Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 5 by Camila Xu and Version 4 by Camila Xu.
Fourier lightfield microscopy (FLMic) is a technique aimed to capture and process 3D information of microscopic samples. Due to its optical design, FLMic has the inherent capacity of capturing a collection of orthographic perspectives of samples in a single shot. Consequently, FLMic is especially suited for capturing and processing 3D images of dynamic processes, being potentially addressed for real-time applications in both life and material sciences.
- microscopy
- light-field
- 3D imaging
- plenoptic
- multi-perspective imaging
1. Introduction
The capture and recovering of the lightfield has been a topic of interest in recent years both in macroscopy and microscopy. Fourier lightfield microscopy (FLMic) [1][2][3][4] represents a reformulation of a previous approach, known as lightfield microscopy [5][6][7][8][9][10][11], both aimed to capture and process 3D information of microscopic samples. The main concept of both methods is to spatially multiplex the angular information by using a microlens array. From this multiplexed information, the 3D image can be recovered computationally in different manners, for instance, by means of backpropagation or deconvolution algorithms. In standard lightfield microscopy the resolution of the reconstructed images is defined by the distance between lenses of the microlens array and varies depending on the refocused distance. The main benefit of FLMic over the previous method is that the system captures directly a set of perspectives of the sample that are diffraction limited and shift-invariant. As a consequence, in FLMic not only the post-processing is eased but also the reconstructed 3D field shows better lateral resolution which, additionally, remains constant along the axial direction [12][13][14][15]. Despite being a relatively recent development, several studies have shown the potential application of FLMic in the study of dynamic processes in biomedical imaging [16][17][18][19][20][21], including 3D real-time imaging of living phytoplankton [23], zebrafish embryos [24], and neuronal activity [25], amongst others.
The standard optical scheme of a FLMic is shown in Figure 1. It consists of a microscope objective, an optical relay system with a field-stop in the common focal plane of both lenses, a microlens array and a digital camera. In addition, at least three illumination modes are suitable for FLMic (brightfield, widefield, and darkfield [1]) that might be used depending on the sample of interest. Some recent contributions have demonstrated the possibility of inserting an add-on to a commercial microscope to transform it into a FLMic [22] or even developing a portable version of FLMic with reduced dimensions and price [26].
2. The Fourier Lightfield Microscopy (FLMic) Design
The FLMic design is based on two conjugation relations and on the key role played by two hard apertures. An MLA is virtually inserted, with the help of a telecentric optical relay, at the aperture stop of an infinity-corrected MO, as illustrated in Figure 1. This adjustment ensures that any microlens captures an orthographic perspective image of the 3D sample volume. The number of microlenses that are fitted in the aperture stop (AS) of the MO equals the number of recorded views, from which the posterior digital processing allows the refocusing of the 3D sample volume. Additionally, the field stop (FS) is set in a plane between the lenses of the relay, so that it is conjugated with the object plane, and also with the pixelated sensor, which is set at the rear focal plane of the microlenses. The task of this second stop is to avoid overlapping between view images, and therefore it determines the field of view of the microscope (Figures 2 and 3).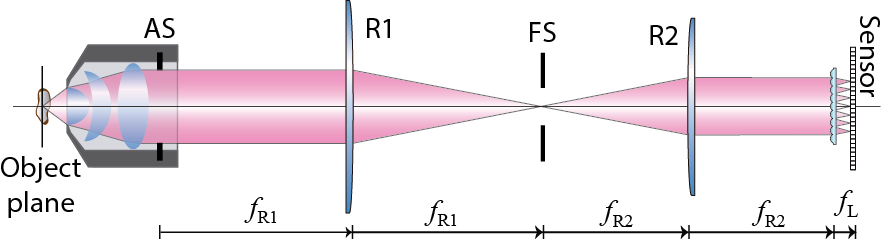
Figure 1. Scheme of the Fourier lightfield microscope.
References
- Llavador, A.; Sola-Pikabea, J.; Saavedra, G.; Javidi, B.; Martínez-Corral, M. Resolution improvements in integral microscopy with Fourier plane recording. Opt. Express 2016, 24, 20792–20798.
- Scrofani, G.; Sola-Pikabea, J.; Llavador, A.; Sanchez-Ortiga, E.; Barreiro, J.C.; Saavedra, G.; Garcia-Sucerquia, J.; Martinez-Corral, M. FIMic: Design for ultimate 3D-integral microscopy of in-vivo biological samples. Biomed. Opt. Express 2018, 9, 335–346.
- Martinez-Corral, M.; Javidi, B. Fundamentals of 3D imaging and displays: A tutorial on integral imaging, light-field, and plenoptic systems. Adv. Opt. Photonics 2018, 10, 512–566.
- Guo, C.; Liu, W.; Hua, X.; Li, H.; Jia, S. Fourier light-field microscopy. Opt. Express 2019, 27, 25573–25594.
- Levoy, M.; Ng, R.; Adams, A.; Footer, M.; Horowitz, M. Light field microscopy. ACM Trans. Graph. 2006, 25, 924–934.
- Levoy, M.; Zhang, Z.; McDowall, I. Recording and controlling the 4D light field in a microscope using microlens arrays. J. Microsc. 2009, 235, 144–162.
- Broxton, M.; Grosenick, L.; Yang, S.; Cohen, N.; Andalman, A.; Deisseroth, K.; Levoy, M. Wave optics theory and 3-D deconvolution for the light field microscope. Opt. Express 2013, 21, 25418–25439.
- Llavador, A.; Scrofani, G.; Saavedra, G.; Martinez-Corral, M. Large depth-of-field integral microscopy by use of a liquid lens. Sensors 2018, 18, 3383.
- Wagner, N.; Norlin, N.; Gierten, J.; de Medeiros, G.; Balázs, B.; Wittbrodt, J.; Hufnagel, L.; Prevedel, R. Instantaneous isotropic volumetric imaging of fast biological processes. Nat. Methods 2019, 16, 497–500.
- Stefanoiu, A.; Page, J.; Symvoulidis, P.; Westmeyer, G.G.; Lasser, T. Artifact-free deconvolution in light field microscopy. Opt. Express 2019, 27, 31644–31666.
- Zhang, Y.; Lu, Z.; Wu, J.; Lin, X.; Jiang, D.; Cai, Y.; Xie, J.; Wang, Y.; Zhu, T.; Ji, X.; et al. Computational optical sectioning with an incoherent multiscale scattering model for light-field microscopy. Nat. Commun. 2021, 12, 6391.
- Sanchez-Ortiga, E.; Scrofani, G.; Saavedra, G.; Martinez-Corral, M. Optical sectioning microscopy through single-shot lightfield protocol. IEEE Access 2020, 8, 14944–14952.
- Liu, F.L.; Kuo, G.; Antipa, N.; Yanny, K.; Waller, L. Fourier diffuserScope: Single-shot 3D Fourier light field microscopy with a diffuser. Opt. Express 2020, 28, 28969–28986.
- Stefanoiu, A.; Scrofani, G.; Saavedra, G.; Martinez-Corral, M.; Lasser, T. What about computational super-resolution in fluorescence Fourier light field microscopy? Opt. Express 2020, 28, 16554–16568.
- Hua, X.; Liu, W.; Jia, S. High-resolution Fourier light-field microscopy for volumetric multi-color live-cell imaging. Optica 2021, 8, 614–620.
- Cong, L.; Wang, Z.; Chai, Y.; Hang, W.; Shang, C.; Yang, W.; Bai, L.; Du, J.; Wang, K.; Wen, Q. Rapid whole brain imaging of neural activity in freely behaving larval zebrafish (Danio rerio). eLife 2017, 6, e28158.
- Yoon, Y.G.; Wang, Z.; Pak, N.; Park, D.; Dai, P.; Kang, J.S.; Suk, H.J.; Symvoulidis, P.; Guner-Ataman, B.; Wang, K.; et al. Sparse decomposition light-field microscopy for high speed imaging of neuronal activity. Optica 2020, 7, 1457–1468.
- Javidi, B.; Carnicer, A.; Arai, J.; Fujii, T.; Hua, H.; Liao, H.; Martínez-Corral, M.; Pla, F.; Stern, A.; Waller, L.; et al. Roadmap on 3D integral imaging: Sensing, processing, and display. Opt. Express 2020, 28, 32266–32293.
- Sims, R.R.; Rehman, S.A.; Lenz, M.O.; Benaissa, S.I.; Bruggeman, E.; Clark, A.; Sanders, E.W.; Ponjavic, A.; Muresan, L.; Lee, S.F.; et al. Single molecule light field microscopy. Optica 2020, 7, 1065–1072.
- Cui, Q.; Park, J.; Ma, Y.; Gao, L. Snapshot hyperspectral light field tomography. Optica 2021, 8, 1552–1558.
- Wang, Z.; Zhu, L.; Zhang, H.; Li, G.; Yi, C.; Yang, Y.; Ding, Y.; Zhen, M.; Gao, S.; Hsiai, T.K.; et al. Real-time volumetric reconstruction of biological dynamics with light-field microscopy and deep learning. Nat. Methods 2021, 18, 551–556.
- Incardona, N.; Tolosa, A.; Scrofani, G.; Martinez-Corral, M.; Saavedra, G. The lightfield microscope eyepiece. Sensors 2021, 21, 6619.
- Scrofani, G.; Saavedra, G.; Martínez-Corral, M.; Sánchez-Ortiga, E. Three-dimensional real-time darkfield imaging through Fourier lightfield microscopy. Opt. Express 2020, 28, 30513–30519.
- Prevedel, R.; Yoon, Y.G.; Hoffmann, M.; Pak, N.; Wetzstein, G.; Kato, S.; Schrödel, T.; Raskar, R.; Zimmer, M.; Boyden, E.S. Simultaneous whole-animal 3D imaging of neuronal activity using light-field microscopy. Nat. Methods 2014, 11, 727–730.
- Ghosh, K.K.; Burns, L.D.; Cocker, E.D.; Nimmerjahn, A.; Ziv, Y.; el Gamal, A.; Schnitzer, M.J. Miniaturized integration of a fluorescence microscope. Nat. Methods 2011, 8, 871.
- Switz, N.A.; D’Ambrosio, M.V.; Fletcher, D.A. Low-Cost Mobile Phone Microscopy with a Reversed Mobile Phone Camera Lens. PLoS ONE 2014, 9, e95330.
- Lu, Q.; Liu, G.; Xiao, C.; Hu, C.; Zhang, S.; Xu, R.X.; Chu, K.; Xu, Q.; Smith, Z.J. A modular, open-source, slide-scanning microscope for diagnostic applications in resource-constrained settings. PLoS ONE 2018, 13, e0194063.
More
