Glioblastoma (GBM) is the primary cancer with the highest incidence within the central nervous system. Natural killer (NK) cells are innate lymphocytes that play an important role in immunosurveillance, acting alongside other immune cells in the response against various types of malignant tumors and the prevention of metastasis. NK cells are being exploited in many ways to treat cancer. The broad arsenal of NK-based therapies includes adoptive transfer of in vitro expanded and activated cells, genetically engineered cells to contain chimeric antigen receptors (CAR-NKs), in vivo stimulation of NK cells (by cytokine therapy, checkpoint blockade therapies, etc.), and tumor-specific antibody-guided NK cells, among others.
- immunotherapy
- natural killer cells
- cancer
- glioblastoma
1. Introduction
Glioblastoma (GBM) considerably reduces patients’ quality of life [2][1]. Its current treatment—including maximal surgical resection, adjuvant radiotherapy, and chemotherapy with temozolomide—significantly improves patient survival [3][2]. However, this therapeutic regimen is still not enough, as half of the patients die within approximately 15 months after diagnosis [4][3], and less than 5% of them remain alive after 5 years [5][4]. Relapse occurs frequently, and death is inevitable.
A broad arsenal of treatments has been established to control or potentially cure neoplasms; more recently, immunotherapy has emerged as a new therapeutic pillar in oncology. Within this treatment modality, most strategies have been centered on either antibodies or T cells, but lately, new studies have sought to take advantage of the antitumor activity of natural killer (NK) cells.
2. Natural Killer Cells
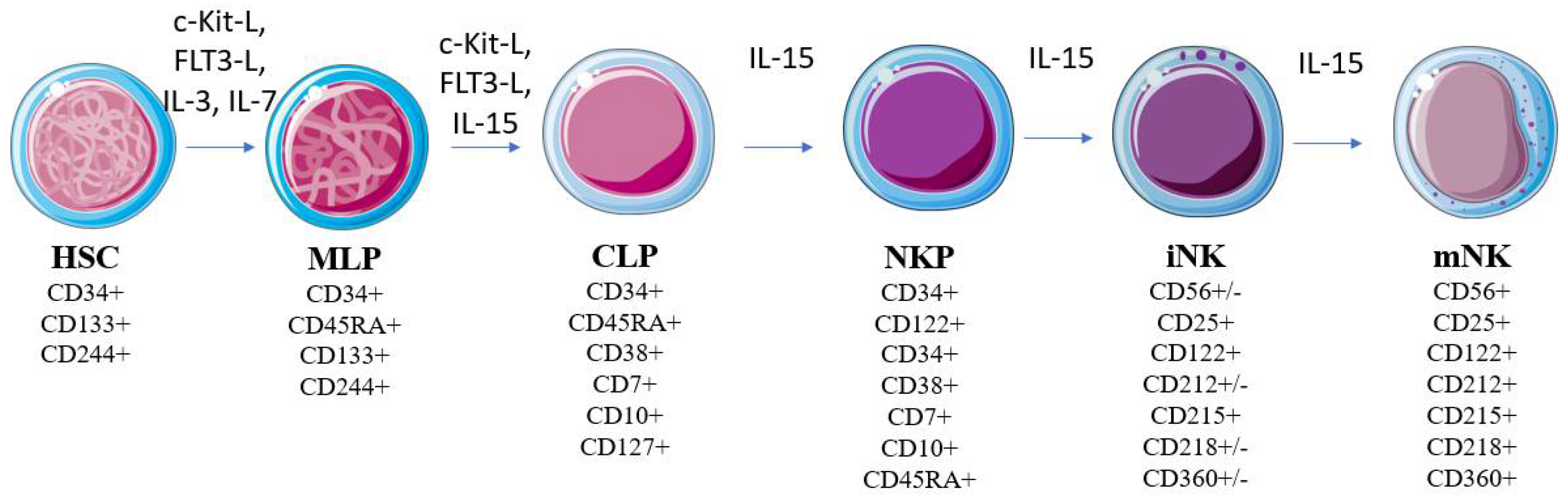
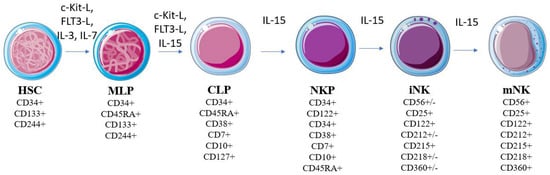 Figure 1. Model of human NK cell development. NK development from HSCs is regulated by multiple cytokines (e.g., FLT3-L, c-Kit ligand, IL-3, IL-7, and IL-15). Modifications of the molecules’ expression patterns are correlated with developmental stages. Abbreviations—Flt3l: FMS-like tyrosine kinase 3 ligand; c-Kit: c-Kit ligand; IL: interleukin; HSC: hematopoietic stem cell; MLP: compromised multipotent lymphoid progenitor; CLP: common lymphoid progenitor; NKP: NK cell lineage precursor; iNK: immature NK cell; mNK: mature NK cell.
Figure 1. Model of human NK cell development. NK development from HSCs is regulated by multiple cytokines (e.g., FLT3-L, c-Kit ligand, IL-3, IL-7, and IL-15). Modifications of the molecules’ expression patterns are correlated with developmental stages. Abbreviations—Flt3l: FMS-like tyrosine kinase 3 ligand; c-Kit: c-Kit ligand; IL: interleukin; HSC: hematopoietic stem cell; MLP: compromised multipotent lymphoid progenitor; CLP: common lymphoid progenitor; NKP: NK cell lineage precursor; iNK: immature NK cell; mNK: mature NK cell.3. NK Cells in Immunosurveillance against Cancer
3.1. Tumor Cell Recognition
NK cells can kill cancer cells while maintaining tolerance against healthy cells [14][13]. As already mentioned, any selective recognition by NK cells depends on the signals integrated by their activating and inhibitory receptors. Unlike most healthy cells, tumor cells may have low or reduced expression levels of the MHC-I molecules on their surface; this prevents the engagement of inhibitory receptors—such as KIR and NKG2A receptors—which would enable NK cell activation over the target cell [100,101,102][37][38][39].3.2. Antitumor Functions
Upon activation, NK cells can exert antitumor activities in two ways: by directly killing malignant cells, or by modulating the activity of other leukocytes (Figure 2). The direct killing is thought to be mediated mainly by perforin/granzyme-dependent cytotoxicity, as noted in numerous experimental models [85,106][27][40]. Moreover, an important role of cytotoxicity mediated by the death receptors FasL and TRAIL has also been observed [107][41]. Time-lapse microscopy analyses indicate that a single NK cell can kill multiple tumor cells in a serial manner [106,108][40][42]. In the serial killing of neoplastic cells susceptible to both death mechanisms, the granzyme-mediated death initially predominates, and the contribution of the pathway dependent on death receptors progressively increases [106][40].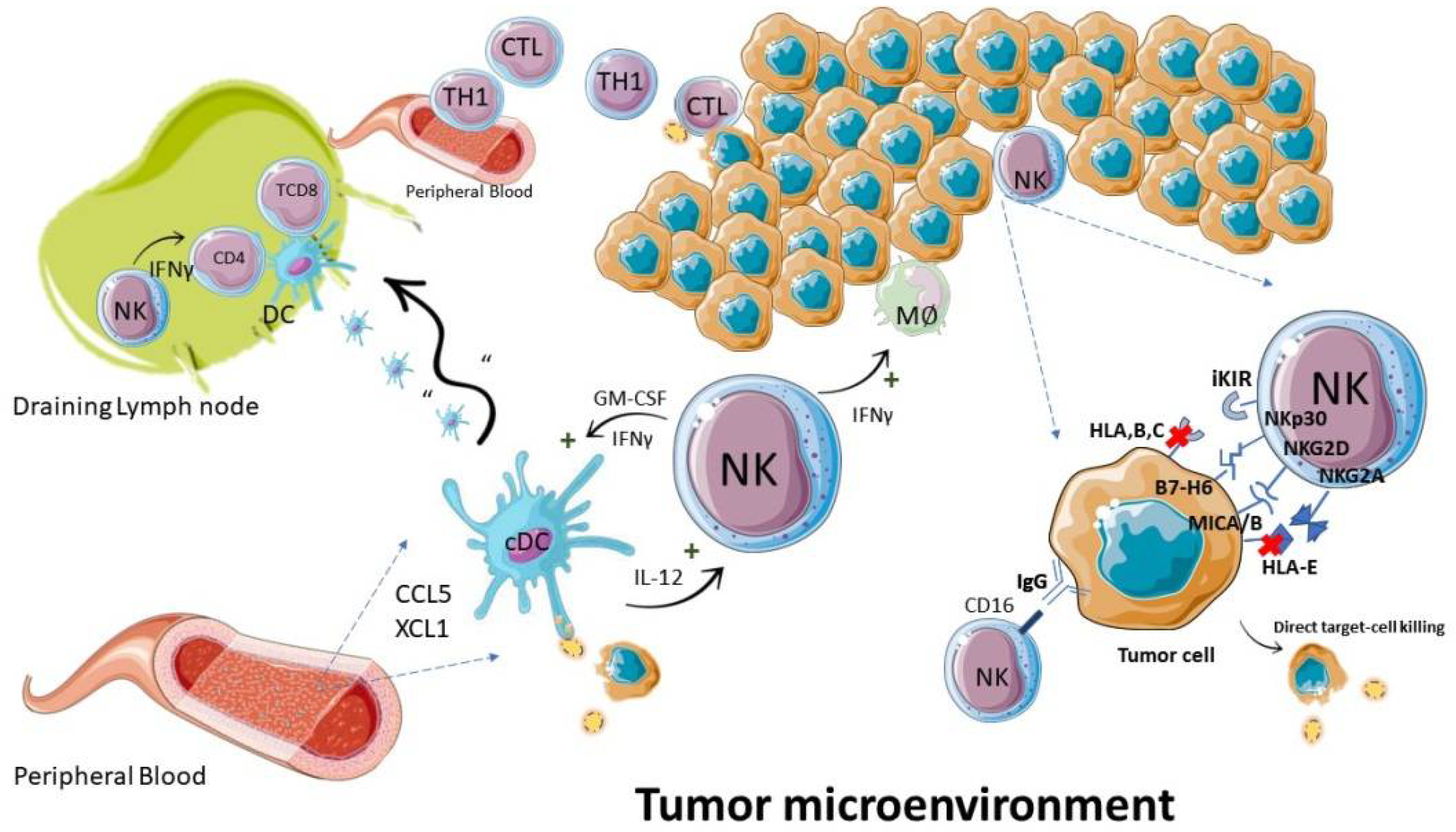
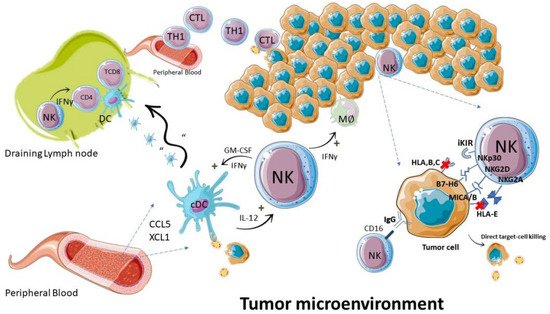 Figure 2. NK cells during an antitumor immune response. The antitumor function of NK cells is executed by direct killing of neoplastic cells and by regulation of immune cells: such as induction of MØ: TH1: and dendritic cell pro-inflammatory responses. Abbreviations—IFN: interferon; CTL: cytotoxic T lymphocyte; TH1: T helper type 1 lymphocyte; cDC: conventional dendritic cell; MØ: macrophage.
Figure 2. NK cells during an antitumor immune response. The antitumor function of NK cells is executed by direct killing of neoplastic cells and by regulation of immune cells: such as induction of MØ: TH1: and dendritic cell pro-inflammatory responses. Abbreviations—IFN: interferon; CTL: cytotoxic T lymphocyte; TH1: T helper type 1 lymphocyte; cDC: conventional dendritic cell; MØ: macrophage.4. NK Cells in GBM
5. NK-Cell-Based Immunotherapy
Cancer immunotherapy has been an elusive goal ever since the proposition of the immunosurveillance hypothesis by Burnet [153][53]. However, due to continuous elucidation of immune and tumor-evasion mechanisms, in recent years there has been a significant change in this previously dismal picture, and cancer immunotherapy has become a concrete option for many patients with various types of cancer [154][54]. Due to the many roles that NK cells can play against cancer, as well as their ability to quickly recognize and attack cancer cells, without the need for previous sensitization [155][55], while also presenting limited reactivity against healthy tissues [156][56]. NK-cell-based immunotherapy presents the possibility of targeting tumors that still lack well-defined antigens for specific response, as well as the possibility of using allogeneic products prepared in advance, and that may be administered in multiple patients without causing graft-versus-host disease (GvHD) [157[57][58][59],158,159], thus potentially leading to less toxicity in comparison to CAR T-cell infusions, for example [156,159][56][59]. Different strategies of NK-cell-based immunotherapy for GBM are summarized in Figure 3; they pursue different strategies, aiming at the number or in vivo persistence of these cells [160][60], the activation or blocking of their inhibitory signals, their cytotoxic potential through adoptive cell therapies [155][55], or sensitizing cancer cells to NK-cell-mediated lysis [161][61].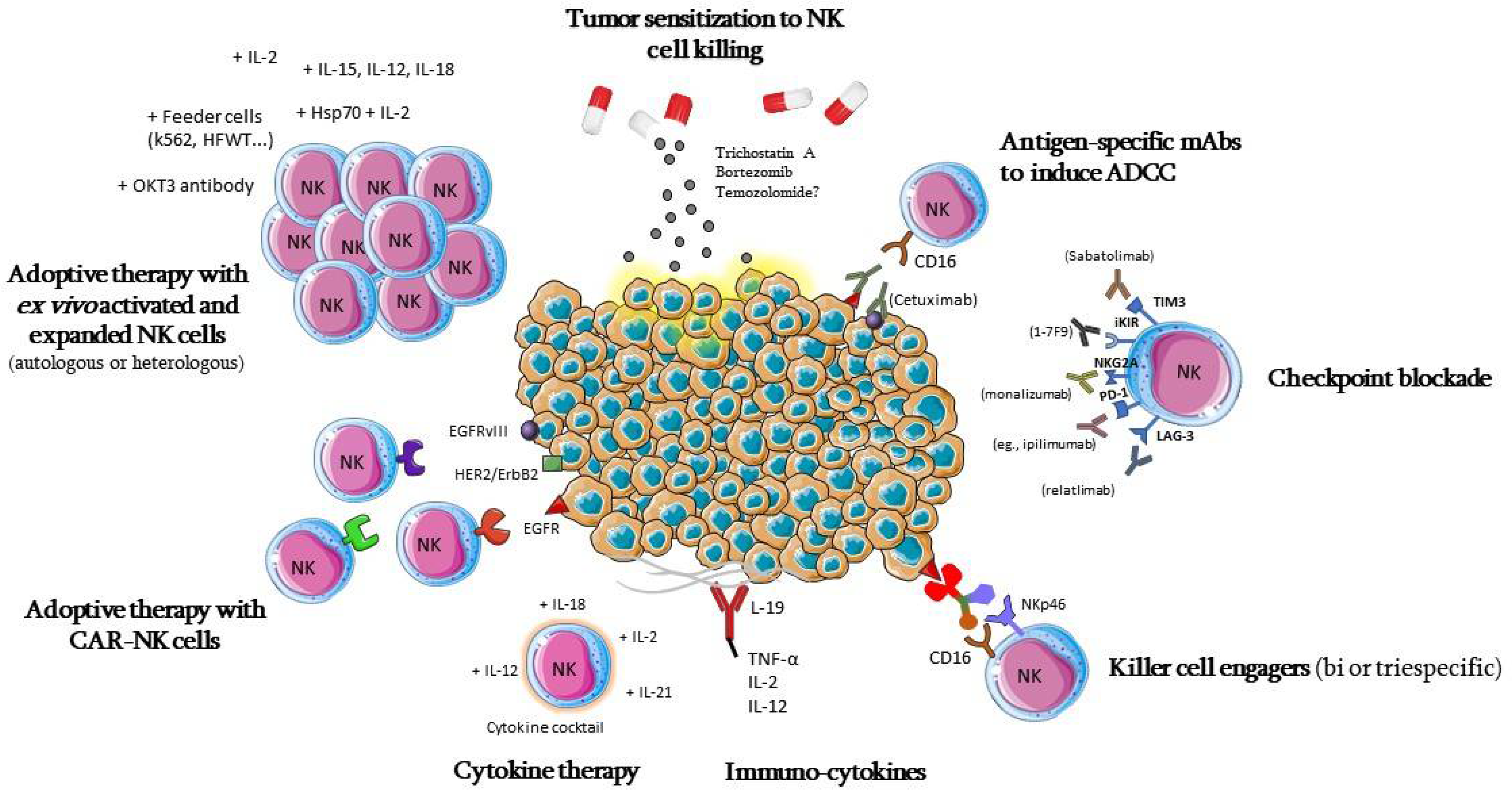
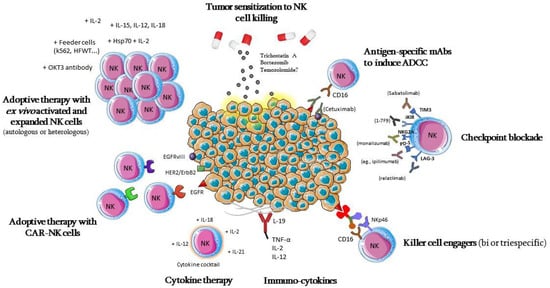
5.1. Adoptive Cell Therapy
5.2. CAR-NK Cells
The success of chimeric antigen receptor (CAR) T-cell therapy against hematological tumors, with three therapies already approved for commercialization by the FDA, has prompted interest in improving the design of CARs, and in expanding this technique to other cell types, such as NK cells. CARs were initially developed to enable T cells to recognize and act against a given tumor antigen, bypassing the need for MHC presentation, which is necessary for recognition by the TCR [205][65]. CARs are engineered molecules composed of four main components (Figure 4): one single-chain variable fragment (scFv), derived from a monoclonal antibody, which binds to the antigen of interest, conferring specificity; the hinge or spacer, which gives support and flexibility to the scFv; a transmembrane domain; and one or more domains of intracellular signaling, responsible for cell activation [206].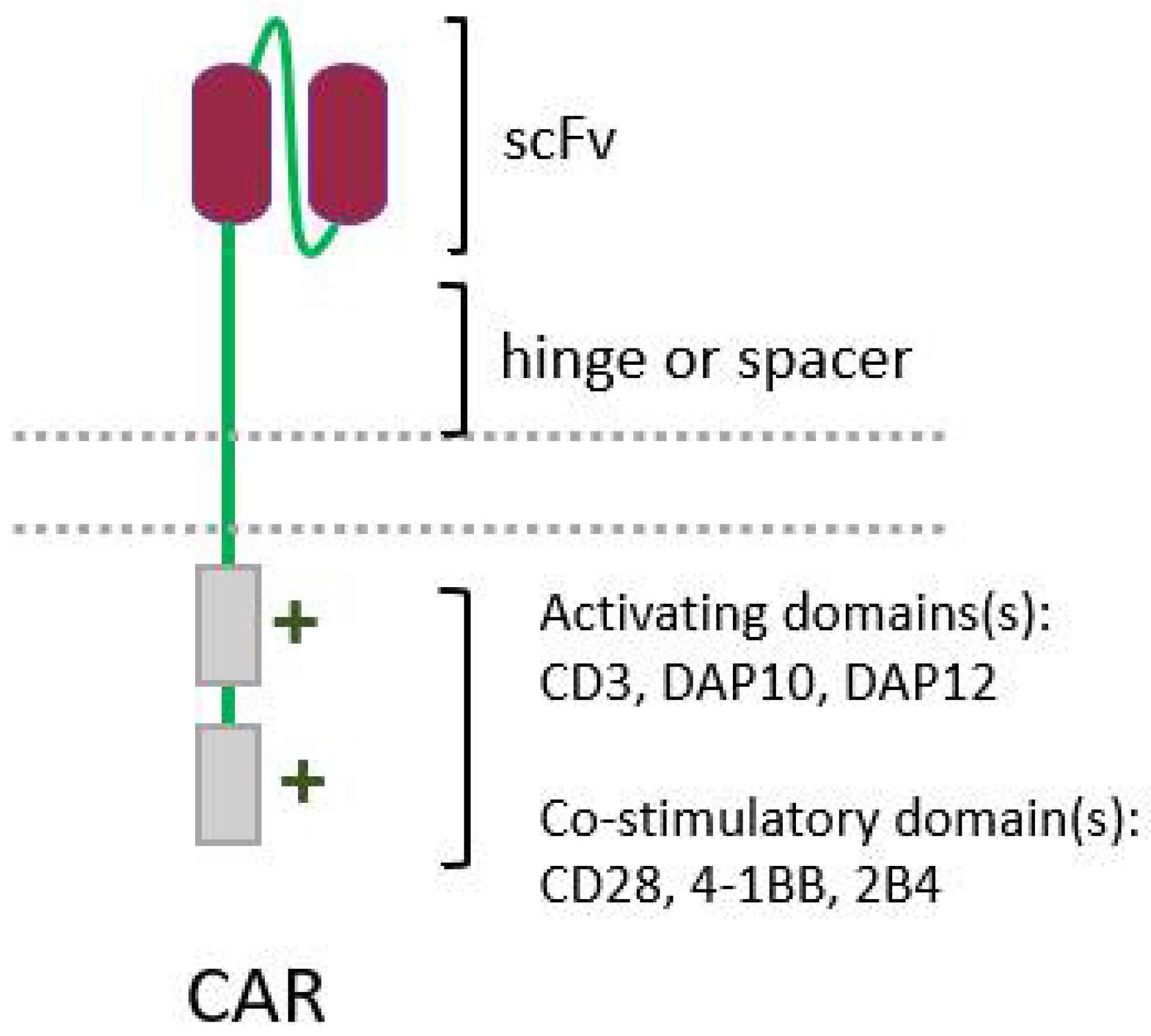
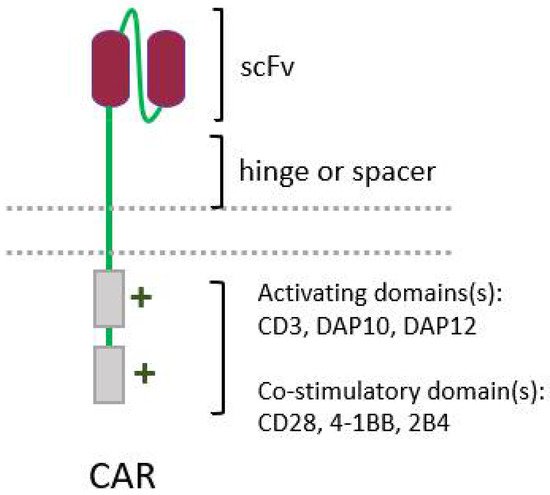 Figure 4. Simplified structure of a conventional chimeric antigen receptor (CAR), showing its four main elements: a single-chain variable fragment (scFv), the hinge, and the intracellular activating domain(s).
Figure 4. Simplified structure of a conventional chimeric antigen receptor (CAR), showing its four main elements: a single-chain variable fragment (scFv), the hinge, and the intracellular activating domain(s).5.3. Cytokine Therapy
5.4. Monoclonal Antibodies, Killer Cell Engagers, Immunocytokines, and Other Antibody-Based Strategies to Boost NK Activation
A broad range of antibody-based therapies have been tested and approved for the treatment of various cancers. Since some of them can trigger NK cell activation, they can be used to overcome the dysfunction and immunosuppressed status of the patients’ NK cells, or even to improve adoptive cell therapy efficiency. One of that strategies consists of the use of monoclonal antibodies (mAbs) targeting tumor cells, rendering them susceptible to ADCC—a process that can be mediated by NK cells [334][66].5.5. Tumor Sensitization
The efficacy of adoptive therapies might be increased by the previous sensitization of the GBM cells to the cytotoxicity mediated by the NK cells. The proteasome inhibitor bortezomib (BTZ), which is an antineoplastic agent already approved and commercialized for the treatment of multiple myeloma and mantle-cell lymphoma [393][67], is an eligible candidate for this function; data regarding its relation to the anti-GBM activity of NK cells have been reported recently by Navarro et al. (2019) [161][61] and Luna et al. (2019) [394][68]; both studies showed that primary GBM cell lines, when treated with BTZ, had a significantly increased membrane expression of ligands for the NK-activating receptor NKG2D, and also for the death receptor TRAIL-R2.6. Conclusions
Despite the constant efforts over the past decades, GBM remains a challenging disease, and the prognosis of patients is still poor. Key characteristics of GBM—such as its intrinsic heterogeneity and the strategies it displays to overcome the immune system, which include several mechanisms of NK cell immunosuppression—make it partially resistant to the various antineoplastic treatments employed. NK cells are lymphocytes of the innate immune system with particularities that could make them advantageous in antitumor responses: their natural cytotoxicity against neoplastic cells, their numerous helper functions and, consequently, their important contribution to immunosurveillance, and to response against established tumors. In agreement with this, NK cell immunotherapy has proven to be a powerful tool for the treatment of various experimental tumors, and initial clinical data from studies in GBM indicate a good safety profile and effectiveness for NK-cell-based immunotherapy. The arsenal of strategies involving NK cells against GBM cells is certainly not limited to those mentioned here, since an increasing number of studies have been showing promising results, and the therapeutic possibilities are being radically expanded. Future strategies will benefit from screening for biomarkers associated with therapeutic efficacy, the combination of multiple therapeutic approaches and, ideally, personalized treatment regimens, considering the unique features of each patient. Thus, even if the contribution of NK cells for antitumor immune response in GBM patients still requires further study, they definitely appear to be a very promising and interesting component of the immune system’s response against GBM, which could be harnessed to improve treatment and, thus, patient care.References
- Wirsching, H.G.; Galanis, E.; Weller, M. Glioblastoma. In Handbook of Clinical Neurology; Berger, M.S., Weller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 134, pp. 381–397. ISBN 9780128029978.
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996.
- Tran, B.; Rosenthal, M.A. Survival comparison between glioblastoma multiforme and other incurable cancers. J. Clin. Neurosci. 2010, 17, 417–421.
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncol. 2014, 16, iv1–iv63.
- Perlmann, P.; Holm, G. Cytotoxic effects of lymphoid cells in vitro. Adv. Immunol. 1969, 11, 117–193.
- Rosenau, W.; Moon, H.D. Lysis of homologous cells by sensitized lymphocytes in tissue culture. J. Natl. Cancer Inst. 1961, 27, 471–483.
- Rosenberg, E.; Herberman, R.; Levine, P.; Halterman, R.; McCoy, J.; Wunderlich, J. Lymphocyte cytotoxicity reactions to leukemia-associated antigens in identical twins. Int. J. Cancer 1972, 9, 648–658.
- Ozer, H.; Strelkauskas, A.J.; Callery, R.T.; Schlossman, S.F. The functional dissection of human peripheral null cells with respect to antibody-dependent cellular cytotoxicity and natural killing. Eur. J. Immunol. 1979, 9, 112–118.
- Trinchieri, G. Biology of natural killer cells. Adv. Immunol. 1989, 47, 187–376.
- Perussia, B.; Acuto, O.; Terhorst, C.; Faust, J.; Lazarus, R.; Fanning, V.; Trinchieri, G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. II. Studies of B73.1 antibody-antigen interaction on the lymphocyte membrane. J. Immunol. 1983, 130, 2142–2148.
- Grossi, C.E.; Cadoni, A.; Zicca, A.; Leprini, A.; Ferrarini, M. Large Granular Lymphocytes in Human Peripheral Blood: Ultrastructural and Cytochemical Characterization of the Granules. Blood 1982, 59, 277–283.
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117.
- Ljunggren, H.G.; Kärre, K. In search of the “missing self”: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244.
- Cerwenka, A.; Lanier, L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016, 16, 112–123.
- Cichocki, F.; Grzywacz, B.; Miller, J.S. Human NK cell development: One road or many? Front. Immunol. 2019, 10, 2078.
- Zhang, Y.; Payne, K.J.; Zhu, Y.; Price, M.A.; Parrish, Y.K.; Zielinska, E.; Barsky, L.W.; Crooks, G.M. SCL Expression at Critical Points in Human Hematopoietic Lineage Commitment. Stem Cells 2005, 23, 852–860.
- Renoux, V.; Zriwil, A.; Peitzsch, C.; Michaëlsson, J.; Friberg, D.; Soneji, S.; Sitnicka, E. Identification of a Human Natural Killer Cell Lineage-Restricted Progenitor in Fetal and Adult Tissues. Immunity 2015, 43, 394–407.
- Freud, A.; Yokohama, A.; Becknell, B.; Lee, M.; Mao, H.; Ferketich, A.; Caligiuri, M. Evidence for discrete stages of human natural killer cell differentiation in vivo. J. Exp. Med. 2006, 203, 1033–1043.
- Scoville, S.; Freud, A.; Caligiuri, M. Modeling Human Natural Killer Cell Development in the Era of Innate Lymphoid Cells. Front. Immunol. 2017, 8, 360.
- Lyman, S.D.; Jacobsen, S.E. c-kit ligand and Flt3 ligand: Stem/progenitor cell factors with overlapping yet distinct activities. Blood 1998, 91, 1101–1134.
- Robin, C.; Ottersbach, K.; Durand, C.; Peeters, M.; Vanes, L.; Tybulewicz, V.; Dzierzak, E. An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev. Cell 2006, 11, 171–180.
- Brusilovsky, M.; Rosental, B.; Shemesh, A.; Appel, M.Y.; Porgador, A. Human NK cell recognition of target cells in the prism of natural cytotoxicity receptors and their ligands. J. Immunotoxicol. 2012, 9, 267–274.
- Vitale, M.; Cantoni, C.; Della Chiesa, M.; Ferlazzo, G.; Carlomagno, S.; Pende, D.; Falco, M.; Pessino, A.; Muccio, L.; De Maria, A.; et al. An Historical Overview: The Discovery of How NK Cells Can Kill Enemies, Recruit Defense Troops, and More. Front. Immunol. 2019, 10, 1415.
- Ochoa, M.C.; Minute, L.; Rodriguez, I.; Garasa, S.; Perez-Ruiz, E.; Inogés, S.; Melero, I.; Berraondo, P. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol. Cell Biol. 2017, 95, 347–355.
- Noh, J.Y.; Yoon, S.R.; Kim, T.D.; Choi, I.; Jung, H. Toll-Like Receptors in Natural Killer Cells and Their Application for Immunotherapy. J. Immunol. Res. 2020, 2020.
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154.
- Malmberg, K.J.; Carlsten, M.; Björklund, A.; Sohlberg, E.; Bryceson, Y.T.; Ljunggren, H.G. Natural killer cell-mediated immunosurveillance of human cancer. Semin. Immunol. 2017, 31, 20–29.
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNγ, and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111.
- Street, S.; Cretney, E.; Smyth, M. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood 2001, 97, 192–197.
- Van den Broek, M.; Kägi, D.; Ossendorp, F.; Toes, R.; Vamvakas, S.; Lutz, W.; Melief, C.; Zinkernagel, R.; Hengartner, H. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 1996, 184, 1781–1790.
- Kaplan, D.; Shankaran, V.; Dighe, A.; Stockert, E.; Aguet, M.; Old, L.; Schreiber, R. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl. Acad. Sci. USA 1998, 95, 7556–7561.
- Cretney, E.; Takeda, K.; Yagita, H.; Glaccum, M.; Peschon, J.J.; Smyth, M.J. Increased Susceptibility to Tumor Initiation and Metastasis in TNF-Related Apoptosis-Inducing Ligand-Deficient Mice. J. Immunol. 2002, 168, 1356–1361.
- Takeda, K.; Smyth, M.; Cretney, E.; Hayakawa, Y.; Kayagaki, N.; Yagita, H.; Okumura, K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J. Exp. Med. 2002, 195, 161–169.
- Smyth, M.; Crowe, N.; Godfrey, D. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int. Immunol. 2001, 13, 459–463.
- Carlsten, M.; Malmberg, K.J.; Ljunggren, H.G. Natural killer cell-mediated lysis of freshly isolated human tumor cells. Int. J. Cancer 2009, 124, 757–762.
- Jouanguy, E.; Gineau, L.; Cottineau, J.; Béziat, V.; Vivier, E.; Casanova, J.L. Inborn errors of the development of human natural killer cells. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 589–595.
- Jobim, M.; Jobim, L. Natural killer cells and immune surveillance. J. Pediatr. 2008, 84, S58–S67.
- Huntington, N.; Cursons, J.; Rautela, J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454.
- Smyth, M.; Godfrey, D.; Trapani, J. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2001, 2, 293–299.
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor–mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127.
- Rossin, A.; Miloro, G.; Hueber, A.O. TRAIL and FasL functions in cancer and autoimmune diseases: Towards an increasing complexity. Cancers 2019, 11, 639.
- Bhat, R.; Watzl, C. Serial Killing of Tumor Cells by Human Natural Killer Cells—Enhancement by Therapeutic Antibodies. PLoS ONE 2007, 2, e326.
- Galea, I.; Bechmann, I.; Perry, V.H. What is immune privilege (not)? Trends Immunol. 2007, 28, 12–18.
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 4196.
- Orrego, E.; Castaneda, C.A.; Castillo, M.; Bernabe, L.A.; Casavilca, S.; Chakravarti, A.; Meng, W.; Garcia-Corrochano, P.; Villa-Robles, M.R.; Zevallos, R.; et al. Distribution of tumor-infiltrating immune cells in glioblastoma. CNS Oncol. 2018, 7, CNS21.
- Yang, I.; Han, S.J.; Sughrue, M.E.; Tihan, T.; Parsa, A.T. Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: Evidence of distinct immunological microenvironments that reflect tumor biology. J. Neurosurg. 2011, 115, 505–511.
- Kmiecik, J.; Poli, A.; Brons, N.H.C.; Waha, A.; Eide, G.E.; Enger, P.Ø.; Zimmer, J.; Chekenya, M. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J. Neuroimmunol. 2013, 264, 71–83.
- González-Tablas Pimenta, M.; Otero, Á.; Arandia Guzman, D.A.; Pascual-Argente, D.; Ruíz Martín, L.; Sousa-Casasnovas, P.; García-Martin, A.; de Oca Roa Montes, J.C.; Villaseñor-Ledezma, J.; Torres Carretero, L.; et al. Tumor cell and immune cell profiles in primary human glioblastoma: Impact on patient outcome. Brain Pathol. 2021, 31, 365–380.
- Tan, W.C.C.; Nerurkar, S.N.; Cai, H.Y.; Ng, H.H.M.; Wu, D.; Wee, Y.T.F.; Lim, J.C.T.; Yeong, J.; Lim, T.K.H. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun. 2020, 40, 135–153.
- Chen, B.; Khodadoust, M.S.; Liu, C.L.; Newman, A.M.; Alizadeh, A.A. Profiling tumor infiltrating immune cells with CIBERSORT. In Cancer Systems Biology. Methods and Protocols; Von Stechow, L., Ed.; Humana Press Inc.: New York, NY, USA, 2018; Volume 1711, pp. 243–259. ISBN 978-1-4939-7492-4.
- Wang, J.; Matosevic, S. NT5E/CD73 as Correlative Factor of Patient Survival and Natural Killer Cell Infiltration in Glioblastoma. J. Clin. Med. 2019, 8, 1526.
- Razavi, S.-M.; Lee, K.E.; Jin, B.E.; Aujla, P.S.; Gholamin, S.; Li, G. Immune Evasion Strategies of Glioblastoma. Front. Surg. 2016, 3, 11.
- Burnet, M. Cancer: A Biological Approach. III. Viruses associated with neoplastic conditions. IV.Pratical aplications. Br. Med. J. 1957, 1, 841–847.
- Helmy, K.Y.; Patel, S.A.; Nahas, G.R.; Rameshwar, P. Cancer immunotherapy: Accomplishments to date and future promise. Ther. Deliv. 2013, 4, 1307–1320.
- Choucair, K.; Duff, J.R.; Cassidy, C.S.; Albrethsen, M.T.; Kelso, J.D.; Lenhard, A.; Staats, H.; Patel, R.; Brunicardi, F.C.; Dworkin, L.; et al. Natural killer cells: A review of biology, therapeutic potential and challenges in treatment of solid tumors. Future Oncol. 2019, 15, 3053–3069.
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218.
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100.
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057.
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553.
- Fang, F.; Xiao, W.; Tian, Z. NK cell-based immunotherapy for cancer. Semin. Immunol. 2017, 31, 37–54.
- Navarro, A.G.; Espedal, H.; Joseph, J.V.; Trachsel-Moncho, L.; Bahador, M.; Gjertsen, B.T.; Kristoffersen, E.K.; Simonsen, A.; Miletic, H.; Enger, P.Ø.; et al. Pretreatment of glioblastoma with bortezomib potentiates natural killer cell cytotoxicity through TRAIL/DR5 mediated apoptosis and prolongs animal survival. Cancers 2019, 11, 996.
- Nianias, A.; Themeli, M. Induced Pluripotent Stem Cell (iPSC)–Derived Lymphocytes for Adoptive Cell Immunotherapy: Recent Advances and Challenges. Curr. Hematol. Malig. Rep. 2019, 14, 261–268.
- Oberoi, P.; Kamenjarin, K.; Ossa, J.; Uherek, B.; Bönig, H.; Wels, W. Directed Differentiation of Mobilized Hematopoietic Stem and Progenitor Cells into Functional NK cells with Enhanced Antitumor Activity. Cells 2020, 9, 811.
- Suck, G.; Odendahl, M.; Nowakowska, P.; Seidl, C.; Wels, W.S.; Klingemann, H.G.; Tonn, T. NK-92: An ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 2015, 65, 485–492.
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724.
- Guedan, S.; Calderon, H.; Posey, A.D.J.; Maus, M.V. Engineering and Design of Chimeric Antigen Receptors. Mol. Ther. Methods Clin. Dev. 2019, 12, 145–156.
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287.
- Raedler, L. Velcade (Bortezomib) Receives 2 New FDA Indications: For Retreatment of Patients with Multiple Myeloma and for First-Line Treatment of Patients with Mantle-Cell Lymphoma. Am. Health Drug Benefits 2015, 8, 135–140.
- Luna, J.I.; Grossenbacher, S.K.; Sturgill, I.R.; Ames, E.; Judge, S.J.; Bouzid, L.A.; Darrow, M.A.; Murphy, W.J.; Canter, R.J. Bortezomib Augments Natural Killer Cell Targeting of Stem-Like Tumor Cells. Cancers 2019, 11, 85.
