Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manuel Martinez-Corral | + 699 word(s) | 699 | 2022-02-16 04:28:07 | | | |
| 2 | Camila Xu | Meta information modification | 699 | 2022-02-21 03:10:46 | | | | |
| 3 | Camila Xu | Meta information modification | 699 | 2022-02-21 03:21:54 | | | | |
| 4 | Camila Xu | + 2 word(s) | 701 | 2022-02-21 03:40:32 | | | | |
| 5 | Camila Xu | Meta information modification | 701 | 2022-02-21 04:02:55 | | | | |
| 6 | Camila Xu | Meta information modification | 701 | 2022-02-21 04:20:22 | | | | |
| 7 | Emilio Sánchez-Ortiga | + 1 word(s) | 702 | 2022-02-21 10:30:49 | | | | |
| 8 | Nicolò Incardona | Meta information modification | 702 | 2022-03-08 17:25:51 | | | | |
| 9 | Camila Xu | Meta information modification | 702 | 2022-03-10 03:29:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Martinez-Corral, M.; Sánchez-Ortiga, E.; Sucerquia, J.; Galdón, L.; Incardona, N. Fourier Lightfield Microscope. Encyclopedia. Available online: https://encyclopedia.pub/entry/19665 (accessed on 07 February 2026).
Martinez-Corral M, Sánchez-Ortiga E, Sucerquia J, Galdón L, Incardona N. Fourier Lightfield Microscope. Encyclopedia. Available at: https://encyclopedia.pub/entry/19665. Accessed February 07, 2026.
Martinez-Corral, Manuel, Emilio Sánchez-Ortiga, Jorge Sucerquia, Laura Galdón, Nicolò Incardona. "Fourier Lightfield Microscope" Encyclopedia, https://encyclopedia.pub/entry/19665 (accessed February 07, 2026).
Martinez-Corral, M., Sánchez-Ortiga, E., Sucerquia, J., Galdón, L., & Incardona, N. (2022, February 21). Fourier Lightfield Microscope. In Encyclopedia. https://encyclopedia.pub/entry/19665
Martinez-Corral, Manuel, et al. "Fourier Lightfield Microscope." Encyclopedia. Web. 21 February, 2022.
Copy Citation
Fourier lightfield microscopy (FLMic) is a technique aimed to capture and process 3D information of microscopic samples. Due to its optical design, FLMic has the inherent capacity of capturing a collection of orthographic perspectives of samples in a single shot. Consequently, FLMic is especially suited for capturing and processing 3D images of dynamic processes, being potentially addressed for real-time applications in both life and material sciences.
microscopy
light-field
3D imaging
plenoptic
multi-perspective imaging
1. Introduction
The capture and recovering of the lightfield has been a topic of interest in recent years both in macroscopy and microscopy. Fourier lightfield microscopy (FLMic) [1][2][3][4] represents a reformulation of a previous approach, known as lightfield microscopy [5][6][7][8][9][10][11], both aimed to capture and process 3D information of microscopic samples. The main concept of both methods is to spatially multiplex the angular information by using a microlens array. From this multiplexed information, the 3D image can be recovered computationally in different manners, for instance, by means of backpropagation or deconvolution algorithms. In standard lightfield microscopy the resolution of the reconstructed images is defined by the distance between lenses of the microlens array and varies depending on the refocused distance. The main benefit of FLMic over the previous method is that the system captures directly a set of perspectives of the sample that are diffraction limited and shift-invariant. As a consequence, in FLMic not only the post-processing is eased but also the reconstructed 3D field shows better lateral resolution which, additionally, remains constant along the axial direction [12][13][14][15]. Despite being a relatively recent development, several studies have shown the potential application of FLMic in the study of dynamic processes in biomedical imaging [16][17][18][19][20][21], including 3D real-time imaging of living phytoplankton [22], zebrafish embryos [23], and neuronal activity [24], amongst others.
The standard optical scheme of a FLMic is shown in Figure 1. It consists of a microscope objective, an optical relay system with a field-stop in the common focal plane of both lenses, a microlens array (MLA) and a digital camera. In addition, at least three illumination modes are suitable for FLMic (brightfield, widefield, and darkfield [23]) that might be used depending on the sample of interest. Some recent contributions have demonstrated the possibility of inserting an add-on to a commercial microscope to transform it into a FLMic [22] or even developing a portable version of FLMic with reduced dimensions and price [25].
2. The Fourier Lightfield Microscopy (FLMic) Design
The FLMic design is based on two conjugation relations and on the key role played by two hard apertures. A MLA is virtually inserted, with the help of a telecentric optical relay, at the aperture stop of an infinity-corrected MO, as illustrated in Figure 1. This adjustment ensures that any microlens captures an orthographic perspective image of the 3D sample volume. The number of microlenses that are fitted in the aperture stop (AS) of the MO equals the number of recorded views, from which the posterior digital processing allows the refocusing of the 3D sample volume. Additionally, the field stop (FS) is set in a plane between the lenses of the relay, so that it is conjugated with the object plane, and also with the pixelated sensor, which is set at the rear focal plane of the microlenses. The task of this second stop is to avoid overlapping between view images, and therefore it determines the field of view of the microscope (Figures 2 and 3).
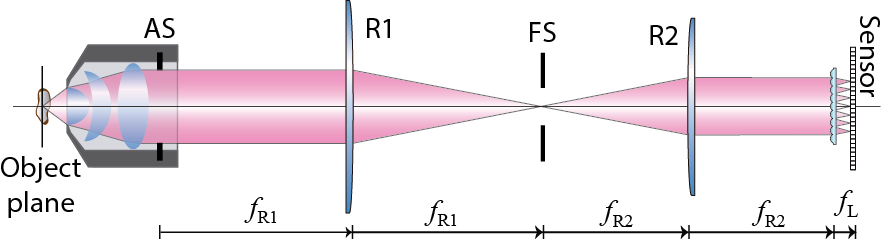
Figure 1. Scheme of the Fourier lightfield microscope.
From the perspective views captured directly with FLMic, stacks of depth images can be calculated and displayed. In the following figures some examples of captured views and computed depth images, are shown.
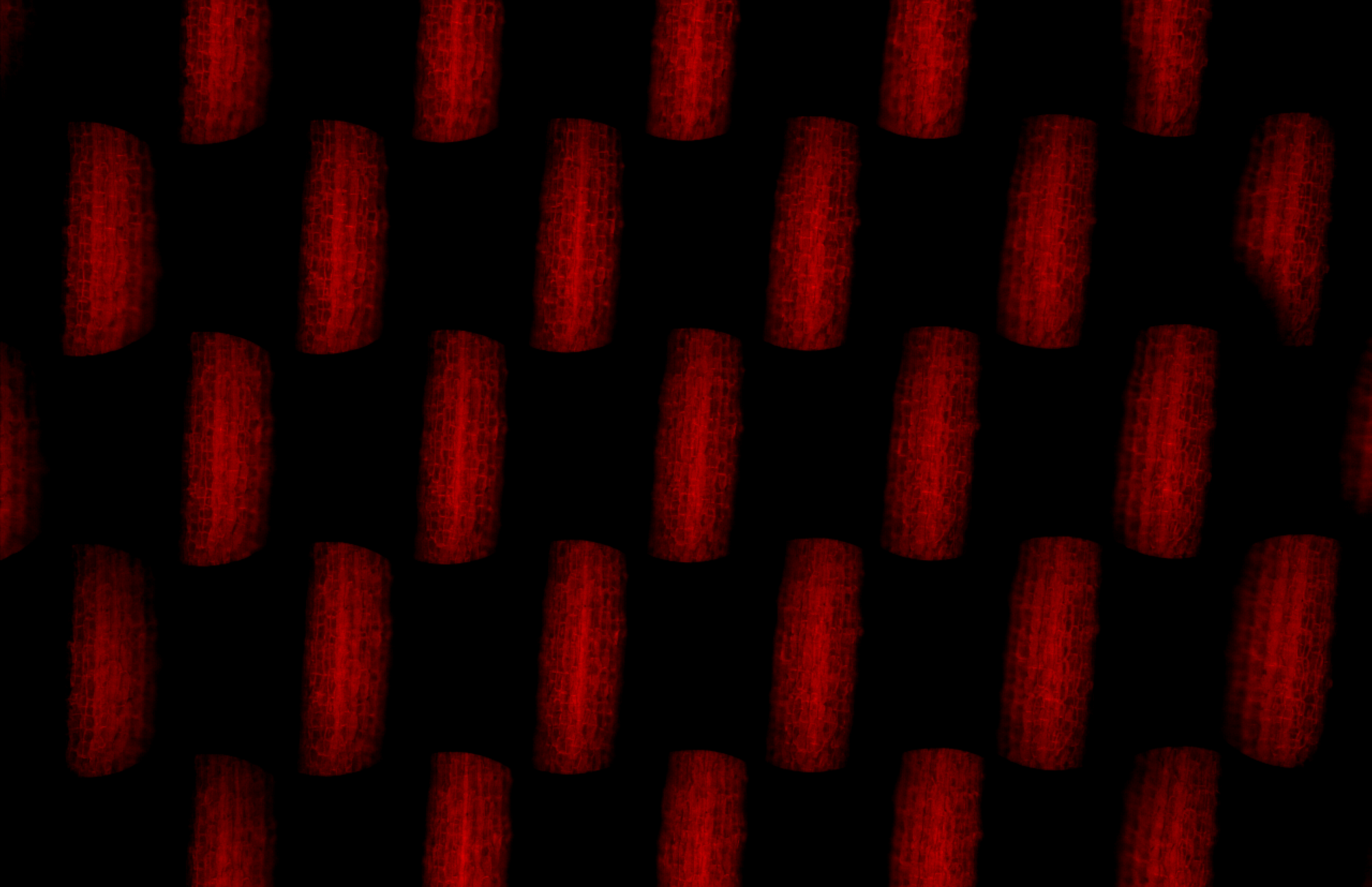
Figure 2. Hypocotyl of Arabidopsis Thaliana imaged with a water immersion objective. On the top, the entire frame registered by the device. On the video, the sample reconstructed at different depths.
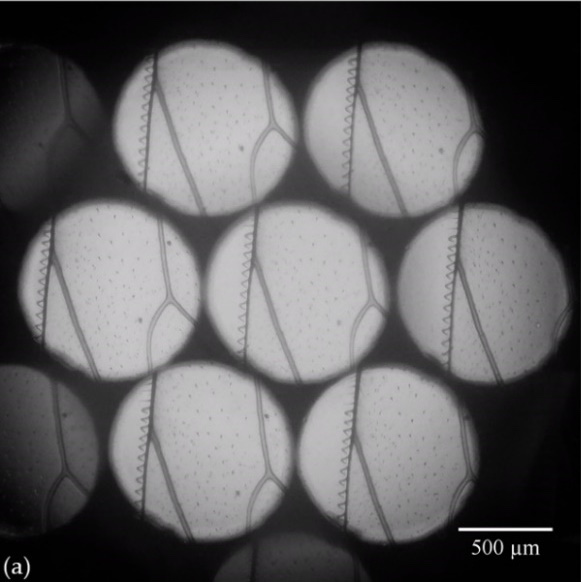
Figure 3. (a) Elemental images of a honeybee wing captured using a handheld and cost-effective FLMic; (b) video with the refocused images at different depth planes.
Currently the company Doit S.L. commercializes an FLMic accessory [22]. Further details about this ultimate modality of Fourier Light Field Microscopy, pushed forward by the groups of 3D Imaging of the University of Valencia and the Optics and Opto-Digital Processing of the Universidad Nacional de Colombia Sede Medellin, can be read in the references included in this entry.
References
- Llavador, A.; Sola-Pikabea, J.; Saavedra, G.; Javidi, B.; Martínez-Corral, M. Resolution improvements in integral microscopy with Fourier plane recording. Opt. Express 2016, 24, 20792–20798.
- Scrofani, G.; Sola-Pikabea, J.; Llavador, A.; Sanchez-Ortiga, E.; Barreiro, J.C.; Saavedra, G.; Garcia-Sucerquia, J.; Martinez-Corral, M. FIMic: Design for ultimate 3D-integral microscopy of in-vivo biological samples. Biomed. Opt. Express 2018, 9, 335–346.
- Martinez-Corral, M.; Javidi, B. Fundamentals of 3D imaging and displays: A tutorial on integral imaging, light-field, and plenoptic systems. Adv. Opt. Photonics 2018, 10, 512–566.
- Guo, C.; Liu, W.; Hua, X.; Li, H.; Jia, S. Fourier light-field microscopy. Opt. Express 2019, 27, 25573–25594.
- Levoy, M.; Ng, R.; Adams, A.; Footer, M.; Horowitz, M. Light field microscopy. ACM Trans. Graph. 2006, 25, 924–934.
- Levoy, M.; Zhang, Z.; McDowall, I. Recording and controlling the 4D light field in a microscope using microlens arrays. J. Microsc. 2009, 235, 144–162.
- Broxton, M.; Grosenick, L.; Yang, S.; Cohen, N.; Andalman, A.; Deisseroth, K.; Levoy, M. Wave optics theory and 3-D deconvolution for the light field microscope. Opt. Express 2013, 21, 25418–25439.
- Llavador, A.; Scrofani, G.; Saavedra, G.; Martinez-Corral, M. Large depth-of-field integral microscopy by use of a liquid lens. Sensors 2018, 18, 3383.
- Wagner, N.; Norlin, N.; Gierten, J.; de Medeiros, G.; Balázs, B.; Wittbrodt, J.; Hufnagel, L.; Prevedel, R. Instantaneous isotropic volumetric imaging of fast biological processes. Nat. Methods 2019, 16, 497–500.
- Stefanoiu, A.; Page, J.; Symvoulidis, P.; Westmeyer, G.G.; Lasser, T. Artifact-free deconvolution in light field microscopy. Opt. Express 2019, 27, 31644–31666.
- Zhang, Y.; Lu, Z.; Wu, J.; Lin, X.; Jiang, D.; Cai, Y.; Xie, J.; Wang, Y.; Zhu, T.; Ji, X.; et al. Computational optical sectioning with an incoherent multiscale scattering model for light-field microscopy. Nat. Commun. 2021, 12, 6391.
- Sanchez-Ortiga, E.; Scrofani, G.; Saavedra, G.; Martinez-Corral, M. Optical sectioning microscopy through single-shot lightfield protocol. IEEE Access 2020, 8, 14944–14952.
- Liu, F.L.; Kuo, G.; Antipa, N.; Yanny, K.; Waller, L. Fourier diffuserScope: Single-shot 3D Fourier light field microscopy with a diffuser. Opt. Express 2020, 28, 28969–28986.
- Stefanoiu, A.; Scrofani, G.; Saavedra, G.; Martinez-Corral, M.; Lasser, T. What about computational super-resolution in fluorescence Fourier light field microscopy? Opt. Express 2020, 28, 16554–16568.
- Hua, X.; Liu, W.; Jia, S. High-resolution Fourier light-field microscopy for volumetric multi-color live-cell imaging. Optica 2021, 8, 614–620.
- Cong, L.; Wang, Z.; Chai, Y.; Hang, W.; Shang, C.; Yang, W.; Bai, L.; Du, J.; Wang, K.; Wen, Q. Rapid whole brain imaging of neural activity in freely behaving larval zebrafish (Danio rerio). eLife 2017, 6, e28158.
- Yoon, Y.G.; Wang, Z.; Pak, N.; Park, D.; Dai, P.; Kang, J.S.; Suk, H.J.; Symvoulidis, P.; Guner-Ataman, B.; Wang, K.; et al. Sparse decomposition light-field microscopy for high speed imaging of neuronal activity. Optica 2020, 7, 1457–1468.
- Javidi, B.; Carnicer, A.; Arai, J.; Fujii, T.; Hua, H.; Liao, H.; Martínez-Corral, M.; Pla, F.; Stern, A.; Waller, L.; et al. Roadmap on 3D integral imaging: Sensing, processing, and display. Opt. Express 2020, 28, 32266–32293.
- Sims, R.R.; Rehman, S.A.; Lenz, M.O.; Benaissa, S.I.; Bruggeman, E.; Clark, A.; Sanders, E.W.; Ponjavic, A.; Muresan, L.; Lee, S.F.; et al. Single molecule light field microscopy. Optica 2020, 7, 1065–1072.
- Cui, Q.; Park, J.; Ma, Y.; Gao, L. Snapshot hyperspectral light field tomography. Optica 2021, 8, 1552–1558.
- Wang, Z.; Zhu, L.; Zhang, H.; Li, G.; Yi, C.; Yang, Y.; Ding, Y.; Zhen, M.; Gao, S.; Hsiai, T.K.; et al. Real-time volumetric reconstruction of biological dynamics with light-field microscopy and deep learning. Nat. Methods 2021, 18, 551–556.
- Incardona, N.; Tolosa, A.; Scrofani, G.; Martinez-Corral, M.; Saavedra, G. The lightfield microscope eyepiece. Sensors 2021, 21, 6619.
- Scrofani, G., Saavedra, G., Martínez-Corral, M., & Sánchez-Ortiga, E. (2020). Three-dimensional real-time darkfield imaging through Fourier lightfield microscopy. Optics Express, 28(21), 30513-30519.
- Prevedel, R.; Yoon, Y.G.; Hoffmann, M.; Pak, N.; Wetzstein, G.; Kato, S.; Schrödel, T.; Raskar, R.; Zimmer, M.; Boyden, E.S. Simultaneous whole-animal 3D imaging of neuronal activity using light-field microscopy. Nat. Methods 2014, 11, 727–730.
- Galdon, L., Yun, H., Saavedra, G., Garcia-Sucerquia, J., Barreiro, J. C., Martinez-Corral, M., & Sanchez-Ortiga, E. (2022). Handheld and Cost-Effective Fourier Lightfield Microscope. Sensors, 22(4), 1459.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
9 times
(View History)
Update Date:
10 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




