Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mingyao Yang | + 2545 word(s) | 2545 | 2022-02-22 04:50:15 | | | |
| 2 | Amina Yu | -6 word(s) | 2539 | 2022-02-23 02:14:52 | | | | |
| 3 | Amina Yu | -6 word(s) | 2539 | 2022-02-23 02:15:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yang, M. Flavonoids for Health and Longevity. Encyclopedia. Available online: https://encyclopedia.pub/entry/19737 (accessed on 10 March 2026).
Yang M. Flavonoids for Health and Longevity. Encyclopedia. Available at: https://encyclopedia.pub/entry/19737. Accessed March 10, 2026.
Yang, Mingyao. "Flavonoids for Health and Longevity" Encyclopedia, https://encyclopedia.pub/entry/19737 (accessed March 10, 2026).
Yang, M. (2022, February 22). Flavonoids for Health and Longevity. In Encyclopedia. https://encyclopedia.pub/entry/19737
Yang, Mingyao. "Flavonoids for Health and Longevity." Encyclopedia. Web. 22 February, 2022.
Copy Citation
Flavonoids are a diverse family of natural phenolic compounds commonly found in fruits, vegetables, tea, wine, and Chinese herbal medicine. Flavonoids have a basic C6–C3–C6 15 carbon skeleton composed of two aromatic rings and one pyran ring. Flavonoid compounds are divided into six subclasses based on their carbon structure and level of oxidation, which are flavones, flavonols, flavanones, isoflavones, flavanol, and anthocyanins.
flavonoids
health span
aging
1. Cellular Senescence Is Driven by Unrepaired Damage
1.1. DNA Damage and Repair
DNA damage has been thought to be a strong candidate as the primary cause of aging [1]. DNA damage includes oxidative modifications, single- and double-strand breaks (DSBs), and mutations, both in vitro and in vivo [2][3]. Many studies have indicated that DNA damage accumulation is associated with aging [4][5]. A complete DNA repair system is also established to repair DNA damage in cells. Prominent DNA repair pathways in mammalian cells are base excision repair (BER), mismatch repair (MMR), nucleotide excision repair (NER), and double-strand break repair (DSBR). It has been observed that the ability to repair DNA damage decreases with aging [6]. Thus, unrepaired DNA damage further accumulates during aging. Unrepaired DNA damage can cause genome instability and induce a signal cascade that leads to cell senescence or death and related cell aging phenotypes [7][8]. More than 50 DNA repair disorders have been described as having varying degrees of overlapping phenotypes with aging, such as neurodegeneration, cancer, and cardiovascular disease [9].
1.2. Protein Damage
Various internal and external factors constantly damage intracellular proteins. Damage to proteins, in turn, may affect myriad intracellular pathways given their abundance. Protein quality control (PQC) is critical to maintaining a functioning proteome. The quality of the protein is guaranteed by the translation mechanism and the activity of auxiliary proteins (including molecular chaperones), while degradation is controlled by autophagy and proteasome functions. The accumulation of protein damage in the aging process is mainly due to (i) decreased translation fidelity [10][11], (ii) downregulation of protein chaperones [12][13], and (iii) decreased proteasome activity [14] and other factors in protein synthesis and quality control. Damaged proteins contribute to proteostatic stress, the accumulation of misfolded/aggregated proteins, and protein toxicity, which further aggravate the senescence of cells.
1.3. Lipid Damage
Lipid damage is mainly due to lipofuscin, a nondegradable protein and lipid oxidation product, which accumulates in senescent cells [15]. Lipofuscin is an autofluorescent lipopigment formed by lipids, metals, and misfolded proteins, which is especially abundant in nerve cells, cardiac muscle cells, and skin [16]. Lipofuscin is emerging as another indicator of senescent cells in culture and in vivo [17][18]. Recent research results indicate that lipofuscin can actively change cell metabolism, cell death, and apoptosis at different levels by inhibiting proteasomes, weakening autophagy and lysosomal degradation, and acting as a metal ion pool to cause ROS generation [19]. In addition, the dispersive nature of the deposits distributed throughout the tissue may support the mechanism of lipofuscin diffusion and seeding of new lipofuscin aggregates [20]. It should be noted that damage accumulation continues even when cell division ceases and can continue for months or even years.
2. Flavonoid Compounds Serve as Anti-Aging Agents
Over the last two decades, flavonoids have drawn attention as promising natural dietary molecules to prevent aging and aging-related diseases. According to their different ways of interfering with aging, anti-aging flavonoids are divided into senolytic flavonoids, senomorphic flavonoids, and antisenescence activit.
2.1. Senolytic Flavonoids
Senescent cells and the senescence-related secretion phenotypes (SASPs) secreted by them are essential factors leading to the aging of tissues and organs [21]. Therefore, therapeutic approaches to specifically kill senescent cells can extend health span and lifespan. “Senolytic” compounds can kill senescent cells [22]. Quercetin is effective against senescent human endothelial cells in combination with dasatinib, which is more effective in eliminating senescent MEFs [23], reducing the expression of SASP factors [24]. Moreover, quercetin plus dasatinib has been proven to enhance health span and lifespan in old mice [21] and improve age-related diseases such as cardiovascular disease and temporomandibular joint degeneration [25]. Furthermore, in an open-label clinical trial, within three weeks, oral quercetin and dasatinib improved the 6-min walking distance, walking speed, and ability to stand up from a chair and shortened the body function battery five days after the last dose [26][27].
In a panel of 10 polyphenols examined, fisetin was potently senolytic in cultured senescent murine and human fibroblasts, while luteolin had a weak effect on clearing senescent cells. Fisetin increased the median and maximum lifespans of aged mice [28]. Notably, fisetin treatment significantly reduced mortality, cellular senescence, and inflammatory markers and increased antiviral antibodies when the SARS-CoV-2-related mouse β-coronavirus was exposed to old mouse pathogens [29]. As fisetin has a good effect against inflammatory factors, it has been used in clinical research to alleviate the dysfunction of COVID-19 and the excessive inflammatory response in the elderly (NCT04537299). Burton et al. showed that luteolin significantly reduced the proportion of microglia stained for IL-1β and IL-6 in LPS-treated adult mice [30].
2.2. Senomorphic Flavonoids
Senomorphics refer to compounds and dietary supplements that can restrain senescence-associated phenotypes by explicitly suppressing the SASP or proinflammatory secretome. Recent research results also show that the flavonoids apigenin, kaempferol, and 4,4′dimethoxychalcone also have such “senomorphic” effects. Apigenin belongs to the flavone subclass of flavonoids and can delay the aging process by activating the Nrf2 pathway [31]. Apigenin partially inhibits SASP by inhibiting IL-1α signaling in human fibroblast cell lines through IRAK1 and IRAK4, p38-MAPK, and NF-κB [32]. Kaempferol is a flavonol, and it significantly inhibited IL-6, IL-8, and IL-1b expression but did not considerably affect senescence itself in bleomycin-induced senescent BJ cells. A cellular mechanism study showed that kaempferol in senescent BJ cells might be mediated, at least in part, by interfering with IRAK1/IkBa/NF-kB p65 signaling [33][34].
2.3. Another Antisenescence Activity of Flavonoids
In addition, an increasing number of flavonoids have been proven to delay the aging process. These compounds include various subsets of flavonoids. The flavonoid 4,4′-dimethoxychalcone (DMC) is derived from Angelica keiskei koidzumi, a plant with longevity- and health-promoting effects in traditional Chinese medicine. DMC extends the lifespan of yeast, worms, and flies and decelerates the senescence of human cell cultures via GATA transcription factors to induce autophagy [35].
Naringenin and nobiletin are widely found in the fruits of Citrus L. plants in the Rutaceae family. Both of them have antioxidant effects and can reduce ROS in senescent cells. In addition, naringenin has a significant impact on reducing cardiovascular markers of damage caused by aging [36]. The lifespan analysis experiment in Drosophila showed that treatment with 400 µm/L of naringenin could prolong lifespan by up to 22.62% [37]. However, nobiletin’s role is mainly in regulating abnormal energy metabolism. Nobiletin targets retinoid acid receptor-related orphan receptors (RORs) to remodel circadian and metabolic gene expression, enhancing the circadian rhythm and preventing metabolic syndrome [38]. Furthermore, nobiletin-RORs have been reported to optimize skeletal muscle mitochondrial respiration and promote healthy aging in high-fat diet mice [39].
Genistein is an isoflavone derived from soy products. Genistein induces autophagy to reduce cell senescence in vascular smooth muscle cells [40]. Genistein reduced age-related increases in NF-κB activity and NF-κB-dependent proinflammatory gene expression in vivo in rats; thus, it can be used as an anti-inflammatory compound [41]. Antisenescence effects have also been reported for epicatechin. Epicatechin induces the reversal of endothelial cell senescence and improves vascular function in rats [42]. Supplementation with epicatechin has been observed to improve the survival rate of elderly mice and age-related phenotypes such as skeletal muscle degeneration [43] and brain dysfunction [44].
Myricetin and dihydromyricetin are produced in several plants, particularly in some commonly consumed fruits and vegetables (strawberries, grapes). They have been approved as food supplements in Europe and the United States. Survival experiments show that both compounds prolong lifespan [45][46]. Interestingly, myricetin and dihydromyricetin have been reported to have anti-AD effects [47].
Rutin, a natural flavonoid glycoside compound, has revealed an extensive anti-aging effect. Rutin can induce autophagy to extend the lifespan of Drosophila treated with HDF [48] and can also effectively improve the metabolic dysfunction associated with aging by regulating the IIS signaling pathway [49]. Moreover, the administration of rutin reduces the expression of ROS and proinflammatory cytokines (TNF-α and IL-1β) in neuronal cells, which can prevent the development of AD and protect the aging brain or slow the neurodegenerative process [50].
Hesperidin is a flavanone glycoside derived from citrus that has been found to possess various pharmacological properties including antioxidant, cholesterol-lowering, and anti-inflammatory ones. Topical application of hesperidin can improve functional abnormalities of the aging epidermis including abnormal epidermal permeability barrier function, epidermal differentiation, lipid production, and stratum corneum acidification [51]. Hesperidin upregulated Nrf2 and reduced ROS, significantly prolonging the replicative lifespan of yeast [52]. Hesperidin treatment also effectively protected the hearts of aged rats by upregulating the protein level of Nrf2 and increasing the activity of enzymatic antioxidants [53]. In addition, some other citrus flavonoids such as naringin, hesperitin, and neohesperidin have also maintained ROS scavenging and potential anti-aging activities in yeast [54].
Theaflavins are derived from the conversion of catechins by endogenous polyphenol oxidase and peroxidase during the production of black tea [55]. Studies have shown that theaflavin can delay the excessive proliferation of intestinal stem cells, prevent intestinal dysbiosis, and inhibit the activation of the Imd signaling pathway, thereby prolonging the lifespan of Drosophila. At the same time, theaflavin is effective in preventing DSS-induced colitis in mice [56]. Moreover, theaflavin can protect against oxidative stress-induced cellular senescence by activating Nrf2 in a mouse osteoarthritis model [57]. Furthermore, treatment of middle-aged mice with theaflavin 3-gallate reduced senescence in hypothalamic neural stem cells while improving senescence-related pathology [58].
In short, flavonoids with anti-aging effects are diverse in both their types and their modes of action. Molecules of the same subclass also have anti-aging targets, showing that more detailed research is needed to reveal their respective regulatory mechanisms.
3. Benefits of Flavonoids in Attenuating Aging Damage
Due to the important impact of damage on cellular and systemic aging, the removal or repair of damage will help re-establish the equilibrium state of damage repair and, thus, slow down the aging rate. Many findings suggest that flavonoids play an essential role in reducing damage and rebuilding tissue homeostasis, as shown in Figure 1.
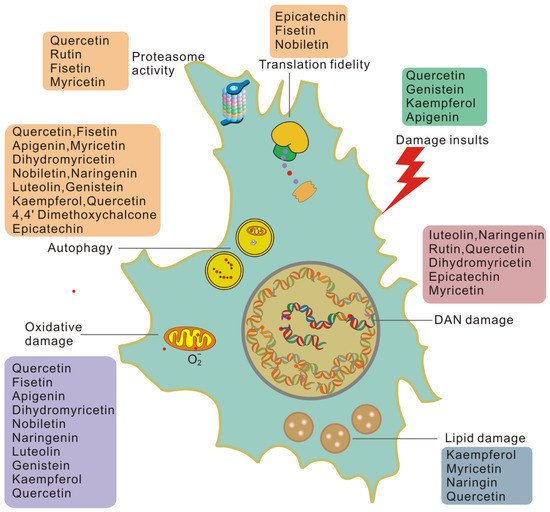
Figure 1. Flavonoids work on each type of damage-dependent trigger of cellular senescence. Cells induced to senesce by damaging insults exhibit higher basal levels of damage than healthy cells and generate damage at a higher rate.
Flavonoids can reduce cellular damage caused by a variety of damage insults. Quercetin protects red blood cells from oxidative stress and genotoxicity in vitro [59]. Quercetin can also protect cells from the stress of misfolded proteins in the endoplasmic reticulum [60]. Genistein may significantly reverse the misfolding of the N-CoR protein induced by PML-RAR by inhibiting the selective phosphorylation-dependent binding of N-CoR and PML-RAR [61]. Kaempferol [62] and apigenin [63] may alter the protein associated with the internal ribosome entry site (IRES) to limit viral infection and inhibit viral IRES-driven translation activities. In this way, flavonoids can reduce cell damage from the source.
Many flavonoids can act on DNA damage in a variety of ways. The flavonoids luteolin, naringenin, and rutin effectively attenuate UVB-induced DNA damage in vitro [64] and in vivo [65]. Quercetin has been reported to effectively reverse 1,2-dimethylhydrazine-mediated oxidative stress and DNA damage by targeting the NRF2/Keap1 signaling pathway in rats [66]. Recently, nanocapsules containing dihydromyricetin were reported to have a 50% sun protection factor (SPF-DNA) against DNA damage caused by UVB radiation and 99.9% protection against DNA damage induction [67]. It was also found that epicatechin protects against DNA damage induced by N-nitrosodibutylamine (NDBA) and N-nitrosopiperidine (NPIP) in human hepatocarcinoma cells [68]. The epicatechin myricetin activates nonhomologous end-joining DNA double-strand break repair in human small intestinal cells [69]. Therefore, flavonoids can reduce DNA damage and enhance the DNA repair ability of cells, thereby reducing the accumulation of unrepaired damage.
Oxidative damage is believed to play a key role in pathological processes related to aging and age-related diseases, and its underlying biochemical mechanisms have been elucidated in detail [70][71]. Antioxidant capacity is an important activity of flavonoids. In APRE-19 cells, the solid dispersion of apigenin upregulates the expression of antioxidant enzymes and upregulates autophagy through the Nrf2 pathway, thereby inhibiting retinal oxidative damage [72]. In a rat natural aging model, fisetin significantly reduces pro-oxidants and increases the level of antioxidants to combat oxidative stress induced by aging [73]. Dihydromyricetin can reduce the oxidative damage of human umbilical vein endothelial cells induced by sodium nitroprusside by activating the PI3K/Akt/FoxO3a signaling pathway [74]. Nobiletin attenuates palmitate-induced ROS and mitochondrial dysfunction in cultured alpha mouse liver 12 cells [75]. In addition, naringenin [76], luteolin [77], genistein [78], kaempferol [79], and quercetin [80] have all been observed to inhibit oxidative damage in a variety of ways. Therefore, flavonoids may eliminate oxidative damage in senescent cells and help cells to overcome aging and aging-related diseases.
Flavonoids are also involved in the process of reducing and removing protein damage. Epicatechin upregulates eukaryotic translation elongation Factor 1A (eEF1A) through the 67 kDa laminin receptor [81]. Fisetin treatment of preadipocytes reduced the phosphorylation of the 70 kDa ribosomal protein S6 kinase 1 (S6K1). Nobiletin significantly blocked the activation of Akt/mTOR signaling and significantly inhibited the phosphorylation of S6K1 and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1) [82]. Phosphorylated S6K targets eIF4B and ribosomal protein S6 (RPS6). At the same time, 4EBP binds to eukaryotic initiation factor 4E (eIF4E) at the eIF4E–eIF4G interaction interface to prevent it from forming the translation initiation complex [83], thereby affecting translation fidelity.
Quercetin can specifically silence the expression level of HSP70. Previous studies have shown that HSP90 inhibitors have senolytic activity [84]. Luteolin can alleviate psoriasis’s pathological changes and symptoms by reversing the effects of IFN-γ and HSP90 expression and exosomal secretion, regulating the proportion of immune cells and inhibiting psoriasis. Myricetin interferes with the binding of HSP90β and TGF-β receptor II, thereby preventing fibroblast activation. This indicates that flavonoids can also regulate the activity of chaperone molecules. Proteasome activity and autophagy are important parts of protein quality control and a meaningful way to eliminate damaged proteins. Myricetin is reported to eliminate neurodegenerative protein aggregation by upregulating the proteasome degradation mechanism [85]. Quercetin and rutin are positive regulators of the Nrf2 transcription factor, which enhances the expression of proteasome catalytic subunits in neurons [86]. Fisetin promotes the survival of nerve cells by enhancing the activity of the proteasome when trophic factors are withdrawn [87].
The removal of lipofuscin in cells results in reduced lipid damage, which is often accompanied by improved aging-related pathology. Anti-aging studies on flavonoids have shown that they also can minimize lipofuscin in cells. Several studies have shown that kaempferol, myricetin, naringin, and quercetin can significantly reduce lipofuscin accumulation in C. elegans, a marker of aging [45][88][89]. However, rutin and fisetin, which also prolong the lifespan of nematodes, cannot delay the accumulation of lipofuscin in cells [88][89]. Quercetin can also inhibit the development of lipofuscin-related autofluorescence in senescent cells [90]. In addition, the accumulation of lipofuscin is closely related to mitochondrial function and lipid metabolism [20]. Flavonoids regulate mitochondrial function; for example, luteolin increases mitochondrial respiration in primary neurons [91]. Flavonoids can reduce lipofuscin in cells and affect the related processes of lipofuscin production.
Collectively, flavonoids effectively reduce the damage of DNA, protein, and lipid macromolecules by reducing the insults of damage. At the same time, they can improve the ability of damage repair or clearance, thereby significantly reducing the rate of unrepaired damage accumulating in cells. Due to the important role of unrepaired damage in inducing cell senescence, cells or tissues can benefit from the anti-damage effects of flavonoids.
References
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703.
- d’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522.
- White, R.; Vijg, J. Do DNA Double-Strand Breaks Drive Aging? Mol. Cell 2016, 63, 729–738.
- Sperka, T.; Wang, J.; Rudolph, K. DNA damage checkpoints in stem cells, ageing and cancer. Nat. Rev. Mol. Cell Biol. 2012, 13, 579–590.
- Rieckher, M.; Garinis, G.; Schumacher, B. Molecular pathology of rare progeroid diseases. Trends Mol. Med. 2021.
- Li, W.; Vijg, J. Measuring genome instability in aging—A mini-review. Gerontology 2012, 58, 129–138.
- Pugh, J.; Foster, S.; Sukhina, A.; Petravic, J.; Uhrlaub, J.; Padilla-Torres, J.; Hayashi, T.; Nakachi, K.; Smithey, M.; Nikolich-Žugich, J. Acute systemic DNA damage in youth does not impair immune defense with aging. Aging Cell 2016, 15, 686–693.
- Tse, K.; Herrup, K. DNA damage in the oligodendrocyte lineage and its role in brain aging. Mech. Ageing Dev. 2017, 161, 37–50.
- Petr, M.A.; Tulika, T.; Carmona-Marin, L.M.; Scheibye-Knudsen, M. Protecting the Aging Genome. Trends Cell Biol. 2020, 30, 117–132.
- Azpurua, J.; Ke, Z.; Chen, I.; Zhang, Q.; Ermolenko, D.; Zhang, Z.; Gorbunova, V.; Seluanov, A. Naked mole-rat has increased translational fidelity compared with the mouse, as well as a unique 28S ribosomal RNA cleavage. Proc. Natl. Acad. Sci. USA 2013, 110, 17350–17355.
- Martinez-Miguel, V.; Lujan, C.; Espie-Caullet, T.; Martinez-Martinez, D.; Moore, S.; Backes, C.; Gonzalez, S.; Galimov, E.; Brown, A.; Halic, M.; et al. Increased fidelity of protein synthesis extends lifespan. Cell Metab. 2021.
- Xilouri, M.; Stefanis, L. Chaperone mediated autophagy in aging: Starve to prosper. Ageing Res. Rev. 2016, 32, 13–21.
- Endicott, S.; Boynton, D.; Beckmann, L.; Miller, R. Long-lived mice with reduced growth hormone signaling have a constitutive upregulation of hepatic chaperone-mediated autophagy. Autophagy 2021, 17, 612–625.
- Koyuncu, S.; Loureiro, R.; Lee, H.; Wagle, P.; Krueger, M.; Vilchez, D. Rewiring of the ubiquitinated proteome determines ageing in C. elegans. Nature 2021, 596, 285–290.
- Sitte, N.; Merker, K.; Grune, T.; von Zglinicki, T. Lipofuscin accumulation in proliferating fibroblasts in vitro: An indicator of oxidative stress. Exp. Gerontol. 2001, 36, 475–486.
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.; Bindewald-Wittich, A.; Spaide, R.; Wolf, S.; Sadda, S.; Holz, F. Fundus autofluorescence imaging. Prog. Retin. Eye Res. 2021, 81, 100893.
- Galanos, P.; Vougas, K.; Walter, D.; Polyzos, A.; Maya-Mendoza, A.; Haagensen, E.; Kokkalis, A.; Roumelioti, F.; Gagos, S.; Tzetis, M.; et al. Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing. Nat. Cell Biol. 2016, 18, 777–789.
- Myrianthopoulos, V.; Evangelou, K.; Vasileiou, P.; Cooks, T.; Vassilakopoulos, T.; Pangalis, G.; Kouloukoussa, M.; Kittas, C.; Georgakilas, A.; Gorgoulis, V. Senescence and senotherapeutics: A new field in cancer therapy. Pharmacol. Ther. 2019, 193, 31–49.
- Reeg, S.; Grune, T. Protein Oxidation in Aging: Does It Play a Role in Aging Progression? Antioxid. Redox Signal. 2015, 23, 239–255.
- Moreno-García, A.; Kun, A.; Calero, O.; Medina, M.; Calero, M. An Overview of the Role of Lipofuscin in Age-Related Neurodegeneration. Front. Neurosci. 2018, 12, 464.
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256.
- Gasek, N.; Kuchel, G.; Kirkland, J.; Xu, M. Strategies for Targeting Senescent Cells in Human Disease. Nat. Aging 2021, 1, 870–879.
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658.
- Ogrodnik, M.; Evans, S.; Fielder, E.; Victorelli, S.; Kruger, P.; Salmonowicz, H.; Weigand, B.; Patel, A.; Pirtskhalava, T.; Inman, C.; et al. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell 2021, 20, e13296.
- Dookun, E.; Passos, J.F.; Arthur, H.M.; Richardson, G.D. Therapeutic Potential of Senolytics in Cardiovascular Disease. Cardiovasc. Drugs Ther. 2020.
- Rufino, A.; Costa, V.; Carvalho, F.; Fernandes, E. Flavonoids as antiobesity agents: A review. Med. Res. Rev. 2021, 41, 556–585.
- Wissler Gerdes, E.; Misra, A.; Netto, J.; Tchkonia, T.; Kirkland, J. Strategies for late phase preclinical and early clinical trials of senolytics. Mech. Ageing Dev. 2021, 200, 111591.
- Yousefzadeh, M.; Zhu, Y.; McGowan, S.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.; Melos, K.; Pirtskhalava, T.; Inman, C.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28.
- Camell, C.; Yousefzadeh, M.; Zhu, Y.; Langhi Prata, L.; Huggins, M.; Pierson, M.; Zhang, L.; O’Kelly, R.; Pirtskhalava, T.; Xun, P.; et al. Senolytics reduce coronavirus-related mortality in old mice. Science 2021, 373, eabe4832.
- Burton, M.; Rytych, J.; Amin, R.; Johnson, R. Dietary Luteolin Reduces Proinflammatory Microglia in the Brain of Senescent Mice. Rejuvenation Res. 2016, 19, 286–292.
- Sang, Y.; Zhang, F.; Wang, H.; Yao, J.; Chen, R.; Zhou, Z.; Yang, K.; Xie, Y.; Wan, T.; Ding, H. Apigenin exhibits protective effects in a mouse model of d-galactose-induced aging via activating the Nrf2 pathway. Food Funct. 2017, 8, 2331–2340.
- Perrott, K.; Wiley, C.; Desprez, P.; Campisi, J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. GeroScience 2017, 39, 161–173.
- Kim, J.; Lee, E.; Kim, D.; Yu, B.; Chung, H. Kaempferol modulates pro-inflammatory NF-kappaB activation by suppressing advanced glycation endproducts-induced NADPH oxidase. Age 2010, 32, 197–208.
- Lim, H.; Park, H.; Kim, H. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem. Pharmacol. 2015, 96, 337–348.
- Carmona-Gutierrez, D.; Zimmermann, A.; Kainz, K.; Pietrocola, F.; Chen, G.; Maglioni, S.; Schiavi, A.; Nah, J.; Mertel, S.; Beuschel, C.; et al. The flavonoid 4,4′-dimethoxychalcone promotes autophagy-dependent longevity across species. Nat. Commun. 2019, 10, 651.
- Hua, Y.; Zeng, Y.; Xu, J.; Xu, X. Naringenin alleviates nonalcoholic steatohepatitis in middle-aged Apoemice: Role of SIRT1. Phytomed. Int. J. Phytother. Phytopharm. 2021, 81, 153412.
- Chattopadhyay, D.; Sen, S.; Chatterjee, R.; Roy, D.; James, J.; Thirumurugan, K. Context- and dose-dependent modulatory effects of naringenin on survival and development of Drosophila melanogaster. Biogerontology 2016, 17, 383–393.
- He, B.; Nohara, K.; Park, N.; Park, Y.; Guillory, B.; Zhao, Z.; Garcia, J.; Koike, N.; Lee, C.; Takahashi, J.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621.
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923.
- Lee, K.; Kim, J.; Choi, H. Genistein-induced LKB1-AMPK activation inhibits senescence of VSMC through autophagy induction. Vasc. Pharmacol. 2016, 81, 75–82.
- Kim, J.; Uehara, Y.; Choi, Y.; Ha, Y.; Ye, B.; Yu, B.; Chung, H. Mechanism of attenuation of pro-inflammatory Ang II-induced NF-κB activation by genistein in the kidneys of male rats during aging. Biogerontology 2011, 12, 537–550.
- Ramirez-Sanchez, I.; Mansour, C.; Navarrete-Yañez, V.; Ayala-Hernandez, M.; Guevara, G.; Castillo, C.; Loredo, M.; Bustamante, M.; Ceballos, G.; Villarreal, F. (-)-Epicatechin induced reversal of endothelial cell aging and improved vascular function: Underlying mechanisms. Food Funct. 2018, 9, 4802–4813.
- Si, H.; Wang, X.; Zhang, L.; Parnell, L.; Admed, B.; LeRoith, T.; Ansah, T.; Zhang, L.; Li, J.; Ordovás, J.; et al. Dietary epicatechin improves survival and delays skeletal muscle degeneration in aged mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 965–977.
- Navarrete-Yañez, V.; Garate-Carrillo, A.; Rodriguez, A.; Mendoza-Lorenzo, P.; Ceballos, G.; Calzada-Mendoza, C.; Hogan, M.; Villarreal, F.; Ramirez-Sanchez, I. Effects of (-)-epicatechin on neuroinflammation and hyperphosphorylation of tau in the hippocampus of aged mice. Food Funct. 2020, 11, 10351–10361.
- Büchter, C.; Ackermann, D.; Havermann, S.; Honnen, S.; Chovolou, Y.; Fritz, G.; Kampkötter, A.; Wätjen, W. Myricetin-mediated lifespan extension in Caenorhabditis elegans is modulated by DAF-16. Int. J. Mol. Sci. 2013, 14, 11895–11914.
- Fan, X.; Zeng, Y.; Fan, Z.; Cui, L.; Song, W.; Wu, Q.; Gao, Y.; Yang, D.; Mao, X.; Zeng, B.; et al. DrosophilaDihydromyricetin promotes longevity and activates the transcription factors FOXO and AOP in. Aging 2020, 13, 460–476.
- Liu, M.; Guo, H.; Li, Z.; Zhang, C.; Zhang, X.; Cui, Q.; Tian, J. Molecular Level Insight Into the Benefit of Myricetin and Dihydromyricetin Uptake in Patients With Alzheimer’s Diseases. Front. Aging Neurosci. 2020, 12, 601603.
- Chattopadhyay, D.; Thirumurugan, K. Longevity-promoting efficacies of rutin in high fat diet fed Drosophila melanogaster. Biogerontology 2020, 21, 653–668.
- Li, T.; Chen, S.; Feng, T.; Dong, J.; Li, Y.; Li, H. Rutin protects against aging-related metabolic dysfunction. Food Funct. 2016, 7, 1147–1154.
- Yu, X.; Li, Y.; Zhang, H.; Su, Y.; Zhou, W.; Zhang, Z.; Wang, S.; Xu, P.; Wang, Y.; Liu, R. Rutin inhibits amylin-induced neurocytotoxicity and oxidative stress. Food Funct. 2015, 6, 3296–3306.
- Man, G.; Mauro, T.; Zhai, Y.; Kim, P.; Cheung, C.; Hupe, M.; Crumrine, D.; Elias, P.; Man, M. Topical hesperidin enhances epidermal function in an aged murine model. J. Investig. Dermatol. 2015, 135, 1184–1187.
- Sun, K.; Xiang, L.; Ishihara, S.; Matsuura, A.; Sakagami, Y.; Qi, J. Anti-aging effects of hesperidin on Saccharomyces cerevisiae via inhibition of reactive oxygen species and UTH1 gene expression. Biosci. Biotechnol. Biochem. 2012, 76, 640–645.
- Elavarasan, J.; Velusamy, P.; Ganesan, T.; Ramakrishnan, S.; Rajasekaran, D.; Periandavan, K. Hesperidin-mediated expression of Nrf2 and upregulation of antioxidant status in senescent rat heart. J. Pharm. Pharmacol. 2012, 64, 1472–1482.
- Guo, C.; Zhang, H.; Guan, X.; Zhou, Z. Saccharomyces CerevisiaeThe Anti-Aging Potential of Neohesperidin and Its Synergistic Effects with Other Citrus Flavonoids in Extending Chronological Lifespan of BY4742. Molecules 2019, 24, 4093.
- Li, S.; Lo, C.; Pan, M.; Lai, C.; Ho, C. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18.
- Cai, Q.; Ji, S.; Li, M.; Zheng, S.; Zhou, X.; Guo, H.; Deng, S.; Zhu, J.; Li, D.; Xie, Z. DrosophilaTheaflavin-regulated Imd condensates control intestinal homeostasis and aging. iScience 2021, 24, 102150.
- Xu, X.; Zheng, G.; Tang, S.; Liu, H.; Hu, Y.; Shang, P. viaTheaflavin protects chondrocytes against apoptosis and senescence regulating Nrf2 and ameliorates murine osteoarthritis. Food Funct. 2021, 12, 1590–1602.
- Xiao, Y.; Yang, M.; Xiao, Y.; Guo, Q.; Huang, Y.; Li, C.; Cai, D.; Luo, X. Reducing Hypothalamic Stem Cell Senescence Protects against Aging-Associated Physiological Decline. Cell Metab. 2020, 31, 534–548.e535.
- Abdallah, F.; Fetoui, H.; Fakhfakh, F.; Keskes, L. Caffeic acid and quercetin protect erythrocytes against the oxidative stress and the genotoxic effects of lambda-cyhalothrin in vitro. Hum. Exp. Toxicol. 2012, 31, 92–100.
- Storniolo, A.; Raciti, M.; Cucina, A.; Bizzarri, M.; Di Renzo, L. Quercetin affects Hsp70/IRE1α mediated protection from death induced by endoplasmic reticulum stress. Oxidative Med. Cell. Longev. 2015, 2015, 645157.
- Ng, A.; Nin, D.; Fong, J.; Venkataraman, D.; Chen, C.; Khan, M. Therapeutic targeting of nuclear receptor corepressor misfolding in acute promyelocytic leukemia cells with genistein. Mol. Cancer Ther. 2007, 6, 2240–2248.
- Tsai, F.; Lin, C.; Lai, C.; Lan, Y.; Lai, C.; Hung, C.; Hsueh, K.; Lin, T.; Chang, H.; Wan, L.; et al. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011, 128, 312–322.
- Qian, S.; Fan, W.; Qian, P.; Zhang, D.; Wei, Y.; Chen, H.; Li, X. Apigenin restricts FMDV infection and inhibits viral IRES driven translational activity. Viruses 2015, 7, 1613–1626.
- Kootstra, A. Protection from UV-B-induced DNA damage by flavonoids. Plant Mol. Biol. 1994, 26, 771–774.
- Wölfle, U.; Esser, P.; Simon-Haarhaus, B.; Martin, S.; Lademann, J.; Schempp, C. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med. 2011, 50, 1081–1093.
- Darband, S.; Sadighparvar, S.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Rahimi, Y.; Mobaraki, K.; Majidinia, M. Quercetin attenuated oxidative DNA damage through NRF2 signaling pathway in rats with DMH induced colon carcinogenesis. Life Sci. 2020, 253, 117584.
- Dalcin, A.; Roggia, I.; Felin, S.; Vizzotto, B.; Mitjans, M.; Vinardell, M.; Schuch, A.; Ourique, A.; Gomes, P. UVB photoprotective capacity of hydrogels containing dihydromyricetin nanocapsules to UV-induced DNA damage. Colloids Surf. B Biointerfaces 2021, 197, 111431.
- Delgado, M.; Haza, A.; García, A.; Morales, P. Myricetin, quercetin, (+)-catechin and (-)-epicatechin protect against N-nitrosamines-induced DNA damage in human hepatoma cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2009, 23, 1292–1297.
- Charles, C.; Nachtergael, A.; Ouedraogo, M.; Belayew, A.; Duez, P. Effects of chemopreventive natural products on non-homologous end-joining DNA double-strand break repair. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2014, 768, 33–41.
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982.
- Zhang, Y.; Yang, Y.; Yu, H.; Li, M.; Hang, L.; Xu, X. Apigenin Protects Mouse Retina against Oxidative Damage by Regulating the Nrf2 Pathway and Autophagy. Oxidative Med. Cell. Longev. 2020, 2020, 9420704.
- Singh, S.; Singh, A.; Garg, G.; Rizvi, S. Fisetin as a caloric restriction mimetic protects rat brain against aging induced oxidative stress, apoptosis and neurodegeneration. Life Sci. 2018, 193, 171–179.
- Zhang, X.; Wang, L.; Peng, L.; Tian, X.; Qiu, X.; Cao, H.; Yang, Q.; Liao, R.; Yan, F. Dihydromyricetin protects HUVECs of oxidative damage induced by sodium nitroprusside through activating PI3K/Akt/FoxO3a signalling pathway. J. Cell. Mol. Med. 2019, 23, 4829–4838.
- Li, S.; Li, X.; Chen, F.; Liu, M.; Ning, L.; Yan, Y.; Shang, Z.; Huang, S.; Tu, C. Nobiletin mitigates hepatocytes death, liver inflammation, and fibrosis in a murine model of NASH through modulating hepatic oxidative stress and mitochondrial dysfunction. J. Nutr. Biochem. 2021, 100, 108888.
- Al-Dosari, D.; Ahmed, M.; Al-Rejaie, S.; Alhomida, A.; Ola, M. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161.
- Chen, H.; Hu, W.; Hung, M.; Ou, H.; Huang, S.; Hsu, P.; Day, C.; Lin, K.; Viswanadha, V.; Kuo, W.; et al. Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutr. Metab. Cardiovasc. Dis. NMCD 2020, 30, 1032–1043.
- Luo, M.; Zheng, L.; Wang, Y.; Huang, J.; Yang, Z.; Yue, Z.; Guo, B. Genistein exhibits therapeutic potential for PCOS mice the ER-Nrf2-Foxo1-ROS pathway. Food Funct. 2021, 12, 8800–8811.
- Yao, X.; Jiang, H.; Yong, N.X.; Piao, X.; Kim, N.H. Kaempferol attenuates mitochondrial dysfunction and oxidative stress induced by H2O2 during porcine embryonic development. Theriogenology 2019, 135, 174–180.
- Zhang, Z.; Yi, P.; Yi, M.; Tong, X.; Cheng, X.; Yang, J.; Hu, Y.; Peng, W. Protective Effect of Quercetin against HO-Induced Oxidative Damage in PC-12 Cells: Comprehensive Analysis of a lncRNA-Associated ceRNA Network. Oxidative Med. Cell. Longev. 2020, 2020, 6038919.
- Umeda, D.; Yano, S.; Yamada, K.; Tachibana, H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J. Biol. Chem. 2008, 283, 3050–3058.
- Qu, Y.; Liu, Y.; Chen, L.; Zhu, Y.; Xiao, X.; Wang, D.; Zhu, Y. Nobiletin prevents cadmium-induced neuronal apoptosis by inhibiting reactive oxygen species and modulating JNK/ERK1/2 and Akt/mTOR networks in rats. Neurol. Res. 2018, 40, 211–220.
- Fan, Q.W.; Nicolaides, T.P.; Weiss, W.A. Inhibiting 4EBP1 in Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 14–21.
- Fuhrmann-Stroissnigg, H.; Ling, Y.; Zhao, J.; McGowan, S.; Zhu, Y.; Brooks, R.; Grassi, D.; Gregg, S.; Stripay, J.; Dorronsoro, A.; et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 2017, 8, 422.
- Joshi, V.; Mishra, R.; Upadhyay, A.; Amanullah, A.; Poluri, K.; Singh, S.; Kumar, A.; Mishra, A. Polyphenolic flavonoid (Myricetin) upregulated proteasomal degradation mechanisms: Eliminates neurodegenerative proteins aggregation. J. Cell. Physiol. 2019, 234, 20900–20914.
- Martín-Aragón, S.; Jiménez-Aliaga, K.L.; Benedí, J.; Bermejo-Bescós, P. Neurohormetic responses of quercetin and rutin in a cell line over-expressing the amyloid precursor protein (APPswe cells). Phytomed. Int. J. Phytother. Phytopharm. 2016, 23, 1285–1294.
- Maher, P. The flavonoid fisetin promotes nerve cell survival from trophic factor withdrawal by enhancement of proteasome activity. Arch. Biochem. Biophys. 2008, 476, 139–144.
- Kampkötter, A.; Gombitang Nkwonkam, C.; Zurawski, R.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch. Toxicol. 2007, 81, 849–858.
- Kampkötter, A.; Nkwonkam, C.; Zurawski, R.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 2007, 234, 113–123.
- Mrvová, N.; Škandík, M.; Bezek, Š.; Račková, L. Protective Effect of Semisynthetic and Natural Flavonoid on Aged Rat Microglia-enriched Cultures. Neurotox. Res. 2019, 36, 844–858.
- Naia, L.; Pinho, C.; Dentoni, G.; Liu, J.; Leal, N.; Ferreira, D.; Schreiner, B.; Filadi, R.; Fão, L.; Connolly, N.; et al. Neuronal cell-based high-throughput screen for enhancers of mitochondrial function reveals luteolin as a modulator of mitochondria-endoplasmic reticulum coupling. BMC Biol. 2021, 19, 57.
More
Information
Subjects:
Gerontology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
3 times
(View History)
Update Date:
23 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





