
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raluca Ioana Stefan-Van Staden | + 1122 word(s) | 1122 | 2022-01-30 08:38:40 | | | |
| 2 | Camila Xu | -5 word(s) | 1117 | 2022-02-22 03:10:40 | | | | |
| 3 | Camila Xu | -5 word(s) | 1117 | 2022-02-22 03:12:07 | | |
Video Upload Options
Two three-dimensional (3D) stochastic microsensors based on immobilization of protoporphyrin IX (PIX) in single-walled carbon nanotubes (SWCNT) and multi-walled carbon nanotubes (MWCNT) decorated with copper (Cu) and gold (Au) nanoparticles were designed and used for the molecular recognition of isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2) in biological samples (brain tumor tissues, whole blood).
1. Introduction
2. Morphological Characterization of the CNT Pastes
The morphology of the pastes (CuAuNP-PIX/SWCNT and CuAuNP-PIX/MWCNT) that contain the necessary channels for the stochastic response is shown in Figure 1 (a.2 and b.2). To evaluate the elemental composition, the quantification of the elements, and their distribution in the material, semi-quantitative analysis was performed by EDX. Moreover, from the mapping, the uniform distribution of the elements in both modified pastes may be seen in Figure 1 (a.1 and b.1).
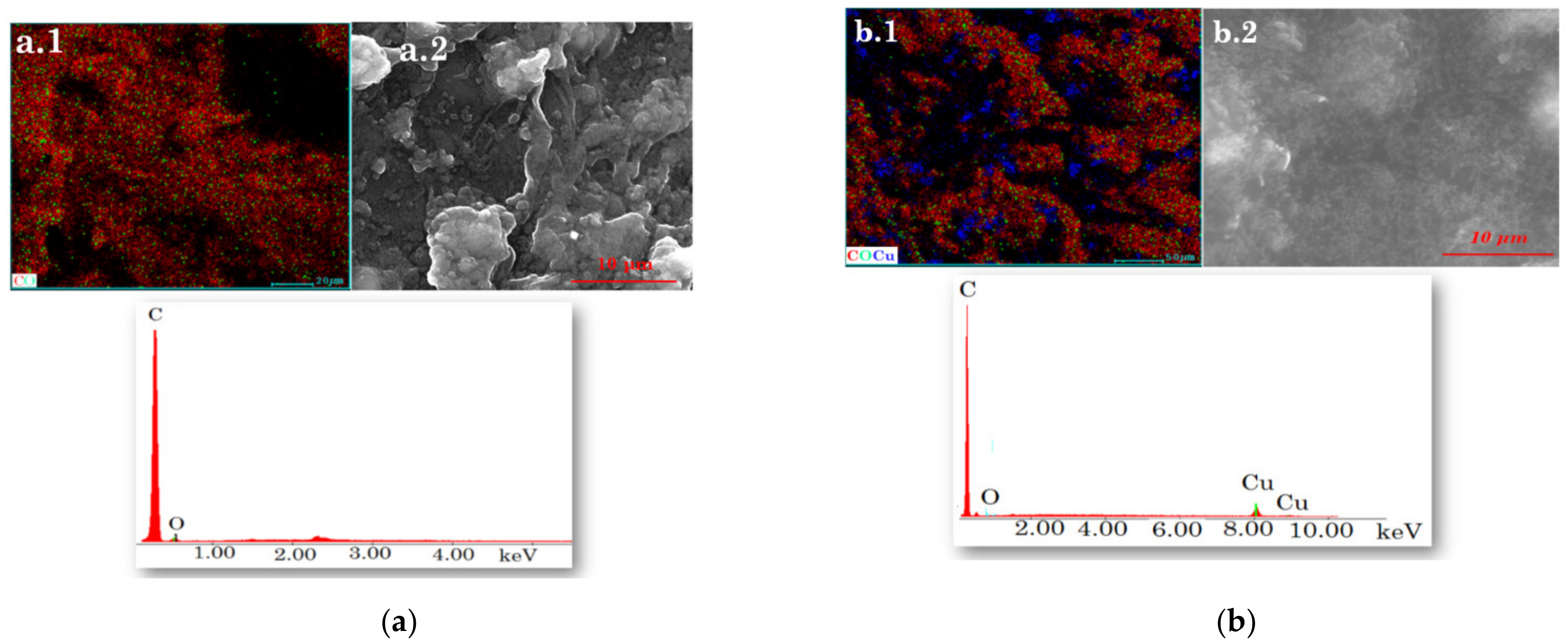 Figure 1. Elemental mapping (a.1, b.1), surface morphology (a.2, b.2), and EDX spectrum of the pastes based on: (a) CuAuNP-PIX/SWCNT and (b) CuAuNP-PIX/MWCNT.
Figure 1. Elemental mapping (a.1, b.1), surface morphology (a.2, b.2), and EDX spectrum of the pastes based on: (a) CuAuNP-PIX/SWCNT and (b) CuAuNP-PIX/MWCNT.
3. Response Characteristics of the Stochastic Microsensors
The response characteristics of the stochastic microsensors used for molecular recognition of IDH1 and IDH2 are shown in Table 1. The signatures obtained for IDH1 and IDH2 were different for each of these microsensors, thus demonstrating the ability of the microsensors to perform the molecular recognition of IDH1 and IDH2 in the biological samples.
Table 1. The response characteristics of the stochastic microsensors used for the molecular recognition of IDH1 and IDH2.
| Stochastic Microsensor Based On |
Signature of IDH toff (s) |
Linear Concentration Range (ng mL−1) | Calibration Equations; The Correlation Coefficient, r * |
Sensitivity (s µg mL−1) |
LOQ (fg mL−1) |
|---|---|---|---|---|---|
| IDH1 | |||||
| CuAuNP-PIX/SWCNT | 0.7 | 1 × 10−5–1 × 102 | 1/ton = 0.03 + 1.48 × C; r = 0.9999 | 1.48 | 10 |
| IDH2 | |||||
| 1.4 | 5 × 10−8–5 × 102 | 1/ton = 0.03 + 7.30 × 104 × C; r = 0.9999 | 7.30 × 104 | 5 × 10−3 | |
| CuAuNP-PIX/MWCNT | IDH1 | ||||
| 1.5 | 1 × 10−5–1 × 102 | 1/ton = 0.04 + 9.58 × 105 × C; r = 0.9989 | 9.58 × 105 | 10 | |
| IDH2 | |||||
| 0.7 | 5 × 10−8–5 × 102 | 1/ton = 0.16 + 1.50 × 107 × C; r = 0.9999 | 1.50 × 107 | 5 × 10−3 | |
* <C-concentration > = µg mL−1; <ton > =s; LOQ—limit of quantification.
Utilization of SWCNT or MWCNT did not influence the linear concentration ranges for the assay of IDH1 (1 × 10−5–1 × 102 ng mL−1) and IDH2 (5 × 10−8- 5 × 102 ng mL−1), as well as the limits of quantification for IDH1 (10 fg mL−1) and IDH2 (5 × 10−3 fg mL−1), but it influenced the sensitivity of the proposed stochastic microsensors: the highest sensitivity was obtained when MWCNT was used for the molecular recognition of IDH1 (9.58 × 105 s µg mL−1) and IDH2 (1.50 × 107 s µg mL−1). Accordingly, the stochastic microsensor of choice for the molecular recognition and quantification of IDH1 and IDH2 is the one based on CuAuNP-PIX/MWCNT.
Compared with the disposable stochastic sensors proposed before [11] (Table 2), a wider linear concentration range and a lower limit of quantification versus the disposable Chitosan/Cu nanolayer-based stochastic sensor was recorded for the assay of IDH1. Moreover, a lower limit of quantification was achieved for the assay of IDH2 with the stochastic sensors based on CNT. Analyses with sensors based on CNT are more cost-effective than those performed using the disposable stochastic sensors because the former can be kept and used continuously for more than one month.
Table 2. The comparison of stochastic microsensors for the assay of IDH1 and IDH2.
| Stochastic Microsensors | Linear Concentration Range (ng mL−1) |
Sensitivity (s µg mL−1) |
LOQ (fg mL−1) |
Reference |
|---|---|---|---|---|
| Disposable Chitosan/Cu nanolayer |
IDH1 | [11] | ||
| 1 × 10−4–1 × 102 | 1.00 × 107 | 102 | ||
| IDH2 | ||||
| 5 × 10−7–5 × 102 | 9.51 × 105 | 5 × 10−1 | ||
| Disposable Chitosan/GR * nanolayer |
IDH1 | |||
| 1 × 10−8–1 × 102 | 3.77 × 107 | 10−2 | ||
| IDH2 | ||||
| 5 × 10−8–5 × 102 | 1.88 × 107 | 5 × 10−2 | ||
| Disposable Chitosan/GR-Cu composite nanolayer |
IDH1 | |||
| 1 × 10−5–1 × 102 | 2.73 × 107 | 10−1 | ||
| IDH2 | ||||
| 5 × 10−8–5 × 102 | 4.44 × 106 | 5 × 10−2 | ||
| CuAuNP-PIX/SWCNT | IDH1 | This work | ||
| 1 × 10−5–1 × 102 | 1.48 | 10 | ||
| IDH2 | ||||
| 5 × 10−8–5 × 102 | 7.30 × 104 | 5 × 10−3 | ||
| CuAuNP-PIX/MWCNT | IDH1 | |||
| 1 × 10−5–1 × 102 | 9.58 × 105 | 10 | ||
| IDH2 | ||||
| 5 × 10−8–5 × 102 | 1.50 × 107 | 5 × 10−3 | ||
Ten of each type of microsensor were designed and used for 1 month for the assay of IDH1 and IDH2. In this period of time, the sensitivities for IDH1 and IDH2 were recorded. For each type of microsensor, the measurements performed during one day showed that the RSD% values for the variation of the sensitivities recorded for 10 microsensors were 0.10% for IDH1 and 0.15% for IDH2 despite the type of microsensor, proving a highly reliable (reproducible) design of the proposed stochastic microsensors. When used for 1 month, the sensitivity variations were 0.37% for the assay of IDH1 and 0.40% for the assay of IDH2 despite the type of microsensor, proving the stability of the microsensors in time.
The selectivity of the stochastic microsensors is given by the signatures (toff values) recorded for different analytes. The signature of the analyte and the possible interference depends on several factors such as molecule size and conformation, deployment capacity, or speed of going in the channel; thus, the signature can act as an element of molecular recognition, contributing to the qualitative analysis of mixtures. The different signatures obtained for analytes such as IDH1, IDH2, heregulin-α, dopamine, epinephrine, and levodopa proved the selectivity of the proposed stochastic microsensor (Table 3).
Table 3. The selectivity of the stochastic microsensors.
| Stochastic Microsensor Based On |
toff (s), Signature | |||||
|---|---|---|---|---|---|---|
| IDH1 | IDH2 | Heregulin-α | Dopamine | Epinephrine | Levodopa | |
| CuAuNP-PIX/SWCNT | 0.7 | 1.4 | 0.2 | 1.9 | 3.0 | 2.5 |
| CuAuNP-PIX/MWCNT | 1.5 | 0.7 | 1.8 | 2.4 | 3.2 | 2.8 |
References
- Oermann, E.K.; Wu, J.; Guan, K.L.; Xiong, Y. Alterations of metabolic genes and metabolites in cancer. Semin. Cell Dev. Biol. 2012, 23, 370–380.
- Dietlein, F.; Weghorn, D.; Taylor-Weiner, A.; Richters, A.; Reardon, B.; Liu, D.; Lander, E.S.; Van Allen, E.M.; Sunyaev, S.R. Identification of cancer driver genes based on nucleotide context. Nat. Genet. 2020, 52, 208–218.
- Tommasini-Ghelfi, S.; Murnan, K.; Kouri, F.M.; Mahajan, A.S.; May, J.L.; Stegh, A.H. Cancer-associated mutation and beyond: The emerging biology of isocitrate dehydrogenases in human disease. Sci. Adv. 2019, 5, eaaw4543.
- Molenaar, R.J.; Maciejewski, J.P.; Wilmink, J.W.; van Noorden, C.J.F. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene 2018, 37, 1949–1960.
- Liu, Y.; Lang, F.; Chou, F.J.; Zaghloul, K.A.; Yang, C. Isocitrate Dehydrogenase Mutations in Glioma: Genetics, Biochemistry, and Clinical Indications. Biomedicines 2020, 8, 294.
- Li, J.J.; Li, R.; Wang, W.; Zhang, B.; Song, X.; Zhang, C.; Gao, Y.; Liao, Q.; He, Y.; You, S.; et al. IDH2 is a novel diagnostic and prognostic serum biomarker for non-small-cell lung cancer. Molec. Oncol. 2018, 12, 602–610.
- Dolecek, T.; Propp, J.; Stroup, N.; Kruchko, C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro. Oncol. 2012, 14, v1–v49.
- Felsberg, J.; Wolter, M.; Seul, H.; Friendensdorf, B.; Goppert, M.; Sabel, M.C.; Reifenberger, G. Rapid and sensitive assessment of the IDH1 and IDH2 mutation status in cerebral gliomas based on DNA pyrosequencing. Acta Neuropathol. 2010, 119, 501–507.
- Shivarov, V.; Ivanova, M.; Hadjiev, E.; Naumova, E. Rapid Detection of DNMT3A R882 Mutations in Hematologic Malignancies Using a Novel Bead-Based Suspension Assay with BNA(NC) Probes. PLoS ONE 2014, 8, e76944.
- Horbinski, C.; Kelly, L.; Nikiforov, Y.E.; Durso, M.B.; Nikiforova, M.N. Detection of IDH1 and IDH2 Mutations by Fluorescence Melting Curve Analysis as a Diagnostic Tool for Brain Biopsies. J. Mol. Diagnosis 2010, 12, 487–492.
- Cioates Negut, C.; Stefan-van Staden, R.-I.; Badulescu, M.; Bita, B. Disposable stochastic sensors obtained by nanolayer deposition of copper, graphene, and copper-graphene composite on silk for the determination of isocitrate dehydrogenases 1 and 2. Anal. Bioanal. Chem. 2022, 414, 1797–1807.
- Dash, D.P.; Dinauer, D. FDA Cleared Companion Diagnostics (CDx) Tests (IDH1, IDH2 and FLT3) for Acute Myeloid Leukemia (AML) Patient Care. Blood 2021, 138, 4442–4443.
- Stefan-van Staden, R.I.; Gheorghe, D.C.; Jinga, V.; Sima, C.S.; Geanta, M. Fast Screening of Whole Blood and Tumor Tissue for Bladder Cancer Biomarkers Using Stochastic Needle Sensors. Sensors 2020, 20, 2420.
- Stefan-van Staden, R.I.; Gheorghe, S.S.; Ilie-Mihai, R.M.; Badulescu, M. Disposable Stochastic Sensor Based on Deposition of a Nanolayer of Silver on Silk for Molecular Recognition of Specific Biomarkers. J. Electrochem. Soc. 2021, 168, 037515.
- Stefan-van Staden, R.I.; Comnea-Stancu, I.R.; Surdu-Bob, C.C. Molecular Screening of Blood Samples for the Simultaneous Detection of CEA, HER-1, NSE, CYFRA 21-1 Using Stochastic Sensors. J. Electrochem. Soc. 2017, 164, B267–B273.
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 99, 1787–1800.
- Britto, P.J.; Santhanam, K.S.V.; Rubio, A.; Alonso, J.A.; Ajayan, P.M. Improved Charge Transfer at Carbon Nanotube Electrodes. Adv. Mater. 1999, 11, 154–157.
- Monsu Scolaro, L.; Castriciano, M.; Romeo, A.; Patane, S.; Cefali, E.; Allegrini, M. Aggregation Behavior of Protoporphyrin IX in Aqueous Solutions: Clear Evidence of Vesicle Formation. J. Phys. Chem. 2002, 106, 2453–2459.




