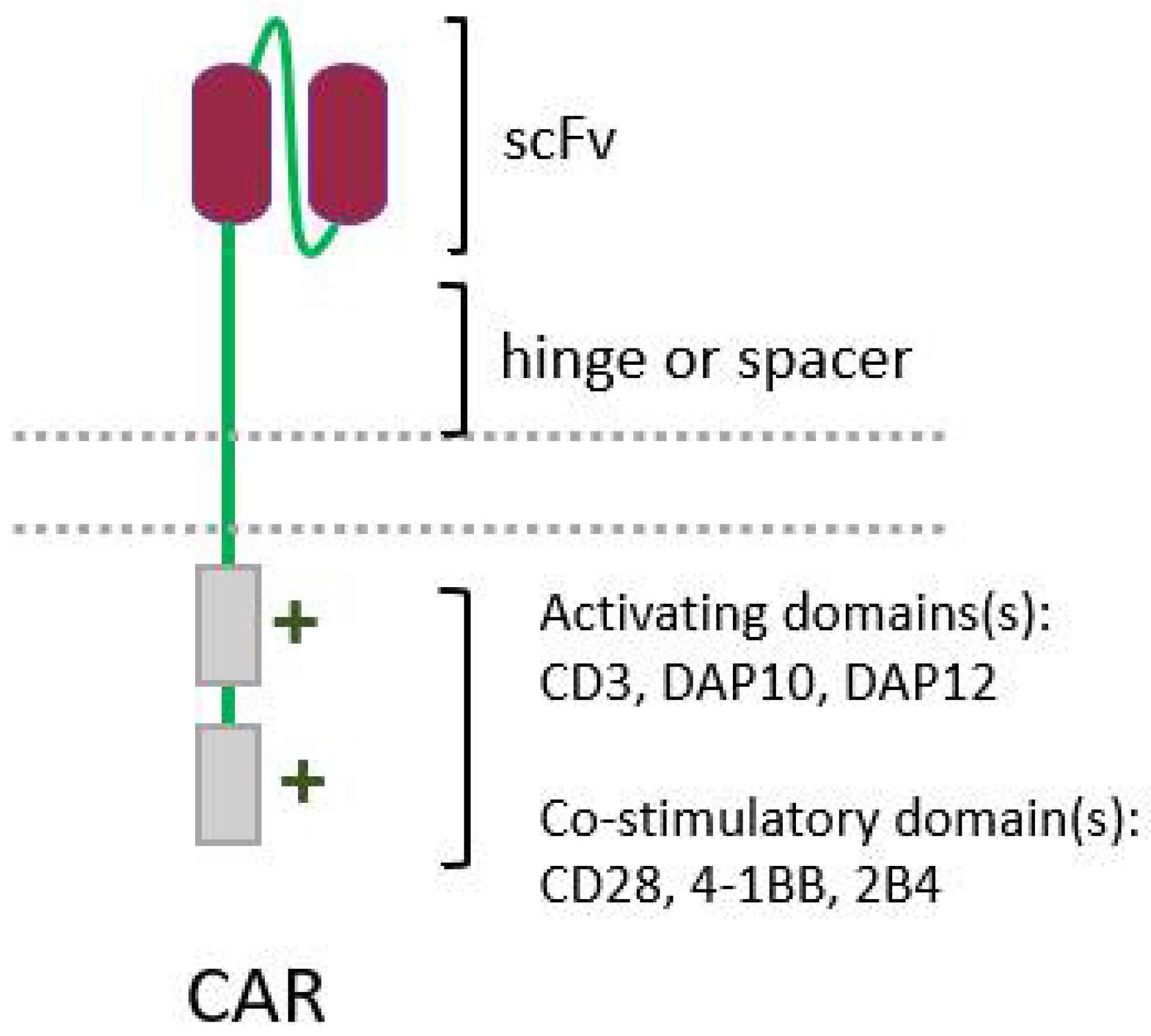
5.2. CAR-NK Cells
5.3. Cytokine Therapy
5.4. Monoclonal Antibodies, Killer Cell Engagers, Immunocytokines, and Other Antibody-Based Strategies to Boost NK Activation
5.5. Tumor Sensitization
6. Conclusions
References
- Wirsching, H.G.; Galanis, E.; Weller, M. Glioblastoma. In Handbook of Clinical Neurology; Berger, M.S., Weller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 134, pp. 381–397. ISBN 9780128029978.
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996.
- Tran, B.; Rosenthal, M.A. Survival comparison between glioblastoma multiforme and other incurable cancers. J. Clin. Neurosci. 2010, 17, 417–421.
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncol. 2014, 16, iv1–iv63.
- Perlmann, P.; Holm, G. Cytotoxic effects of lymphoid cells in vitro. Adv. Immunol. 1969, 11, 117–193.
- Rosenau, W.; Moon, H.D. Lysis of homologous cells by sensitized lymphocytes in tissue culture. J. Natl. Cancer Inst. 1961, 27, 471–483.
- Rosenberg, E.; Herberman, R.; Levine, P.; Halterman, R.; McCoy, J.; Wunderlich, J. Lymphocyte cytotoxicity reactions to leukemia-associated antigens in identical twins. Int. J. Cancer 1972, 9, 648–658.
- Ozer, H.; Strelkauskas, A.J.; Callery, R.T.; Schlossman, S.F. The functional dissection of human peripheral null cells with respect to antibody-dependent cellular cytotoxicity and natural killing. Eur. J. Immunol. 1979, 9, 112–118.
- Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 1989, 47, 187–376.
- Perussia, B.; Acuto, O.; Terhorst, C.; Faust, J.; Lazarus, R.; Fanning, V.; Trinchieri, G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. II. Studies of B73.1 antibody-antigen interaction on the lymphocyte membrane. J. Immunol. 1983, 130, 2142–2148.
- Grossi, C.E.; Cadoni, A.; Zicca, A.; Leprini, A.; Ferrarini, M. Large Granular Lymphocytes in Human Peripheral Blood: Ultrastructural and Cytochemical Characterization of the Granules. Blood 1982, 59, 277–283.
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117.
- Ljunggren, H.G.; Kärre, K. In search of the “missing self”: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244.
- Cerwenka, A.; Lanier, L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016, 16, 112–123.
- Cichocki, F.; Grzywacz, B.; Miller, J.S. Human NK cell development: One road or many? Front. Immunol. 2019, 10, 2078.
- Zhang, Y.; Payne, K.J.; Zhu, Y.; Price, M.A.; Parrish, Y.K.; Zielinska, E.; Barsky, L.W.; Crooks, G.M. SCL Expression at Critical Points in Human Hematopoietic Lineage Commitment. Stem Cells 2005, 23, 852–860.
- Renoux, V.; Zriwil, A.; Peitzsch, C.; Michaëlsson, J.; Friberg, D.; Soneji, S.; Sitnicka, E. Identification of a Human Natural Killer Cell Lineage-Restricted Progenitor in Fetal and Adult Tissues. Immunity 2015, 43, 394–407.
- Freud, A.; Yokohama, A.; Becknell, B.; Lee, M.; Mao, H.; Ferketich, A.; Caligiuri, M. Evidence for discrete stages of human natural killer cell differentiation in vivo. J. Exp. Med. 2006, 203, 1033–1043.
- Scoville, S.; Freud, A.; Caligiuri, M. Modeling Human Natural Killer Cell Development in the Era of Innate Lymphoid Cells. Front. Immunol. 2017, 8, 360.
- Lyman, S.D.; Jacobsen, S.E. c-kit ligand and Flt3 ligand: Stem/progenitor cell factors with overlapping yet distinct activities. Blood 1998, 91, 1101–1134.
- Robin, C.; Ottersbach, K.; Durand, C.; Peeters, M.; Vanes, L.; Tybulewicz, V.; Dzierzak, E. An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev. Cell 2006, 11, 171–180.
- Brusilovsky, M.; Rosental, B.; Shemesh, A.; Appel, M.Y.; Porgador, A. Human NK cell recognition of target cells in the prism of natural cytotoxicity receptors and their ligands. J. Immunotoxicol. 2012, 9, 267–274.
- Vitale, M.; Cantoni, C.; Della Chiesa, M.; Ferlazzo, G.; Carlomagno, S.; Pende, D.; Falco, M.; Pessino, A.; Muccio, L.; De Maria, A.; et al. An Historical Overview: The Discovery of How NK Cells Can Kill Enemies, Recruit Defense Troops, and More. Front. Immunol. 2019, 10, 1415.
- Ochoa, M.C.; Minute, L.; Rodriguez, I.; Garasa, S.; Perez-Ruiz, E.; Inogés, S.; Melero, I.; Berraondo, P. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol. Cell Biol. 2017, 95, 347–355.
- Noh, J.Y.; Yoon, S.R.; Kim, T.D.; Choi, I.; Jung, H. Toll-Like Receptors in Natural Killer Cells and Their Application for Immunotherapy. J. Immunol. Res. 2020, 2020.
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154.
- Malmberg, K.J.; Carlsten, M.; Björklund, A.; Sohlberg, E.; Bryceson, Y.T.; Ljunggren, H.G. Natural killer cell-mediated immunosurveillance of human cancer. Semin. Immunol. 2017, 31, 20–29.
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNγ, and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111.
- Street, S.; Cretney, E.; Smyth, M. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 2001, 97, 192–197.
- Van den Broek, M.; Kägi, D.; Ossendorp, F.; Toes, R.; Vamvakas, S.; Lutz, W.; Melief, C.; Zinkernagel, R.; Hengartner, H. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 1996, 184, 1781–1790.
- Kaplan, D.; Shankaran, V.; Dighe, A.; Stockert, E.; Aguet, M.; Old, L.; Schreiber, R. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA 1998, 95, 7556–7561.
- Cretney, E.; Takeda, K.; Yagita, H.; Glaccum, M.; Peschon, J.J.; Smyth, M.J. Increased Susceptibility to Tumor Initiation and Metastasis in TNF-Related Apoptosis-Inducing Ligand-Deficient Mice. J. Immunol. 2002, 168, 1356–1361.
- Takeda, K.; Smyth, M.; Cretney, E.; Hayakawa, Y.; Kayagaki, N.; Yagita, H.; Okumura, K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J. Exp. Med. 2002, 195, 161–169.
- Smyth, M.; Crowe, N.; Godfrey, D. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int. Immunol. 2001, 13, 459–463.
- Carlsten, M.; Malmberg, K.J.; Ljunggren, H.G. Natural killer cell-mediated lysis of freshly isolated human tumor cells. Int. J. Cancer 2009, 124, 757–762.
- Jouanguy, E.; Gineau, L.; Cottineau, J.; Béziat, V.; Vivier, E.; Casanova, J.L. Inborn errors of the development of human natural killer cells. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 589–595.
- Jobim, M.; Jobim, L. Natural killer cells and immune surveillance. J. Pediatr. 2008, 84, S58–S67.
- Huntington, N.; Cursons, J.; Rautela, J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454.
- Smyth, M.; Godfrey, D.; Trapani, J. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2001, 2, 293–299.
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor–mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127.
- Rossin, A.; Miloro, G.; Hueber, A.O. TRAIL and FasL functions in cancer and autoimmune diseases: Towards an increasing complexity. Cancers 2019, 11, 639.
- Bhat, R.; Watzl, C. Serial Killing of Tumor Cells by Human Natural Killer Cells—Enhancement by Therapeutic Antibodies. PLoS ONE 2007, 2, e326.
- Galea, I.; Bechmann, I.; Perry, V.H. What is immune privilege (not)? Trends Immunol. 2007, 28, 12–18.
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 4196.
- Orrego, E.; Castaneda, C.A.; Castillo, M.; Bernabe, L.A.; Casavilca, S.; Chakravarti, A.; Meng, W.; Garcia-Corrochano, P.; Villa-Robles, M.R.; Zevallos, R.; et al. Distribution of tumor-infiltrating immune cells in glioblastoma. CNS Oncol. 2018, 7, CNS21.
- Yang, I.; Han, S.J.; Sughrue, M.E.; Tihan, T.; Parsa, A.T. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: Evidence of distinct immunological microenvironments that reflect tumor biology. J. Neurosurg. 2011, 115, 505–511.
- Kmiecik, J.; Poli, A.; Brons, N.H.C.; Waha, A.; Eide, G.E.; Enger, P.Ø.; Zimmer, J.; Chekenya, M. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J. Neuroimmunol. 2013, 264, 71–83.
- González-Tablas Pimenta, M.; Otero, Á.; Arandia Guzman, D.A.; Pascual-Argente, D.; Ruíz Martín, L.; Sousa-Casasnovas, P.; García-Martin, A.; de Oca Roa Montes, J.C.; Villaseñor-Ledezma, J.; Torres Carretero, L.; et al. Tumor cell and immune cell profiles in primary human glioblastoma: Impact on patient outcome. Brain Pathol. 2021, 31, 365–380.
- Tan, W.C.C.; Nerurkar, S.N.; Cai, H.Y.; Ng, H.H.M.; Wu, D.; Wee, Y.T.F.; Lim, J.C.T.; Yeong, J.; Lim, T.K.H. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 2020, 40, 135–153.
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling tumor infiltrating immune cells with CIBERSORT. In Cancer Systems Biology. Methods and Protocols; Von Stechow, L., Ed.; Humana Press Inc.: New York, NY, USA, 2018; Volume 1711, pp. 243–259. ISBN 978-1-4939-7492-4.
- Wang, J.; Matosevic, S. NT5E/CD73 as Correlative Factor of Patient Survival and Natural Killer Cell Infiltration in Glioblastoma. J. Clin. Med. 2019, 8, 1526.
- Razavi, S.-M.; Lee, K.E.; Jin, B.E.; Aujla, P.S.; Gholamin, S.; Li, G. Immune Evasion Strategies of Glioblastoma. Front. Surg. 2016, 3, 11.
- Burnet, M. Cancer: A Biological Approach. III. Viruses associated with neoplastic conditions. IV.Pratical aplications. Br. Med. J. 1957, 1, 841–847.
- Helmy, K.Y.; Patel, S.A.; Nahas, G.R.; Rameshwar, P. Cancer immunotherapy: Accomplishments to date and future promise. Ther. Deliv. 2013, 4, 1307–1320.
- Choucair, K.; Duff, J.R.; Cassidy, C.S.; Albrethsen, M.T.; Kelso, J.D.; Lenhard, A.; Staats, H.; Patel, R.; Brunicardi, F.C.; Dworkin, L.; et al. Natural killer cells: A review of biology, therapeutic potential and challenges in treatment of solid tumors. Future Oncol. 2019, 15, 3053–3069.
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218.
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100.
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057.
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553.
- Fang, F.; Xiao, W.; Tian, Z. NK cell-based immunotherapy for cancer. Semin. Immunol. 2017, 31, 37–54.
- Navarro, A.G.; Espedal, H.; Joseph, J.V.; Trachsel-Moncho, L.; Bahador, M.; Gjertsen, B.T.; Kristoffersen, E.K.; Simonsen, A.; Miletic, H.; Enger, P.Ø.; et al. Pretreatment of glioblastoma with bortezomib potentiates natural killer cell cytotoxicity through TRAIL/DR5 mediated apoptosis and prolongs animal survival. Cancers 2019, 11, 996.
- Nianias, A.; Themeli, M. Induced Pluripotent Stem Cell (iPSC)–Derived Lymphocytes for Adoptive Cell Immunotherapy: Recent Advances and Challenges. Curr. Hematol. Malig. Rep. 2019, 14, 261–268.
- Oberoi, P.; Kamenjarin, K.; Ossa, J.; Uherek, B.; Bönig, H.; Wels, W. Directed Differentiation of Mobilized Hematopoietic Stem and Progenitor Cells into Functional NK cells with Enhanced Antitumor Activity. Cells 2020, 9, 811.
- Suck, G.; Odendahl, M.; Nowakowska, P.; Seidl, C.; Wels, W.S.; Klingemann, H.G.; Tonn, T. NK-92: An ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 2015, 65, 485–492.
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724.
- Guedan, S.; Calderon, H.; Posey, A.D.J.; Maus, M.V. Engineering and Design of Chimeric Antigen Receptors. Mol. Ther. Methods Clin. Dev. 2019, 12, 145–156.
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287.
- Raedler, L. Velcade (Bortezomib) Receives 2 New FDA Indications: For Retreatment of Patients with Multiple Myeloma and for First-Line Treatment of Patients with Mantle-Cell Lymphoma. Am. Health Drug Benefits 2015, 8, 135–140.
- Luna, J.I.; Grossenbacher, S.K.; Sturgill, I.R.; Ames, E.; Judge, S.J.; Bouzid, L.A.; Darrow, M.A.; Murphy, W.J.; Canter, R.J. Bortezomib Augments Natural Killer Cell Targeting of Stem-Like Tumor Cells. Cancers 2019, 11, 85.




