
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gregorio Vargas | + 5335 word(s) | 5335 | 2022-01-18 09:40:40 | | | |
| 2 | Conner Chen | -1 word(s) | 5334 | 2022-02-23 03:41:27 | | | | |
| 3 | Conner Chen | -1 word(s) | 5334 | 2022-02-23 03:42:30 | | | | |
| 4 | Conner Chen | -52 word(s) | 5283 | 2022-02-23 03:44:12 | | | | |
| 5 | Conner Chen | Meta information modification | 5283 | 2022-02-24 07:53:46 | | | | |
| 6 | Conner Chen | Meta information modification | 5283 | 2022-02-24 09:03:43 | | |
Video Upload Options
In humid environments, the formation of biofilms and microfouling are known to be the detrimental processes that first occur on stainless steel surfaces. This is known as biofouling. Subsequently, the conditions created by metabolites and the activity of organisms trigger corrosion of the metal and accelerate corrosion locally, causing a deterioration in, and alterations to, the performance of devices made of stainless steel. The microorganisms which thus affect stainless steel are mainly algae and bacteria.
1. Introduction
When a biofilm forms on a surface, and is subsequently colonized by micro and macroorganisms, this is known as biofouling, and it can lead to the deterioration of the surface. It is a process that occurs naturally in many areas of human activity, including marine structures, food processing, and in the use of medical implants and devices [1][2]. The negative effects of biofouling on the performance and maintenance of stainless steel surface components may reduce their lifetime.
Stainless steels are widely used for their anti-corrosion properties in different wet environments. However, in the presence of electrolytes, stainless steels are susceptible to the formation of biofilms and consequently to biofouling and microbiologically induced corrosion There are three main areas where increasing the useful life of stainless steel components would be beneficial: for components placed in seawater, those used in food processing, and in biomedical devices [3][4][5].
Stainless steel is resistant to corrosion due to the passive chromium oxide layer that forms on its surface. However, in certain operating conditions, microorganisms can colonize the surface, [6][7], accelerating corrosion reactions, and/or changing the corrosion mechanisms. This effect is commonly referred to as “microbiologically influenced corrosion” (MIC) [6].
The US National Association of Corrosion Engineers (NACE) [8] estimates that annual losses associated with the corrosion of consumer and industrial goods make up around 2–4% of the GDP of all nations. Other estimates indicate that microbial processes are directly, or indirectly, responsible for about 30% of these losses [9].
The nanotechnology plays important roles on biofouling mitigation:
A large surface area is available for the interaction of nanoparticles with the cells of microorganisms [10][11][12];
Due to the smaller size of nanoparticles, they are easier to transport into the cells of microorganisms, which facilitates their elimination or the inhibition of their development [10][11][12];
The wide spectrum of nanoparticles available with different mechanisms of biocidal action allows them to be used synergistically to inhibit the formation of biofilms and consequently avoid micro and macro fouling [13];
The controlled release capacity of “smart”/stimuli-responsive nanomaterials. Mesoporous silica nanocapsules, layered double hydroxides, halloysite nanotubes, and surface functionalization can increase antifouling activity time by up to one year through the controlled release of biocides. Controlled release can also reduce the toxicity of biocides relative to their application in free form. Furthermore, it was demonstrated that some of these “smart” nanomaterials exhibit eco-friendly properties since the controlled release capacity ensures a significant reduction in toxicity and environmental hazards compared with the conventional booster biocides [14][15].
2. Environments in Which Biofouling Occurs
Stainless steel is widely used in marine environments, food processing, and in biomedical implants and devices. Figure 1 shows the most important factors influencing the biofouling of stainless steel surfaces in these three environments. Each of these factors is briefly explained below.
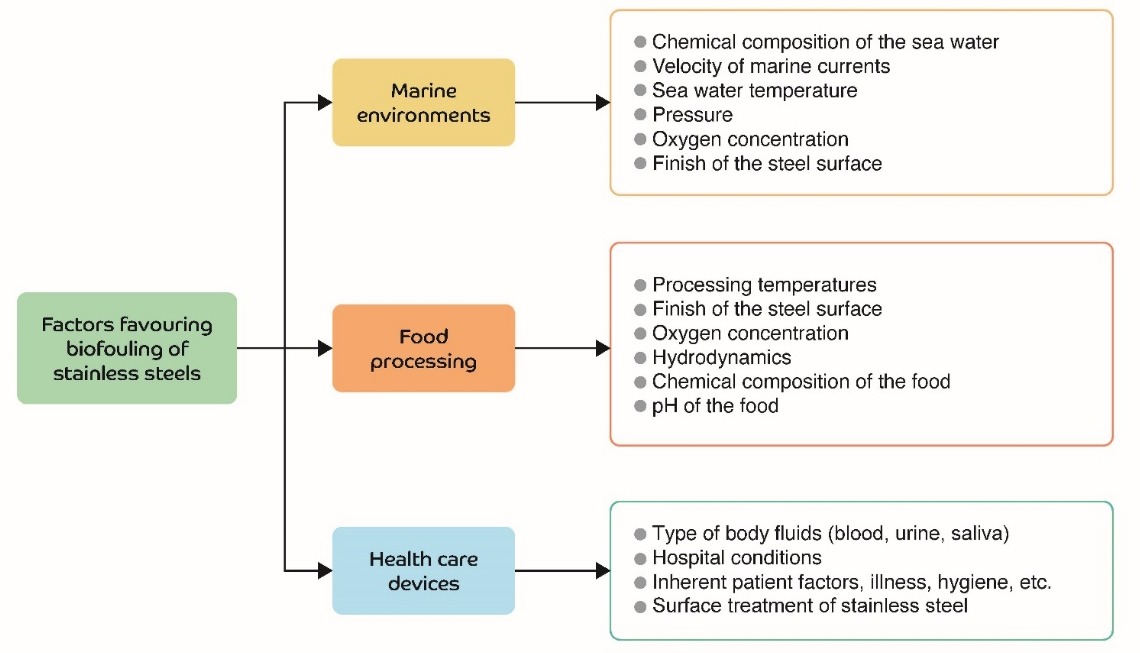
Figure 1. Biofouling factors in stainless steels devices used in the environments discussed
2.1. Marine Environments
At the interfaces between interacting bodies and/or between surfaces and their environment, deterioration may occur. This degradation may be purely mechanical, such as erosion and abrasion, or may involve significant chemical aspects, such as corrosion of a metal, or tribocorrosion (chemical and mechanical processes occurring simultaneously). Corrosion, therefore, cannot be defined without reference to the characteristics of the material and the environment in which it is found.
The oceans are environments that enable the creation of life and the conversion of energy, but they are also extremely aggressive environments. Seawater is a complex mixture of various salts, dissolved gases, trace elements, suspended solids, decomposed organic matter, and living organisms [16]. In seawater, the behaviour of metals is linked to oxygen content, the velocity of currents, temperature, pollution, marine organisms, and the position of the materials with respect to the mean sea tide [17][18]. The contribution of each degradation mechanism, both individually and in combination with each other, in marine environments can be seen in Figure 2.
According to Little and De Palma [19], the factors that affect the rate of biofouling are: the chemical composition of the water [19], marine current flow [20][21], pressure [22][23], shear stress [22], physical and chemical characteristics of the substrate [19], the photosynthetic activity of aquatic plants [19], changes in salinity [19], seasonal changes [19], turbidity [19], oxygen levels [19], and the depth of the metal surface relative to sea level [19].
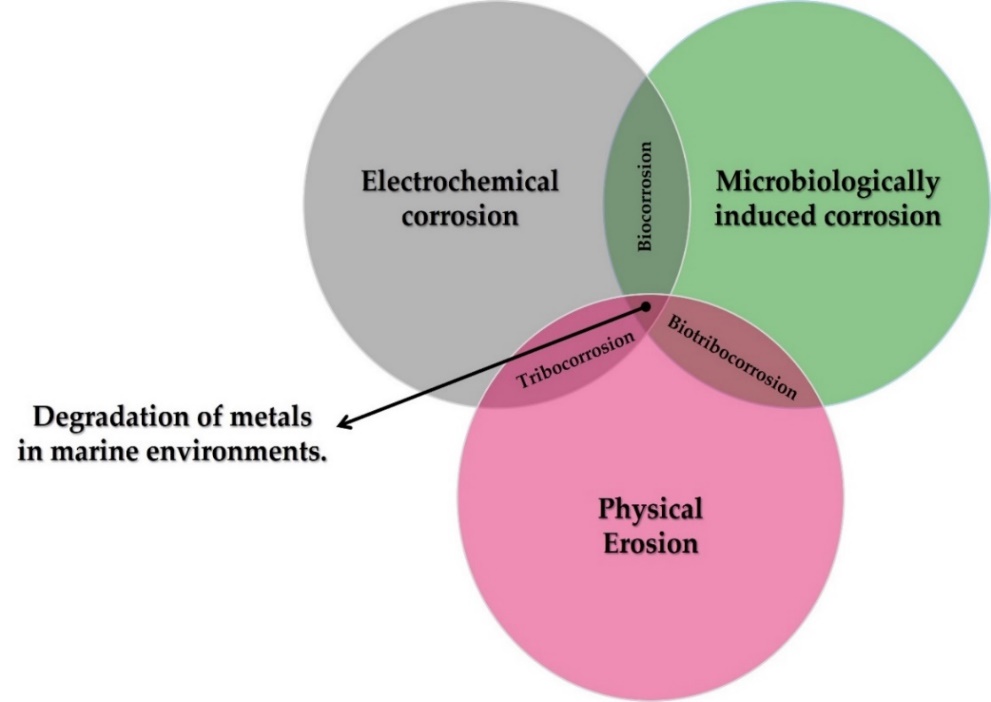
Figure 2. Different forms of metal degradation in marine environments.
2.2. Food Processing
Factors that have a significant impact on the biofouling of steel surfaces in the food processing industry [24][25][26] include: the substrate surface finish [27][28][29][30][31], processing temperatures [25][32][33][34][35], oxygen concentrations [36][37] pH [25] and the nature and chemical composition of the food being processed [25][30][38].
2.3. Implant Devices
The main factors [39] in this area which affect the biofouling process are: the type of body fluid, the surface finish of the steel, nosocomial sanitary conditions and factors inherent to the recipient patient.
3. The Biofouling Process
In its initial stages, the biofouling process is governed by physicochemical forces such as electron transfer, Brownian motion, electrostatic interactions, and Van der Waals forces [2]. The four stages in the biofouling process on seawater devices are described below:
3.1. Stage 1. Biofilm Formation
One minute after immersion of the component, adhesion of organic compounds (proteins and polysaccharides) will have occurred. These proteins and polysaccharides come from the existing nutrients in seawater and are formed from nitrogen and carbon from biological cycles (excretions and the death of marine organisms). These nutrients in turn attract the first colonizing organisms forming a biofilm. A biofilm is a set of immobilized cells embedded in a dense and complex extracellular polymeric matrix produced by the microorganisms themselves, on a substrate [1]. Biofouling is a very complex phenomenon that is not yet fully understood, formed through mechanisms such as crystallization, particle contamination, chemical precipitation, corrosion, and solidification [40]. The microorganisms that may be involved in marine biofouling are mainly the sticky, or sessile, forms present in shallower waters along the coast [2]. Zhang et al. [41] identified more than 7300 biofilm forming species in the sea, of these it is as yet uncertain how many can adapt to the conditions on human infrastructure and adhere to them. These organisms have also adapted to fluctuations in environmental conditions such as temperature, ocean current flow, and salinity [2][42].
The metabolic activity of the cells within the biofilm leads to a reduction in the oxygen concentration on the metal surface, producing a differential aeration cell underneath the biofilm, and generating an anode. On the other hand, in the uncolonized areas exposed to maximum oxygen concentrations, a cathode is generated. The basic corrosion mechanism involves the flow of electrons from an anode to a cathode region, where the electron acceptor is oxygen [7].
The biofilm is made up of a cell mass that represents only 2–5% of the total weight, the rest is made up of an extracellular polymeric substance. This substance comprises various polysaccharides, proteins, nucleic acids, glycoproteins, phospholipids, water, and other surfactants. These polymers are mainly polysaccharide fibrils based on glucose and fructose [43]. The proportion of excreted extracellular substances depends on the type of microorganism [44].
Both the extracellular adhesive substances and the roughness of the steel surface help to trap more particles and organisms [2], protecting them against environmental stresses, including: desiccation; changes in temperature, pH predators, and toxins (in 10 to 1000 times higher concentrations); UV exposure; and facilitating the capture of necessary nutrients (thanks to the polymer gel matrix in which they are embedded) [45]. This access to nutrients and organic molecules is probably the main advantage that bacteria within biofilms have [3][46][47]. Once established on the metal, the marine biofilm rapidly colonizes surfaces such as stainless steel, accelerating their corrosion [48].
3.2. Stage 2. Primary Colonization
The colony formation of microorganisms by bacteria or algae occurs during the first 24 h. At this stage the biofilm becomes a more complex community that generally includes multicellular organisms, herbivores, and decaying organisms. Bacterial adhesion occurs through interactions with planktonic cells, such as electrostatic interactions, gravity, and water flow. After the initial reversible adsorption, bacteria use extracellular polymers to temporarily attach themselves to the surface.
There is a significant difference in biofilm formation depending on whether it occurs in marine environments, or in hospitals and food processing machines [49][50][51][52]. Cell-to-cell communication plays an essential role in the synchronization of processes within the biofilm and is carried out through the quorum sensing system (QS). This system regulates gene expression in response to cell population density. This communication process is carried out by compounds called “autoinducers”, which serve as a chemical signal to induce gene expression in the cell collective [53].
Gram-positive and gram-negative bacteria use quorum sensing communication circuits to regulate a wide variety of physiological activities, such as symbiosis, virulence, competition, conjugation, antibiotic production, motility, sporulation, etc. In general, gram-negative bacteria use acyl-homoserine lactone as an autoinducer, whereas gram-positive bacteria use processed oligopeptides as autoinducers [53]. The quorum sensing communication circuit organizes the microorganisms inside the biofilm and helps it to mature.
In medical devices [54][55], bacteria can adhere to the metal surfaces, causing severe diseases in patients when they mature [2][54]. Moreover, as the biofilm blocks the patient’s defence mechanisms, the action of antibiotics and other chemical agents is inhibited [31][32]. Body fluids can also cause corrosion of a steel device, releasing chromium and nickel ions, which can accumulate in the tissues and put the patient’s health at risk [56].
3.3. Stage 3. Secondary Colonization (Microfouling)
3.3.1. Marine Environments
The adhesion of microalgae and protozoa spores occurs during the first week. Colonization by microorganisms in the sea begins after about 1 h. Both bacteria and algae can attach to marine structures such as ships, pipelines, and heat exchangers [2], reducing operational efficiency and increasing maintenance costs [57]. Dang et al. [46] and Dang and Lovell [57] found that bacteria colonize metal surfaces found in the sea, and that their cells can determine the structure and function of a mature biofilm. Railkin et al. [58] have determined that the observed ratio in marine biofilms of bacteria, diatoms and flagellates is 640:4:1. Some species of molluscs and crustaceans also colonize such surfaces, which leads to a considerable increase in greenhouse gas emissions [59].
Colonization of unicellular eukaryotes (e.g., diatoms, yeasts, and protozoa) usually begins several days after immersion [46]. Later, colonization by multicellular eukaryotes occurs. The main eukaryotic microorganisms are diatoms, fungi, and protozoa, with diatoms being the dominant microorganisms. Diatom adhesion is a more complicated process than bacterial adhesion, since most diatoms lack flagella, so they cannot actively approach a specific surface, but passively precipitate on the substrate. Benthic diatoms approach surfaces through gravitational effects or ocean currents. Planktonic diatoms, which have almost the same specific gravity as seawater, precipitate on surfaces through turbulence mainly. In addition, electrostatic interactions such as Coulomb attraction and electrostatic contact potential are involved [59][60].
After the diatoms precipitate on the steel surface, they form a reversible bond called primary adhesion through the secretion of extracellular polymeric substances. Subsequently, they reorient themselves and move across the surface to positions more suited to their preferences, a process called diatom gliding. The extracellular polymeric substance of diatoms is composed of sulphated carboxylic acid polysaccharides, which are involved in primary adhesion, and proteoglycans, which are involved in diatom gliding and stabilizing the cross-linking of the biofilm matrix [60].
To mitigate the action of microorganisms, it is essential to specify which ones are present, as well as their growth mechanisms. Among the bacteria are: sulphate reducing bacteria (SRB) [61], acid producing bacteria (APB) [62][63] manganese reducing bacteria (MRBn) [61][62], and iron oxidizing bacteria (IOB) [61][63], which generally settle in the biofilms formed on the surface of the steel.
Figueroa de Gil et al. [64] studied the effect of SRB (Desulfotomaculum thermoacetoxidians) on the corrosion of 316L stainless steel; the combined effect of oxygen and sulphide ions on the passive film of the steel, produced localized corrosion on the steel surface. This effect is still greater if hydrogen sulphide (H2S) is formed, as this reduces the pH in the affected zone, leading to pitting on the steel surface. A similar effect was also described by Santander Morales et al. [65] with the bacterium Desulfovibrio desulfuricans. They too observed the colonization of the surface of 316L stainless steel and pitting.
Emerson et al. [66] made an analysis of Fe-oxidizing bacteria (FeOB), explaining that this type of bacteria is oxygen-dependent, develops in neutral pH and belongs to the group of proteobacteria. Acting under aerobic conditions they consume oxygen, leaving conditions conducive for SRB to act under anaerobic conditions. FeOB also provide iron and sulphide ions, which is important for the pitting mechanism mentioned above by promoting the presence of anodic sites.
In marine environments, the dominant species is Mariprofundus ferroxydans. This is the most documented bacterium; it does not use sulphur, hydrogen, and ammonium compounds, nor organic substrates for its growth. It is a mesophilic microorganism with a growth limit temperature of over 30 °C, with genes to perform autotrophy. Marinobacter aquaeolei, some pseudoalteromonas and pseudomonas bacteria were also identified.
3.3.2. Food Processing
Microorganisms can adhere to equipment, pipes, conveyor systems, and tanks in the food industry [25][27] reducing heat transfer, generating energy losses, increasing the frictional resistance of fluids, and accelerating the corrosion process [33][34]. In addition, their adhesion can cause pathogenic diseases transmitted through contaminated food [24][25].
The environmental conditions in the food processing industry favour the proliferation of various types of microbes that can form biofilms, such as the bacteria Listeria monocytogenes, Salmonella enterica, Escherichia coli, or Pseudomonas aeruginosa and Staphylococcus aureus [24][26].
3.3.3. Implants and Biomedical Devices
In prostheses and in medical implants, such as pacemakers, insulin pumps, operating room monitors, and defibrillators (coronary stents, valves and catheters are those most referred to in the literature) colonization will depend on the type of device in question. Biofilm formed on a urinary catheter may contain Staphylococcus epidermidis, Enterococcus faecalis, Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, Klebsiella pneumoniae, and other gram-negative bacteria [67]. The biofilm formed on a central venous catheter may contain Staphylococcus epidermidis, Staphylococcus aureus, Candida albicans, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Enterococcus faecalis bacteria. All these bacteria commonly originate from the patient’s skin, medical device, or healthcare personnel [68][69]. Both Staphylococcus aureus and Staphylococcus epidermidis are estimated to cause about 40–50% of prosthetic heart valve infections, 50–70% of catheter biofilm infections, and 87% of bloodstream infections [68].
3.4. Stage 4. Tertiary Colonization (Macrofouling)
During the second and third week, occurs the adhesion of organisms as larvae, crustaceans, cnidarians, molluscs, polychaetes, and tunicates. Macroorganisms such as algae, spores, barnacles, cnidarians, marine fungi and protozoa, polychaetes, tunicates, coelenterates, and other molluscs, as well as spores, can be attracted by sensory stimuli or can be trapped by the polymeric matrix of the biofilm. This is known as macrofouling [43][70]. The macroorganisms settle on the surface of stainless steel and this is the most noticeable and disturbing phase of biofouling, increasing the weight of the structure. It can only happen after microfouling [43][70]. Typically, macrofouling species have rapid metamorphosis and growth rates, a low degree of substrate preference, and adapt well to different environments.
The settlement and growth of marine microorganisms involve invertebrates, such as mussels and barnacles, along with the growth of macroalgae [43][48]. Some of the most common macrofouling are shown in table 1.
Table 1. Macrofouling species.
|
Macro Fouling Species |
Description |
Reference |
|
Crustacean Balanus amphitrite Semibalanus balanoides Balanus improvises |
Barnacles are common and are particularly difficult to remove. They respond in complex ways to a variety of signals, particularly the quorum signals of their species. Balanus improvisus tends to prefer hydrophobic metal surfaces and relatively low flow velocities. Balanus amphitrite prefers hydrophilic surfaces and prefers to settle on surfaces exposed to flows of medium velocity. |
|
|
Bryozoa Bugula simplex Bugula stolonifera Bugula turrita Bugula neritina Bugula flabellata |
They tend to settle on substrates with mature biofilms; however, biofilms inhibit the settlement of Bugula flabellata. |
|
|
Tunicates or urochordates Diplosoma listerianum Didemnum candidum Ascidia mentula Ciona intestinalis |
Settlement of some tunicates is facilitated or attracted by biofilms and generally increases as biofilms age. |
[78] |
|
Cnidarians Clava multicornis Porites astreoides Balanophyllia elegans Alcyonium siderium Dynamena pumila |
Their ideal place of settlement is often in algae already settled on the material, where there is less sediment, in the bottom part of the algae. |
|
|
Annelids Spirorbis spirorbis Spirorbis tridentatus Pomatoceros lamarckii Hydroides elegans |
They tend to settle in large amounts on the surface of the material at low tide, once the biofilm has dried. |
[80] |
|
Sponges or beads Reneira Cliona celata |
Sponge larvae do not feed. They are ephemeral and have limited dispersal. Since sponges lack adhesive glands, to adhere to a surface their ectodermal cells secrete adhesive. |
[83] |
|
Molluscs Dreissena polymorpha Mytilus edulis |
Molluscs, unlike other organisms, are able to metamorphose in other places and move towards the material. The blue mussel, Mytilus edulis, prefers rough biofilms and hydrophobic surfaces for settlement. |
[84] |
Names highlighted in bolt for species identification only.
4. Use of Nanotechnology to Mitigate the Biofouling Process on Stainless Steel
To mitigate biofilm formation and the adhesion of microorganisms on steel surfaces in seawater, antifouling paints based on copper or tin compounds have been used. According to Gipperth [71], tributyl tin (TBT) has been used since the 1970s due to its long shelf life, and has is very effective against the microorganisms mentioned. These compounds were banned in 2008 [2] as they cause abnormal development in non-target marine biota, such as oysters and mussels. The banning of paints made from tributyl tin (TBT) has brought about a significant change in antifouling coatings for marine structures and components.
Table 2 shows the microorganisms whose adhesion was inhibited using nanotechnology. Significant inhibitory effects were observed for 18 different bacteria (17 reported in marine environments, four in food processing and five in health care). Adhesion was also inhibited in five types of algae in marine environments.
The bacteria most studied were Staphylococcus aureus (gram-positive) and Escherichia coli (gram-negative); they are the most common cause of disease and infection in humans [72][73]. Analysis of them could be used as an inhibition model for gram-positive and gram-negative bacteria. Zotolla and Sasahara [74] reported that biofilm formation occurs when the number of adherent cells is 106 to 107 CFU/cm2.
Three lines of research on the use of nanotechnology to reduce biofouling of stainless-steel surfaces were identified:
- the use of metallic nanoparticles in organic matrix coatings;
- the generation of nano textures on the surface.
Table 2. Microorganisms that have shown reduction or inhibition of adhesion/growth on stainless steel surfaces because of nanotechnology treatment.
|
Type of Stainless Steel |
Inhibited Bacteria |
Inhibited Algae |
Suggested Treatment |
Work Environment |
Reference |
|
Not specified |
GR + Staphylacococcus aureus GR − Escherichia coli |
- |
Vanadium pentoxide (V2O5) particles |
Marine |
[85] |
|
Not specified |
GR+ Listeria monocytogenes GR − Pseudomonas aeruginosa |
- |
Copper particles |
Food and medicine |
[86] |
|
SS 304 |
GR + Staphylacococcus aureus GR − Escherichia coli |
Phaeodactylum triconutum |
Copper particles (Polidopamide matrix) |
Marine |
[87] |
|
SS 316L |
GR − Halomonas aquamarina GR − Vibro aesturianus GR - Pseudoalteromonas elyakovii |
Halamphora coffeaeformis Cylindrotheca closterium |
Zinc particles (Calcium alginate matrix) |
Marine |
[88] |
|
Not specified |
GR − Pseudomonas aeruginosa GR+ Staphylocuccus aureus GR−Escherichia coli |
- |
Anatase particles (TiO 2−Ag) |
Medicine |
[[13]] |
|
SS 316L |
GR − Escherichia coli GR+ Staphylococcus aureus |
- |
TiO2-PTFE (Polytetrafluoroethylene) |
Medicine |
[89] |
|
SS 304 |
GR+ Staphylocuccus aureus |
- |
TiO 2-CID (Diamond-like carbon) |
Medicine |
[90] |
|
SS 304 |
GR − Escherichia coli |
- |
Ag-APTES (3-aminopropyl triethoxysilane) |
Food |
[91] |
|
SS 304 |
GR − Escherichia coli GR + Staphylococcus aureus |
Chlorella pyrenoidosa Phaeodactylum tricornutum Naviculaceae spp. |
Silver nanoparticles in a polidopamine matrix |
Marine |
[92] |
|
SS 304 |
GR − Escherichia coli GR − Flavobacterium sp. GR − Pseudomonas aeruginosa GR − Aeromonas sp. GR − Vibrio cholerae GR − Salmonella sp. GR − Shigella sp. GR − Enterobacter aerogenes GR − Klebsiella sp. GR − Chromohalobacter GR + Bacillus sp. GR + Micrococcus sp. GR + Corynebacterium sp. GR + Bacillus litoralis GR + Staphylococcus aureus |
- |
Silver particles—algae turbine ornate |
Marine |
[44] |
|
SS 316L |
GR + Staphylococcus aureus GR − Escherichia coli |
- |
Generation of nanotextures |
Food and medicine |
[93] |
The inhibition mechanisms generated by these modifications on stainless steel are described below.
4.1. Use of Metallic Nanoparticles in Organic Matrix Coatings
The use of nanoparticles to inhibit biofilm formation on stainless steel surfaces has been reported, killing up to 18 types of bacteria [44][88][90] and five types of algae [89][90][94]. The main difficulties in this are the homogeneous distribution of nanoparticles on the substrate surface and the controlled release of active ions. An advantage of the use of nanoparticles is the large surface area available for interaction with the microorganisms, becoming more cytotoxic to them [11][12]. This is possible as the particle is much smaller than the main compounds that form the structure of the cell of the microorganisms, making the interaction easy. Four nanoparticles used in treatments to reduce biofouling are described below.
Generally, to obtain the inhibitory mechanism of these metal nanoparticles, it is necessary to form an organic coating on the stainless steel surfaces. This coating helps to have a homogenious distribution and their correct join of these nanoparticles
Vanadium. According to Natalio et al. [87], the use of nanowires of V2O5 showed inhibitory activity against bacteria Staphylococcus aureuos and Escherichia coli without affecting marine biota. They associate the biocidal mechanism of V2O5 nanowires to their bromination activity, such as the functioning of vanadium haloperoxidase (V-HPO) enzymes, which produce hypobromous acid (HBrO) at pH 8–8.3 (pH commun of seawater). The nanowires catalyze the oxidation of bromide ions (Br−) to HBrO in the presence of hydrogen peroxide (H2O2), forming a reactive oxygen molecule which exerts vigorous antibacterial activity and interferes with the bacterium quorum sensing system. This effect is potentialized mixing vanadium nanowires with antifouling paint.
Copper. The research of Cao et al. [89] indicated that copper nanoparticles reduce the adhesion of Escherichia coli and Staphylococcus aureus while the research of Ghesemian et al. [88] showed the inhibition of Listeria monocytogenes and Pseudomonas aeruginosa too. They and Tsai, suggest as inhibitory mechanisms the formation of stable copper-protein complexes causes interference in the transport of essential elements and causes oxidative stress, generating different cellular dysfunctions, such as the suppression of cell division and an increase in membrane permeability, and the destruction of phospholipid layer of the cell wall of bacteria.
Ghesemian et al. [88], stated that the antibacterial effect of copper nanoparticles is mainly due to the surface area available for the copper-bacteria interaction, that is, the size of the particle. When particles have more surface area available to interact with bacteria, their antibacterial effect tends to increase, and they become more cytotoxic to microorganisms [10][11][12].
Zinc. Abi Nassif et al. [90] showed good bacterial inhibition of zinc nanoparticles mixed with calcium alginate. This system inhibit Halomonas aquamarine, Vibrio aesturianus, Pseudoalteromonas elyakovii bacteria and Halomphora coffeaeformis and Cylindrotheca Closterium algae.
Stanic et. Al. [95] explain the antibacterial and anti-algae activity of Zn2+ ions indicating that these ions tend to form strong bonds with the thiol or sulfhydryl (−SH), imidazole (C3H4N2), amino (−NH2) and carboxyl (−COOH) groups of the membrane proteins of microorganism. Structural changes in the membrane (increased permeability) mean that microorganisms are unable to properly regulate the transport of essential elements, leading to cell death.
Silver. Research of Krishnan et al. [44], Chen et al. [96] and Cao et al. [94] showed that silver nanoparticles inhibit and reduce de adhesion of 16 different bacteria and 3 types of algae. The inhibition mechanism of these nanoparticles causes first loss of membrane integrity and cell lysis [97][98]. Chen et al. [96] suggests that when Ag+ ions encounter bacteria they interact with the sulphur, nitrogen, or phosphorus atoms in the membrane, inhibiting growth and even killing the bacteria. Cao et al. [94] indicated the antibacterial activity of these nanoparticles is associated with direct damage to the cell wall by the imminent contact. Thiel et al. [99] suggest that nanoparticles bind to thiol or sulfhydryl groups of enzymes and proteins in the cell membrane, thus affecting protein biosynthesis and consequently the DNA and RNA of bacterial cells. Feng et al. [100] demonstrated that Ag+ ions interact with thiol groups of proteins and DNA bases, leading to a respiratory inhibition of bacteria or unwinding of DNA, resulting in bacterial death or inhibition.
The inhibitory mechanism in algae suggested on the research of Cao et al. [94] that the Ag+ ion released on the surface of the nanoparticles interacts with the algae, triggering the release of proteins and polysaccharides in the algal cells, thus killing them, or inhibiting their growth [101][102].
Anatase (TiO2). Tallósy et al. [13], Zhang et al. [91] and Lopes et al. [92] showed the inhibition of Staphylococcus aureus and Escherichia coli using anatase nanoparticles. Anatase posses broad-spectrum bactericide has excellent biocompatibility and corrosion resistance [103][104]. TiO2 particles are photoreactive and can kill or inhibit bacterial growth through cell wall penetration. The reactive oxygen species (ROS), produced as a result of the photoreactive effect interact with bacterial cells, killing them or inhibiting their growth [105][106]. Among the reactive species produced, -OH radicals stand out. These can destroy the bacterial cell wall by breaking covalent bonds and inhibiting the formation of crosslinks in the peptidoglycan layer, which is mainly responsible for the stability of the cell wall in bacteria [107][108].
Li et al. [109] reported that TiO2 can eliminate both gram-negative and gram-positive bacteria, due to the tendency of reactive oxygen species (such as −OH ions) to attack the peptidoglycan layer.
Tallósy et al. [13] observed the degradation of peptidoglycan and the outer membrane of bacterium cells by photocatalysis in the studied bacteria. Finally, the addition of silver nanoparticles gave a 15% improvement in bactericidal activity compared to pure TiO2 coatings. These results indicate that the use of TiO2−Ag coatings in marine structures could be very useful.
4.2. Generation of Nanotextures on Stainless Steel
Jang et al. [93] observed that the surface of 316L stainless steel nanotextured by electrochemical pickling reduced adhesion of Staphylococcus aureus and Escherichia coli. The antibacterial mechanism of the nanotexture is attributed to the formation of controlled nanopores and nanoprotrusions with a diameter of 20 nm. The sharp edges can induce mechanical stress on the membrane of adherent bacteria, resulting in cell death without the application of antibiotics, metallic, or polymeric coatings [110]. Competition between cell membrane elasticity and the capillarity of nanopores on the surface of steels can also improve the deformation and tension of the bacterial membranes [93].
To test the effectiveness of nanotexturing, Choi et al. [111] found that an increase in surface roughness from nanometres to microns does not significantly reduce bacterial adhesion on 316L stainless steel compared to untreated 316L stainless steel.
5. Nanomaterials with Potential Use to Inhibit Biofouling on Stainless Steel
It is important to consider other alternatives that have been reported for the surfaces of different materials that could be used on stainless steel. Some works that show promise for marine environments, but that could hardly be used in food processing and in the human body, are described below.
Several materials with good biocidal and antifouling activity have been reported [14][15][112][113][114][115][116][117][118]. Among these, the following stand out: halloysite clay nanotubes, mesoporous silica nanocapsules, and layered double hydroxides. These nanomaterials showed their efficiency in marine environments on target species with low toxicity on non-target species, when accompanied by biocidal agents such as DCOIT (4,5-Dichloro-2-octyl- 3-isothiazolone), zinc and copper pyrithiones, and silver nanoparticles. These materials are called smart nano-containers, they are nanostructured materials that release the active compounds in a controlled way, avoiding contact between the active species and the coating matrix, providing a protective barrier [119]. The antifouling mechanisms of these nanomaterials are briefly explained below:
5.1. Mesoporous Silica Nanocapsules
These spherical nanoparticles typically have a diameter of 100–200 nm and the predominant release mechanism is based on the diffusion of the active compound through the porous layer [120][121].
Unloaded silica nanocapsules. Unloaded silica nanocapsules inhibited the growth of the alga P. tricornutum [14]. Gutner-Hoch et al. [15] observed a mild toxic effect on the nauplii of the crustacean Artemia salina and the sea urchin Paracentrotus lividus. This can be attributed to the presence, within its porous structure, of a quaternary ammonium compound (CTAB Hexadecyltrimethylammonium bromide), which is used as an emulsion stabilizer during the synthesis of nanocapsules [118][121]. This compound was catalogued as a toxic compound for a wide range of aquatic organisms [122].
Silica nanocapsules loaded with biocide. The copper pyrithione-filled nanocapsules were very found to be effective against diatom growth; however, acute toxicity was observed for non-target species [116]. The release of zinc and copper pyrithione is limited by the low solubility of biocides in seawater [123][124]. When applying a DCOIT biocide load, Santos et al. [118] observed that in gametes of the bivalve P. perna, the application of nanocapsules loaded with DCOIT was 137 times less toxic than free DCOIT. This substantial difference is related to the controlled release of the biocide that occurs gradually over time, and by predefined stimuli.
The toxicity of the encapsulated biocides was lower for all the species evaluated, and the toxicity of free DCOIT was 214 times higher than encapsulated DCOIT. The toxicity of the encapsulates may be due to physical/mechanical effects on certain species, such as [115][121][125][126][127]:
- Particles can adhere to gills, interfering with filtration/respiration, leading to sublethal effects that ultimately cause the death;
- Particles can adhere to the body surface, affecting mobility which can cause starvation and death;
- Particles can cause a shadowing effect on the microalgae, which can interfere with the photosynthesis process.
5.2. Layered Double Hydroxides
These plates typically have a thickness of 20–40 nm, and the release mechanism of the main active compound is anion exchange [120].
Unloaded layered double hydroxides. According to Avelelas et al. [14], when applied alone, double layer hydroxides have low toxicity for target species (such as P. Tricornutum and the mollusk Mytilus edulis) as against non-target species (such as algae Tetraselmis chuii). Gutner-Hoch et al. [117] also observed that hydroxides without biocidal loading have a more pronounced antifouling effect on bryozoan larvae than on adult mussels.
Layered double hydroxides loaded with biocide. When these hydroxides are loaded with zinc and copper pyrithiones, there are more limitations with respect to silica nanocapsules due to the possibility of having a significant amount of pyrithione molecules chemically bound to the surface of the hydroxide. In the case of nanocapsules, pyrithiones are only physically trapped and can diffuse more easily, despite having a lower charge content [14].
The double layer hydroxide with zinc pyrithione proved to be even more effective in terms of antifouling properties, showing a higher acute toxicity against the mussel M. edulis (macro-fouling species) compared to free zinc pyrithione [14]. Gutner-Hoch et al. [15] observed that hydroxides loaded with pyrithiones are the most effective antimacroincrustants for mussels and bryozoans, with those loaded with zinc pyrithione being more effective for mussels, and those loaded with copper pyrithione being more effective for bryozoans.
Gutner-Hoch et al. [15], observed that the presence of zinc hydroxide-pyrithione can contribute to the formation and release of ionized pyrithione and Zn2+ ion. Although intracellular zinc levels can pose risks to the cell, pyrithione ions are highly reactive and tend to react with metals and generate new compounds, thus presenting a more toxic chemical mixture [[14]]. Pyrithiones are known to be powerful inhibitors of various cellular processes, such as membrane transport, regulation of ATP levels, and protein synthesis [128].
5.3. Halloysite nanotubes
Halloysite is a naturally occurring mineral, widely available at low cost. It is a tubular material of rolled layers of aluminosilicates with an outer diameter of 50 to 60 nm, a lumen of 10 to 15 nm, and a length of 0.5 to 1 m [114]. It is like kaolinite in composition, with more water between the layers adjacent to the walls [114]. The outer surface of negatively charged silica and the lower surface of positively charged alumina in halloysite allow for the selective charging of chemicals [129][130].
These nanotubes can be used as containers for the encapsulation and controlled release of antifouling active agents. Sustained release can even be achieved for years. These inexpensive nanotubes can be used to contain antifouling agents such as DCOIT and serve as a template for the formation of silver particles, preventing their undesirable aggregation [131][132].
Fu et al. [113], developed an epoxy antifouling coating doped with halloysite nanotubes loaded with the biocide (DCOIT) or silver, which provided prolonged protection against the proliferation of the marine bacterium Vibrio natriegens and this effect can be potentialized using silver nanoparticles.
References
- Dong, B.; Manolache, S.; Somers, E.B.; Lee Wong, A.C.; Denes, F.S.; Generation of antifouling layers on stainless steel surfaces by plasma-enhanced crosslinking of polyethylene glycol. J. Appl. Polym. Sci 2005, 97, 485, doi.org/10.1002/app.21766..
- Diego Meseguer Yebra; Søren Kiil; Kim Dam-Johansen; Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Progress in Organic Coatings 2004, 50, 75-104, 10.1016/j.porgcoat.2003.06.001.
- J W Costerton; Z Lewandowski; D E Caldwell; D R Korber; H M Lappin-Scott; Microbial Biofilms. Annual Review of Microbiology 1994, 49, 711-745, 10.1146/annurev.micro.49.1.711.
- Chityal Ganesh Kumar; S.K Anand; Significance of microbial biofilms in food industry: a review. International Journal of Food Microbiology 1998, 42, 9-27, 10.1016/s0168-1605(98)00060-9.
- Vicente J.D. Rascio; ANTIFOULING COATINGS: WHERE DO WE GO FROM HERE. Corrosion Reviews 2000, 18, 133-154, 10.1515/corrrev.2000.18.2-3.133.
- Little, B. J.; Lee, J. S.. Microbiologically Influenced Corrosion. In kirk-Othmer Encyclopedia of Chemical Technology; John Willey & Sons, Inc.: Hoboken, Nueva Jersey, USA, 2009; pp. 1-42.
- W A Hamilton; Microbially Influenced Corrosion as a Model System for the Study of Metal Microbe Interactions: A Unifying Electron Transfer Hypothesis. Biofouling 2003, 19, 65-76, 10.1080/0892701021000041078.
- International measures of prevention, application, and economics of corrosion technologies study . NACE Int.. Retrieved 2022-2-10
- Impact of Biofilm and Bioufouling on Materials and Processes . CORDIS. Retrieved 2022-2-10
- Jose Ruben Morones; Jose Luis Elechiguerra; Alejandra Camacho; Katherine Holt; Juan Kouri; Jose Ruben Morones-Ramirez; Miguel Jose Yacaman; The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346-2353, 10.1088/0957-4484/16/10/059.
- Muhammad Raffi; Saba Mehrwan; Tariq Mahmood Bhatti; Javed Iqbal Akhter; Abdul Hameed; Wasim Yawar; M. Masood Ul Hasan; Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Annals of Microbiology 2010, 60, 75-80, 10.1007/s13213-010-0015-6.
- B.G. Cousins; Heather Allison; P.J. Doherty; C. Edwards; M.J. Garvey; D.S. Martin; Rachel Williams; Effects of a nanoparticulate silica substrate on cell attachment of Candida albicans. Journal of Applied Microbiology 2006, 102, 757-765, 10.1111/j.1365-2672.2006.03124.x.
- Szabolcs Péter Tallósy; László Janovák; Elisabeth Nagy; Ágota Deák; Ádám Juhász; Edit Csapó; Norbert Buzás; Imre Dékány; Adhesion and inactivation of Gram-negative and Gram-positive bacteria on photoreactive TiO2/polymer and Ag–TiO2/polymer nanohybrid films. Applied Surface Science 2016, 371, 139-150, 10.1016/j.apsusc.2016.02.202.
- Francisco Avelelas; Roberto Martins; Tânia Oliveira; Frederico Maia; Eliana Malheiro; Amadeu Soares; Susana Loureiro; João Tedim; Efficacy and Ecotoxicity of Novel Anti-Fouling Nanomaterials in Target and Non-Target Marine Species. Marine Biotechnology 2017, 19, 164-174, 10.1007/s10126-017-9740-1.
- Eldad Gutner-Hoch; Roberto Martins; Tania Oliveira; Frederico Maia; Amadeu M. V. M. Soares; Susana Loureiro; Chen Piller; Iris Preiss; Michal Weis; Severine B. Larroze; et al.Tania TeixeiraJoão TedimYehuda Benayahu Antimacrofouling Efficacy of Innovative Inorganic Nanomaterials Loaded with Booster Biocides. Journal of Marine Science and Engineering 2018, 6, 6, 10.3390/jmse6010006.
- Robert E. Melchers. Springer Handbookoƒ Ocean Engineering; Manhar R. Dhanak, Nikolaos I. Xiros , Eds.; Springer-Verlag GmbH Berlin Heidelberg: Leipzig, Germany, 2016; pp. 111-121.
- Corrosion Impact of Offshore Platforms, Structures, and Vessel . Materials Performance. Retrieved 2022-2-10
- Robert E. Melchers; Robert Jeffrey; Corrosion of long vertical steel strips in the marine tidal zone and implications for ALWC. Corrosion Science 2012, 65, 26-36, 10.1016/j.corsci.2012.07.025.
- Little B. J., De Palma J. T.. Marine Biofouling. In Materials for Marine Systems and Structures: Treatise on Materials Science and Technology; Hasson, D.F., Crowe, C., Eds.; Academic Press Inc.: London, 1988; pp. 90-117.
- Relini, G.; Dabini-Oliva, G. In Proceedings of the Third International Congress On Marine Corrosion and Fouling, National Bureau of Standards, Gaithersburg, Maryland, October 2-6, 1972; pp. 757–766
- Huve, P. Étude expérimentale du peuplement de surfaces rocheuses immergées, en Méditerranée occidentale. Comptes Rendus Hebd. Séances L’académie Sci. 1953, 236, 419–422.
- H W Jannasch; K Eimhjellen; C O Wirsen; A Farmanfarmaian; Microbial Degradation of Organic Matter in the Deep Sea. Science 1971, 171, 672-675, 10.1126/science.171.3972.672.
- Berger L. R.; Enzyme kinetics, microbial respiration, and active transport at increased hydrostatic pressure. Rev. Phys. Chem. Japan 1974, Special Issue, 639-642.
- Brigitte Carpentier; Olivier Cerf; Biofilms and their consequences, with particular reference to hygiene in the food industry. Journal of Applied Bacteriology 1993, 75, 499-511, 10.1111/j.1365-2672.1993.tb01587.x.
- Diego García-Gonzalo; Rafael Pagán; Influence of Environmental Factors on Bacterial Biofilm Formation in the Food Industry: A Review. postdoc journal 2015, 3, 3-13, 10.14304/surya.jpr.v3n6.2.
- Sanz, M. Multi-Species Biofilms in the Food Industry. Available online: https://www.betelgeux.es/blog/2019/11/08/biofilms-multiespecie-en-la-industria-alimentaria/#:~:text=The%20biofilms%20act%20C3%BAan%20as%20an%20extracellular%20that%20they%20myself%20generate
- C. Mariani; Nadia Oulahal; J.-F. Chamba; F. Dubois-Brissonnet; E. Notz; Romain Briandet; Inhibition of Listeria monocytogenes by resident biofilms present on wooden shelves used for cheese ripening. Food Control 2011, 22, 1357-1362, 10.1016/j.foodcont.2011.02.012.
- Gun Wirtanen; Ulrika Husmark; Tiina Mattila-Sandholm; Microbial Evaluation of the Biotransfer Potential from Surfaces with Bacillus Biofilms after Rinsing and Cleaning Procedures in Closed Food-Processing Systems. Journal of Food Protection 1996, 59, 727-733, 10.4315/0362-028x-59.7.727.
- Yuehuei H. An; Richard J. Friedman; Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. Journal of Biomedical Materials Research 1997, 43, 338-348, 10.1002/(SICI)1097-4636(199823)43:3%3C338::AID-JBM16%3E3.0.CO;2-B.
- M Katsikogianni; Yf Missirlis; Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. European Cells and Materials 2004, 8, 37-57, 10.22203/ecm.v008a05.
- Hisao Morisaki; Hidenori Tabuchi; Bacterial attachment over a wide range of ionic strengths. Colloids and Surfaces B: Biointerfaces 2009, 74, 51-55, 10.1016/j.colsurfb.2009.06.023.
- Patrick Chavant; Brigitte Martinie; Thierry Meylheuc; Marie-Noëlle Bellon-Fontaine; Michel Hebraud; Listeria monocytogenes LO28: Surface Physicochemical Properties and Ability To Form Biofilms at Different Temperatures and Growth Phases. Applied and Environmental Microbiology 2002, 68, 728-737, 10.1128/aem.68.2.728-737.2002.
- Pierluigi Di Ciccio; A. Vergara; A.R. Festino; D. Paludi; Emanuela Zanardi; Sergio Ghidini; A. Ianieri; Biofilm formation by Staphylococcus aureus on food contact surfaces: Relationship with temperature and cell surface hydrophobicity. Food Control 2015, 50, 930-936, 10.1016/j.foodcont.2014.10.048.
- Trevor Roger Garrett; Manmohan Bhakoo; Zhibing Zhang; Bacterial adhesion and biofilms on surfaces. Progress in Natural Science 2008, 18, 1049-1056, 10.1016/j.pnsc.2008.04.001.
- Agnès Villain-Simonnet; Michel Milas; Marguerite Rinaudo; A new bacterial exopolysaccharide (YAS34). II. Influence of thermal treatments on the conformation and structure. Relation with gelation ability. International Journal of Biological Macromolecules 2000, 27, 77-87, 10.1016/s0141-8130(99)00119-1.
- Anderson, G.; O’toole, G.. Innate and induced resistance mechanisms of bacterial biofilms. In Bacterial Biofilms; Romeo, T., Eds.; Springer: Berlin/Heidelberg: Leipzig, Germiny, 2008; pp. 85-105.
- Carlos Moreno-Castilla; Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 2003, 42, 83-94, 10.1016/j.carbon.2003.09.022.
- W.G. Characklis; M.G. Trulear; J.D. Bryers; N. Zelver; Dynamics of biofilm processes: methods. Water Research 1982, 16, 1207-1216, 10.1016/0043-1354(82)90139-7.
- Lo Vetri, K.; Gawande, P.V.; Yakandawala, N.; Madhyastha, S. . Biofouling and Anti-Fouling of Medical Devices. In Biofouling: Types, Impact and Anti-Fouling; Chan, J., Wong, S, Eds.; Nova Science Publishers, Incorporated: New York, USA, 2010; pp. 105–127.
- Puhakka, E.; Riihimäki, M.; Keiski, R. Fouling mechanisms by ab initio calculations condensation reactions on the rutile (101) surface and adsorption of ions on the Cr2O3 surfaces. In Proceedings of the ECI Symposium Series, Volume RPS: Proceedings of 7th International Conference on Heat Exchanger Fouling and Cleaning VII RP5, Tomar, Portugal, 1–6 July 2007; pp. 300–307. https://dc.engconfintl.org/heatexchanger2007
- Weipeng Zhang; Wei Ding; Yong-Xin Li; Chunkit Tam; Salim Bougouffa; Ruojun Wang; Bite Pei; Hoyin Chiang; Pokman Leung; Yanhong Lu; et al.Jin SunHe FuVladimir B BajicHongBin LiuNicole S. WebsterPei-Yuan Qian Marine biofilms constitute a bank of hidden microbial diversity and functional potential. Nature Communications 2019, 10, 1-10, 10.1038/s41467-019-08463-z.
- Ghiya, S. Self-polishing antifoulings. Paintindia 1987, 37, 19–31
- S Abarzua; S Jakubowski; Biotechnological investigation for the prevention of biofouling. I. Biological and biochemical principles for the prevention of biofouling. Marine Ecology Progress Series 1994, 123, 301-312, 10.3354/meps123301.
- Muthukumar Krishnan; Vignesh Sivanandham; Dahms Hans-Uwe; Santhosh Gokul Murugaiah; Palanichamy Seeni; Subramanian Gopalan; Arthur James Rathinam; Antifouling assessments on biogenic nanoparticles: A field study from polluted offshore platform. Marine Pollution Bulletin 2015, 101, 816-825, 10.1016/j.marpolbul.2015.08.033.
- Flemming, H.C.; Griebe, T.; Schaule, G.; Antifouling strategies in technical systems -- a short review. Water Science and Technology 1995, 34, 51792.1, 10.1016/0273-1223(96)00687-7.
- Hongyue Dang; Tiegang Li; Mingna Chen; Guiqiao Huang; Cross-Ocean Distribution of Rhodobacterales Bacteria as Primary Surface Colonizers in Temperate Coastal Marine Waters. Applied and Environmental Microbiology 2007, 74, 52-60, 10.1128/aem.01400-07.
- Paula Furey; Antonia Liess; Sylvia Lee; Substratum-Associated Microbiota. Water Environment Research 2017, 89, 1634-1675, 10.2175/106143017x15023776270610.
- Brenda J. Little; Jason S. Lee; Richard I. Ray; The influence of marine biofilms on corrosion: A concise review. Electrochimica Acta 2008, 54, 2-7, 10.1016/j.electacta.2008.02.071.
- Romain Briandet; Thierry Meylheuc; Catherine Maher; Marie Noëlle Bellon-Fontaine; Listeria monocytogenes Scott A: Cell Surface Charge, Hydrophobicity, and Electron Donor and Acceptor Characteristics under Different Environmental Growth Conditions. Applied and Environmental Microbiology 1999, 65, 5328-5333, 10.1128/aem.65.12.5328-5333.1999.
- J. Harvey; K.P. Keenan; A. Gilmour; Assessing biofilm formation by Listeria monocytogenes strains. Food Microbiology 2007, 24, 380-392, 10.1016/j.fm.2006.06.006.
- Carol Potera; J. Han; H. G. Craighead; Forging a Link Between Biofilms and Disease. Science 1999, 283, 1837-1839, 10.1126/science.283.5409.1837.
- Philip Stewart; Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrobial Agents and Chemotherapy 1996, 40, 2517-2522, 10.1128/aac.40.11.2517.
- Lami Raphael. Chapter 3—Quorum Sensing in Marine Biofilms and Environments. In Quorum Sensing; Tommonaro, G., Eds.; Academic Press: Cambridge, Massachusetts, USA, 2019; pp. 55-96.
- Zw Teo Wendy; C Schalock Peter; W Teo; Pc Schalock; Hypersensitivity Reactions to Implanted Metal Devices: Facts and Fictions. Journal of Investigational Allergy and Clinical Immunology 2016, 26, 279-294, 10.18176/jiaci.0095.
- Arnout J. van der Borden; Patrick G.M. Maathuis; Eefje Engels; Gerhard Rakhorst; Henny C. van der Mei; Henk J. Busscher; Prashant Kumar Sharma; Prevention of pin tract infection in external stainless steel fixator frames using electric current in a goat model. Biomaterials 2007, 28, 2122-2126, 10.1016/j.biomaterials.2007.01.001.
- Sajjad Habibzadeh; Ling Li; Dominique Shum-Tim; Elaine C. Davis; Sasha Omanovic; Electrochemical polishing as a 316L stainless steel surface treatment method: Towards the improvement of biocompatibility. Corrosion Science 2014, 87, 89-100, 10.1016/j.corsci.2014.06.010.
- Hongyue Dang; Charles R. Lovell; Microbial Surface Colonization and Biofilm Development in Marine Environments. Microbiology and Molecular Biology Reviews 2016, 80, 91-138, 10.1128/mmbr.00037-15.
- Railkin, A.I. Marine Biofouling: Colonization Processes and Defenses; CRC press: Boca Raton, Florida, USA, 2003
- John A. Finlay; Maureen E. Callow; Linnea K. Ista; Gabriel P. Lopez; James A. Callow; The Influence of Surface Wettability on the Adhesion Strength of Settled Spores of the Green Alga Enteromorpha and the Diatom Amphora. Integrative and Comparative Biology 2002, 42, 1116-1122, 10.1093/icb/42.6.1116.
- Ille C. Gebeshuber; Herbert Stachelberger; Manfred Drack; Diatom Bionanotribology—Biological Surfaces in Relative Motion: Their Design, Friction, Adhesion, Lubrication and Wear. Journal of Nanoscience and Nanotechnology 2004, 5, 79-87, 10.1166/jnn.2005.018.
- Congmin Xu; Yaoheng Zhang; Guangxu Cheng; Wensheng Zhu; Localized corrosion behavior of 316L stainless steel in the presence of sulfate-reducing and iron-oxidizing bacteria. Materials Science and Engineering: A 2007, 443, 235-241, 10.1016/j.msea.2006.08.110.
- Lewandowskiy, Z.; Dickinsony, W.; Leey, W.; Electrochemical interactions of biofilms with metal surfaces. Water Science and Technology 1996, 36, 295-302, 10.1016/s0273-1223(97)00336-3.
- I. G. Chamritski; G. R. Burns; B. J. Webster; N. J. Laycock; Effect of Iron-Oxidizing Bacteria on Pitting of Stainless Steel. Corrosion 2004, 60, 658-669, 10.5006/1.3287842.
- Figueroa de Gil, Y.F.; Camero, S.; Prin, J.L.; Réquiz, R.; Evaluación de la corrosión inducida por bacteria sulfato reductora en un acero inoxidable 316L. Revista Latinoamericana de Metalurgia Y Materiales 2008, 28, 60-72.
- Santander, C. Estudio Experimental de Corrosión en Metales de uso Industrial por Desulfovibrio Desulfuricans; Memory to get a Civil Engineer in Biotechnology degree. Univeridad de Chile, Santiago de Chile, 2008. https://repositorio.uchile.cl/handle/2250/103133
- David Emerson; Emily J. Fleming; Joyce M. McBeth; Iron-Oxidizing Bacteria: An Environmental and Genomic Perspective. Annual Review of Microbiology 2010, 64, 561-583, 10.1146/annurev.micro.112408.134208.
- Gregory D. Bixler; Bharat Bhushan; Biofouling: lessons from nature. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2012, 370, 2381-2417, 10.1098/rsta.2011.0502.
- Meng Chen; Qingsong Yu; Hongmin Sun; Novel Strategies for the Prevention and Treatment of Biofilm Related Infections. International Journal of Molecular Sciences 2013, 14, 18488-18501, 10.3390/ijms140918488.
- Rodney Donlan; Biofilms and Device-Associated Infections. Emerging Infectious Diseases 2001, 7, 277-281, 10.3201/eid0702.010226.
- Shan Cao; Jiadao Wang; HaoSheng Chen; Darong Chen; Progress of marine biofouling and antifouling technologies. Chinese Science Bulletin 2010, 56, 598-612, 10.1007/s11434-010-4158-4.
- Lena Gipperth; The legal design of the international and European Union ban on tributyltin antifouling paint: Direct and indirect effects. Journal of Environmental Management 2009, 90, S86-S95, 10.1016/j.jenvman.2008.08.013.
- Arias-Flores, R.; Rosado-Quiab, U.; Vargas-Valerio, A.; Grajales-Muñiz, C. Los microorganismos causantes de infecciones nosocomiales en el Instituto Mexicano del Seguro Social. Rev. Med. IMSS 2016, 54, 5
- WHO. World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed . World Health Organization. Retrieved 2022-2-11
- Edmund A. Zottola; Kyle C. Sasahara; Microbial biofilms in the food processing industry—Should they be a concern?. International Journal of Food Microbiology 1994, 23, 125-148, 10.1016/0168-1605(94)90047-7.
- C Andre; Per Jonsson; M Lindegarth; Predation on settling bivalve larvae by benthic suspension feeders: the role of hydrodynamics and larval behaviour. Marine Ecology Progress Series 1992, 97, 183-192, 10.3354/meps097183.
- Mia Dahlström; Henrik Jonsson; Per R. Jonsson; Hans Elwing; Surface wettability as a determinant in the settlement of the barnacle Balanus Improvisus (DARWIN). Journal of Experimental Marine Biology and Ecology 2004, 305, 223-232, 10.1016/j.jembe.2003.12.013.
- Ritchie Head; Koos Overbeke; Job Klijnstra; Rens Biersteker; Jeremy Thomason; The Effect of Gregariousness in Cyprid Settlement Assays. Biofouling 2003, 19, 269-278, 10.1080/0892701031000101502.
- Per R. Jonsson; Kent M. Berntsson; Ann I. Larsson; LINKING LARVAL SUPPLY TO RECRUITMENT: FLOW-MEDIATED CONTROL OF INITIAL ADHESION OF BARNACLE LARVAE. Ecology 2004, 85, 2850-2859, 10.1890/03-0565.
- M. S. Brancato; R. M. Woollacott; Effect of microbial films on settlement of bryozoan larvae (Bugula simplex, B. stolonifera and B. turrita). Marine Biology 1982, 71, 51-56, 10.1007/bf00396992.
- Dj Marshall; Mj Keough; Variation in the dispersal potential of non-feeding invertebrate larvae: the desperate larva hypothesis and larval size. Marine Ecology Progress Series 2002, 255, 145-153, 10.3354/meps255145.
- Pei-Yuan Qian; D Rittschof; B Sreedhar; Macrofouling in unidirectional flow: miniature pipes as experimental models for studying the interaction of flow and surface characteristics on the attachment of barnacle, bryozoan and polychaete larvae. Marine Ecology Progress Series 1999, 207, 109-121, 10.3354/meps207109.
- John P. Hamer; Graham Walker; Avoidance of dried biofilms on slate and algal surfaces by certain spirorbid and bryozoan larvae. Journal of the Marine Biological Association of the United Kingdom 2001, 81, 167-168, 10.1017/s0025315401003526.
- Anthony Pires; Robert M. Woollacott; Joshua T. Vogelstein; Youngser Park; Tomoko Ohyama; Rex Kerr; James W. Truman; Carey E. Priebe; Marta Zlatic; A Direct and Active Influence of Gravity on the Behavior of a Marine Invertebrate Larva. Science 1983, 220, 731-733, 10.1126/science.220.4598.731.
- Sergio Rossi; Josep Maria Gili; R. G. Hugues; The effects of exposure to wave action on the distribution and morphology of the epiphytic hydrozoans Clava multicornis and Dynamena pumila. Scientia Marina 2000, 64, 135-140, 10.3989/scimar.2000.64s1135.
- Manuel Maldonado; Cm Young; Effects of physical factors on larval behavior, settlement and recruitment of four tropical demosponges. Marine Ecology Progress Series 1995, 138, 169-180, 10.3354/meps138169.
- Chia, F.; Bickell, L. Mechanisms of larval attachment and the induction of settlement and metamorphosis in coelenterates: A Review. In Settlement and Metamorphosis of Marine Invertebrate Larvae; Chia, F.-S., Rice, M.E., Eds.; Elsevier, New York, USA. 1978
- Filipe Natalio; Rute André; Aloysius F. Hartog; Brigitte Stoll; Klaus Peter Jochum; Ron Wever; Wolfgang Tremel; Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nature Nanotechnology 2012, 7, 530-535, 10.1038/nnano.2012.91.
- Ehsan Ghasemian; Ali Naghoni; Helya Rahvar; Mahsa Kialha; Bahman Tabaraie; Evaluating the Effect of Copper Nanoparticles in Inhibiting Pseudomonas aeruginosa and Listeria monocytogenes Biofilm Formation. Jundishapur Journal of Microbiology 2015, 8, e17430, 10.5812/jjm.8(5)2015.17430.
- Pan Cao; Zhimin Cao; Chengqing Yuan; Stainless steel coated by Cu NPs via dopamine coupling for antifouling application. Surface and Interface Analysis 2019, 51, 809-816, 10.1002/sia.6654.
- L. Abi Nassif; S. Rioual; W. Farah; M. Fauchon; Y. Toueix; C. Hellio; M. Abboud; B. Lescop; Electrophoretic deposition of zinc alginate coatings on stainless steel for marine antifouling applications. Journal of Environmental Chemical Engineering 2020, 8, 104246, 10.1016/j.jece.2020.104246.
- Shuai Zhang; Xinjin Liang; Geoffrey Michael Gadd; Qi Zhao; Advanced titanium dioxide-polytetrafluorethylene (TiO2-PTFE) nanocomposite coatings on stainless steel surfaces with antibacterial and anti-corrosion properties. Applied Surface Science 2019, 490, 231-241, 10.1016/j.apsusc.2019.06.070.
- F.S. Lopes; J.R. Oliveira; J. Milani; Luciane Oliveira; J.P.B. Machado; V.J. Trava-Airoldi; Anderson Oliveira Lobo; F.R. Marciano; Biomineralized diamond-like carbon films with incorporated titanium dioxide nanoparticles improved bioactivity properties and reduced biofilm formation. Materials Science and Engineering: C 2017, 81, 373-379, 10.1016/j.msec.2017.07.043.
- Yeongseon Jang; Won Tae Choi; Christopher T. Johnson; Andrés J. García; Preet M. Singh; Victor Breedveld; Dennis W. Hess; Julie A. Champion; Inhibition of Bacterial Adhesion on Nanotextured Stainless Steel 316L by Electrochemical Etching. ACS Biomaterials Science & Engineering 2017, 4, 90-97, 10.1021/acsbiomaterials.7b00544.
- Pan Cao; Xiaoyan He; Jinfei Xiao; Chengqing Yuan; Xiuqin Bai; Covalent bonding of AgNPs to 304 stainless steel by reduction in situ for antifouling applications. Applied Surface Science 2018, 452, 201-209, 10.1016/j.apsusc.2018.04.227.
- Vojislav Stanić; Suzana Dimitrijevic-Brankovic; Jelena Antic Stankovic; Miodrag Mitrić; Bojan Jokić; Ilija B. Plećaš; Slavica Raičević; Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Applied Surface Science 2010, 256, 6083-6089, 10.1016/j.apsusc.2010.03.124.
- Limei Chen; Lin Zheng; Yaohui Lv; Hong Liu; Guancong Wang; Na Ren; Duo Liu; Jiyang Wang; Robert I. Boughton; Chemical assembly of silver nanoparticles on stainless steel for antimicrobial applications. Surface and Coatings Technology 2010, 204, 3871-3875, 10.1016/j.surfcoat.2010.05.003.
- Sondi, I.; Salopek-Sondi, B.; Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative. J. Colloid Interface Sci. 2004, 275, 177-182, 10.1016/j.jcis.2004.02.012.
- Hong Xu; Xue Shi; Hui Ma; Yihang Lv; Linping Zhang; Zhiping Mao; The preparation and antibacterial effects of dopa-cotton/AgNPs. Applied Surface Science 2011, 257, 6799-6803, 10.1016/j.apsusc.2011.02.129.
- J. Thiel; L. Pakstis; S. Buzby; M. Raffi; C. Ni; D. J. Pochan; S. Ismat Shah; Antibacterial Properties of Silver-Doped Titania. Small 2007, 3, 799-803, 10.1002/smll.200600481.
- Q. L. Feng; J. Wu; G. Q. Chen; F. Z. Cui; T. N. Kim; J. O. Kim; A mechanistic study of the antibacterial effect of silver ions onEscherichia coli andStaphylococcus aureus. Journal of Biomedical Materials Research 2000, 52, 662-668, 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3.
- Ai-Jun Miao; Kathleen A. Schwehr; Chen Xu; Sai-Jin Zhang; Zhiping Luo; Antonietta Quigg; Peter H. Santschi; The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environmental Pollution 2009, 157, 3034-3041, 10.1016/j.envpol.2009.05.047.
- Enrique Navarro; Flavio Piccapietra; Bettina Wagner; Fabio Marconi; Ralf Kaegi; Niksa Odzak; Laura Sigg; Renata Behra; Toxicity of Silver Nanoparticles to Chlamydomonas reinhardtii. Environmental Science & Technology 2008, 42, 8959-8964, 10.1021/es801785m.
- Xiaojing He; Guannan Zhang; Xin Wang; Ruiqiang Hang; Xiaobo Huang; Lin Qin; Bin Tang; Xiangyu Zhang; Biocompatibility, corrosion resistance and antibacterial activity of TiO2/CuO coating on titanium. Ceramics International 2017, 43, 16185-16195, 10.1016/j.ceramint.2017.08.196.
- Thomas Verdier; Marie Coutand; Alexandra Bertron; Christine Roques; Antibacterial Activity of TiO2 Photocatalyst Alone or in Coatings on E. coli: The Influence of Methodological Aspects. Coatings 2014, 4, 670-686, 10.3390/coatings4030670.
- Gaëlle Carré; Erwann Hamon; Saïd Ennahar; Maxime Estner; Marie-Claire Lett; Peter Horvatovich; Jean-Pierre Gies; Valérie Keller; Nicolas Keller; Philippe Andre; et al. TiO 2 Photocatalysis Damages Lipids and Proteins in Escherichia coli. Applied and Environmental Microbiology 2014, 80, 2573-2581, 10.1128/aem.03995-13.
- Pin-Ching Maness; Sharon Smolinski; Daniel M. Blake; Zheng Huang; Edward J. Wolfrum; William A. Jacoby; Bactericidal Activity of Photocatalytic TiO 2 Reaction: toward an Understanding of Its Killing Mechanism. Applied and Environmental Microbiology 1999, 65, 4094-4098, 10.1128/aem.65.9.4094-4098.1999.
- Linke Ge; Guangshui Na; Siyu Zhang; Kai Li; Peng Zhang; Honglei Ren; Ziwei Yao; New insights into the aquatic photochemistry of fluoroquinolone antibiotics: Direct photodegradation, hydroxyl-radical oxidation, and antibacterial activity changes. Science of The Total Environment 2015, 527-528, 12-17, 10.1016/j.scitotenv.2015.04.099.
- Gaëtan Gogniat; Sam Dukan; TiO 2 Photocatalysis Causes DNA Damage via Fenton Reaction-Generated Hydroxyl Radicals during the Recovery Period. Applied and Environmental Microbiology 2007, 73, 7740-7743, 10.1128/aem.01079-07.
- Qilin Li; Shaily Mahendra; Delina Lyon; Lena Brunet; Michael V. Liga; Dong Li; Pedro J.J. Alvarez; Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Research 2008, 42, 4591-4602, 10.1016/j.watres.2008.08.015.
- Luting Liu; Batur Ercan; Linlin Sun; Katherine S. Ziemer; Thomas J. Webster; Understanding the Role of Polymer Surface Nanoscale Topography on Inhibiting Bacteria Adhesion and Growth. ACS Biomaterials Science & Engineering 2015, 2, 122-130, 10.1021/acsbiomaterials.5b00431.
- Won Tae Choi; KkochNim Oh; Preet M. Singh; Victor Breedveld; Dennis W. Hess; Wettability control of stainless steel surfaces via evolution of intrinsic grain structures. Journal of Materials Science 2016, 51, 5196-5206, 10.1007/s10853-016-9821-y.
- Marios Michailidis; Eldad Gutner-Hoch; Reut Wengier; Rob Onderwater; Raechelle A. D'sa; Yehuda Benayahu; Anton Semenov; Vladimir Vinokurov; Dmitry G. Shchukin; Highly Effective Functionalized Coatings with Antibacterial and Antifouling Properties. ACS Sustainable Chemistry & Engineering 2020, 8, 8928-8937, 10.1021/acssuschemeng.0c00998.
- Ye Fu; Wencai Wang; Liqun Zhang; Vladimir Vinokurov; Anna Stavitskaya; Yuri Lvov; Development of Marine Antifouling Epoxy Coating Enhanced with Clay Nanotubes. Materials 2019, 12, 4195, 10.3390/ma12244195.
- Yuri M Lvov; Wencai Wang; Liqun Zhang; Rawil F Fakhrullin; Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Advanced Materials 2015, 28, 1227-1250, 10.1002/adma.201502341.
- Joana Figueiredo; Susana Loureiro; Roberto Martins; Hazard of novel anti-fouling nanomaterials and biocides DCOIT and silver to marine organisms. Environmental Science: Nano 2020, 7, 1670-1680, 10.1039/d0en00023j.
- Joana Figueiredo; Tânia Oliveira; Violeta Ferreira; Alesia Sushkova; Sara Silva; Diana Carneiro; Diogo Cardoso; Sandra F. Gonçalves; Frederico Maia; Claudia Rocha; et al.João TedimSusana LoureiroRoberto Martins Toxicity of innovative anti-fouling nano-based solutions to marine species. Environmental Science: Nano 2019, 6, 1418-1429, 10.1039/c9en00011a.
- Eldad Gutner-Hoch; Roberto Martins; Frederico Maia; Tânia Oliveira; Muki Shpigel; Michal Weis; João Tedim; Yehuda Benayahu; Toxicity of engineered micro- and nanomaterials with antifouling properties to the brine shrimp Artemia salina and embryonic stages of the sea urchin Paracentrotus lividus. Environmental Pollution 2019, 251, 530-537, 10.1016/j.envpol.2019.05.031.
- Juliana Vitoria Nicolau Dos Santos; Roberto Martins; Mayana Karoline Fontes; Bruno Galvão De Campos; Mariana Bruni Marques Do Prado E Silva; Frederico Maia; Denis Moledo De Souza Abessa; Fernando Cesar Perina; Can Encapsulation of the Biocide DCOIT Affect the Anti-Fouling Efficacy and Toxicity on Tropical Bivalves?. Applied Sciences 2020, 10, 8579, 10.3390/app10238579.
- Max-Planck-Gesellschaft Zur Forderung Der Wissenschaften Ev. The Community Research and Development Information Service (CORDIS) Project 0660523: No Biofouling Surfaces. Germany, 2015-2017
- J. Tedim; Sergey Poznyak; Alena Kuznetsova; D. Raps; T. Hack; Mikhail Zheludkevich; Mario Ferreira; Enhancement of Active Corrosion Protection via Combination of Inhibitor-Loaded Nanocontainers. ACS Applied Materials & Interfaces 2010, 2, 1528-1535, 10.1021/am100174t.
- Frederico Maia; João Tedim; Aleksey D. Lisenkov; Andrei N. Salak; Mikhail L. Zheludkevich; Mário G. S. Ferreira; Silica nanocontainers for active corrosion protection. Nanoscale 2012, 4, 1287-1298, 10.1039/c2nr11536k.
- Chang Zhang; Fang Cui; Guang-Ming Zeng; Min Jiang; Zhong-Zhu Yang; Aaron Yu; Meng-Ying Zhu; Liu-Qing Shen; Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Science of The Total Environment 2015, 518-519, 352-362, 10.1016/j.scitotenv.2015.03.007.
- Yamada, H.M.; Behaviour Occurrence and Aquatic Toxicity of New Antifouling Biocides and Preliminary Assessment of Risk to Aquatic Ecosystems.. Bull. Fish. Res 2008, 21, 31-45.
- Kv Thomas; The environmental fate and behaviour of antifouling paint booster biocides: A review. Biofouling 2001, 17, 73-86, 10.1080/08927010109378466.
- Pierre-Emmanuel Buffet; Olivia Fossi Tankoua; Jin-Fen Pan; Deborah Berhanu; Christine Herrenknecht; Laurence Poirier; Claude Amiard-Triquet; Jean-Claude Amiard; Jean-Baptiste Bérard; Christine Risso; et al.Marielle GuibboliniMichèle RoméoPaul ReipEugenia Valsami-JonesCatherine Mouneyrac Behavioural and biochemical responses of two marine invertebrates Scrobicularia plana and Hediste diversicolor to copper oxide nanoparticles. Chemosphere 2011, 84, 166-174, 10.1016/j.chemosphere.2011.02.003.
- Singh, A.K. Engineered Nanoparticles: Structure, Properties and Mechanisms of Toxicity; Academic Press: Cambridge, Massachusetts, USA, 2016
- A. Wegner; E. Besseling; E.M. Foekema; P. Kamermans; A.A. Koelmans; Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulisL.). Environmental Toxicology and Chemistry 2012, 31, 2490-2497, 10.1002/etc.1984.
- Carol J. Chandler; Irwin H. Segel; Mechanism of the Antimicrobial Action of Pyrithione: Effects on Membrane Transport, ATP Levels, and Protein Synthesis. Antimicrobial Agents and Chemotherapy 1978, 14, 60-68, 10.1128/aac.14.1.60.
- Mingliang Du; Baochun Guo; Demin Jia; Newly emerging applications of halloysite nanotubes: a review. Polymer International 2010, 59, 574-582, 10.1002/pi.2754.
- Yuri M. Lvov; Dmitry Shchukin; Helmuth Moehwald; Ronald R. Price; Halloysite Clay Nanotubes for Controlled Release of Protective Agents. ACS Nano 2008, 2, 814-820, 10.1021/nn800259q.
- Vladimir Vinokurov; Anna V. Stavitskaya; Aleksandr Glotov; Andrei A. Novikov; Anna V. Zolotukhina; Mikhail Kotelev; Pavel Gushchin; Evgenii Ivanov; Yusuf Darrat; Yuri M. Lvov; et al. Nanoparticles Formed onto/into Halloysite Clay Tubules: Architectural Synthesis and Applications. The Chemical Record 2018, 18, 858-867, 10.1002/tcr.201700089.
- Vladimir A. Vinokurov; Anna V. Stavitskaya; Yaroslav A. Chudakov; Evgenii V. Ivanov; Lok Kumar Shrestha; Katsuhiko Ariga; Yusuf A. Darrat; Yuri M. Lvov; Formation of metal clusters in halloysite clay nanotubes. Science and Technology of Advanced Materials 2017, 18, 147-151, 10.1080/14686996.2016.1278352.




