| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Federico Vancheri | + 2402 word(s) | 2402 | 2020-09-07 10:36:33 | | | |
| 2 | Nicole Yin | Meta information modification | 2402 | 2020-09-10 05:04:14 | | |
Video Upload Options
Coronary microvascular dysfunction (CMD) is defined as the clinical syndrome of angina, electrocardiographic ischemic changes in the absence of obstructive CAD. The pathophysiological basis is impaired microvascular vasodilatation, leading to inadequate increase in blood flow to match myocardial oxygen needs (previously referred to in the literature as “cardiac syndrome X”).
1. Introduction
CMD is functionally expressed as reduced coronary flow reserve (CFR), which is the maximum increase in coronary blood flow above the resting value after pharmacological coronary vasodilatation. Reduced CFR due to functional and/or structural abnormalities of the microcirculation has been reported in about 50% of patients with chronic coronary syndromes and up to 20% of those with acute coronary syndromes, in the absence of epicardial coronary flow obstruction[1][2][3][4][5].
Until recently, the prognosis of non-obstructive CAD was thought to be benign, and patients were often inappropriately reassured, without further investigation, despite clinical features requiring coronary angiography. Instead, this condition represents a major cause for myocardial ischemia and is associated with a high risk of major adverse cardiovascular events, including MI, progressive heart failure, stroke, and even sudden death[6][7][8][9][10][11][12]. Moreover, in most patients there is evidence for close interactions between microvascular dysfunction and atherosclerotic epicardial coronary disease[13][14]. CMD is a strong determinant of prognosis even in patients with coronary stenosis of intermediate severity[15].
2. Pathophysiology of CMD
In contrast to coronary epicardial atherosclerosis, CMD does not develop atheroma. However, coronary microcirculation can develop structural and functional atherosclerotic changes, particularly in patients with cardiovascular (CV) risk factors. CMD is expressed as either the inability of the coronary arteries to dilate appropriately to meet myocardial oxygen demand (vasodilator abnormality) and/or as the abrupt reduction in coronary blood flow (coronary microvascular spasm). The underlying mechanism for coronary vasomotor dysfunction may be endothelium-dependent or endothelium-independent. Endothelium-dependent dysfunction is a consequence of an imbalance between endothelium-derived relaxing factors, such as nitric oxide (NO), and endothelium-derived constrictors, such as endothelin. Endothelium-independent function is based on myocyte tone. Moreover, CMD can occur in the absence and/or in the presence of obstructive epicardial coronary artery disease[16]. The myocardial consequences of coronary blood flow abnormalities related to CMD are different from those caused by epicardial flow-limiting stenosis. In the latter, the impairment in myocardial perfusion is homogeneously distributed in the region perfused by the stenosed artery, resulting in segmental impairment of wall contraction. In contrast, the myocardial ischemia in CMD may not involve all microvessels originating from an epicardial artery but may have a patchy distribution. This explains why these patients may have symptoms without myocardial wall contraction abnormalities[17].
2.1. Endothelial Dysfunction
Despite the fact that endothelial structure and function may vary considerably among different vascular regions, they have a common feature in secreting substances that regulate vascular tone and permeability, thus contributing to vascular protection[18]. The endothelium has a crucial role in CV homeostasis, in particular in the myocardial capillaries where endothelial cells are directly in contact with adjacent cardiomyocytes[19]. Normally, under physiological stimuli such as exercise, the vascular endothelial cells modulate an appropriate dilatation of coronary arteries by locally releasing vasodilator substances, in particular NO[20][21][22]. NO also protects the integrity of the endothelium through its anti-inflammatory properties by inhibiting fibrosis, platelet aggregation, and apoptosis and by promoting angiogenesis.
CMD is initiated by the low-grade inflammatory response to CV risk factors, similar to those of obstructive CAD, although they have been shown to account for <20% of variability in microvascular function, leaving a large proportion unexplained[23][24][25]. The inflammatory response reduces NO bioavailability, which is the link between atherosclerosis and microvascular dysfunction, leading to impairment of both endothelium-dependent and endothelium-independent coronary microvascular vasomotor function, thus increasing the risk of myocardial ischemia. Functional changes induced by endothelial dysfunction may be simultaneously present in coronary microcirculation and in the epicardial arteries, as well as peripheral vascular sites, thus representing a systemic disorder[26][27]. Inflammation-induced endothelial dysfunction is thought to be mediated by elevated C-reactive protein (CRP) levels[23][28][29][30]. However, it is still controversial whether high CRP concentration is cause or effect of atherosclerosis, or confounding factor[31][32][33].
The inflammatory response of endothelial cells is strongly influenced by WSS, which is the tangential component of the mechanical friction exerted by the flowing blood on the vascular endothelial surface[34][35]. Specific biomechanical receptors in the glycocalyx, a surface proteoglycan layer, translate WSS into biochemical signals that modulate the vascular tone, platelet activity, leukocyte adhesion, and endothelial permeability[36]. While physiological WSS promotes the maintenance of anti-inflammatory properties of endothelial barrier, low WSS induces inflammation, and atherogenesis through decreased endothelial production of NO[37][38]. This results in impairment of dilator function and blunted coronary blood flow augmentation in response to myocardial metabolic demands. Impaired endothelial function may also release vasoconstrictor substances (endothelin, prostaglandin, and thromboxane), thus shifting the vasomotor response to physiological stimuli from dilatation to constriction, reducing the blood flow[39]. Vasomotor abnormalities in patients with CMD include coronary spasm, which affects large and small coronary arteries. Following intracoronary infusion of acetylcholine, about 50% of patients with exertional angina and no evidence of obstructive CAD develop coronary microvascular spasm, defined as angina and ischemic ECG changes without changes in epicardial coronary artery diameter[40].
The decreased production of NO by impaired endothelial cells also increases collagen deposition, reduces angiogenesis and collateral development, and promotes the conversion of endothelial cells into mesenchymal cells, leading to microvascular rarefaction defined as loss of perfused microvessels, fibrosis, and hypertrophy[41][42]. Moreover, while under normal conditions endothelial cells produce anti-proliferative and antithrombotic mediators, inflammation promotes a switch in endothelial function from a quiescent to an activated state, involving platelet activation, lipid oxidation, and leukocyte adhesion and migration which, in turn, accelerate inflammation[43][44]. Microvascular inflammation and reduced NO availability directly promote proliferation of fibroblasts, which increase the production of fibronectin and collagen leading to cardiac remodeling following myocardial infarction, pressure overload, and myocarditis[45][46][47]. Additionally, proliferation of fibroblast and increased content of extracellular matrix proteins increase the distance of oxygen diffusion between capillaries and myocytes, exposing the myocardium to the risk of hypoxia under the condition of reduced blood flow.
Microvascular functional abnormalities, characterized by persistent changes in microvascular tone, are also associated with the development of structural abnormalities, which include luminal narrowing, due to inward remodeling of intramyocardial arterioles, rarefaction of microvessels, and microembolization after atherosclerotic plaque rupture or during coronary intervention. Remodeling is mainly caused by medial wall thickening because of proliferated smooth muscle cells, surrounded by perivascular fibrosis[48][49]. Both arteriolar remodeling and decrease in capillary density (estimated as capillary number per unit area) contribute to increased microvascular resistance and reduced CFR[50][51][52][53]. Coronary microvascular microembolization by debris from epicardial atherosclerotic plaques together with thrombogenic and inflammatory substances may develop acutely following plaque rupture or erosion[54][55]. This may occur spontaneously or may be induced during percutaneous coronary intervention after myocardial infarction. Release of debris and thrombogenic substances from atherosclerotic epicardial lesions may also be chronic and asymptomatic, leading to progressive microvascular dysfunction.
2.2. Autonomic Nervous System
Sympathetic and parasympathetic nerves can modulate arterial tone directly acting on vascular smooth muscle cells or stimulating the release of NO from the endothelium[56]. The physiological role of coronary blood flow control by the vagus nerve is uncertain. However, under controlled conditions, the intracoronary infusion of acetylcholine results in dilatation of resistance vessels and a three to four-fold increase in blood flow. In contrast, in patients with coronary atherosclerosis, the response is attenuated or results in epicardial or microvascular coronary spam[40]. Such effects of acetylcholine on the coronary endothelium are used to test the endothelium-dependent coronary function.
At rest, the sympathetic control of coronary vasomotor tone is negligible and depends on the balance between β-adrenergic mediated arterial dilatation, which is prevalent, and α-adrenergic mediated vasoconstriction that has only a limited effect[56]. During exercise, coronary tone is modulated by sympathetic activation through release of norepinephrine by sympathetic nerves of coronary circulation, as well as by circulating norepinephrine and epinephrine. β-adrenergic activation, involving mainly β2-adrenoceptors, contributes to coronary vasodilatation to compensate for the increased myocardial oxygen consumption, accounting for approximately 25% of exercise hyperemia[57]. However, when the coronary circulation is impaired by atherosclerotic endothelial dysfunction, the α1-adrenergic mediated vasoconstriction becomes more intense, reduces blood flow, and may lead to myocardial ischemia[58][59].
3. Clinical Presentation
Conditions presented as symptoms and signs of myocardial ischemia without significant coronary artery stenosis (<50% diameter stenosis) are termed “ischemia with non-obstructive coronary artery disease” (INOCA)[2][11][60][61]. Endothelial dysfunction accounts for approximately two-thirds of INOCA [99]. Among these patients, the clinical feature of microvascular angina (MVA), defined as the clinical manifestation of myocardial ischemia due to CMD in the absence of flow-limiting CAD[62][63], is present in 52%, vasospastic angina (VSA) defined as a manifestation of epicardial spasm in 17%, and mixed forms in 20%[64][65].
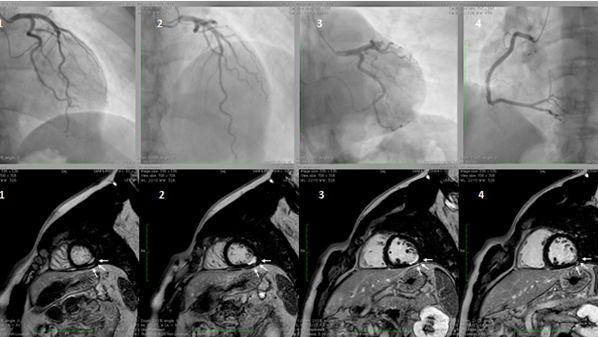
Myocardial infarction with non-obstructive coronary artery disease (≤50% diameter stenosis in a major epicardial artery) is termed MINOCA (Figure 1). This is a heterogeneous group of conditions including epicardial and microvascular causes of myocardial ischemia, such as plaque disruption, epicardial coronary spasm, spontaneous coronary dissection, microvascular spasm, and coronary distal embolization[66][67][68][69][70][71][72]. The features of Takotsubo cardiomyopathy and myocarditis, initially included in the definition, were subsequently excluded[66]. The prevalence of MINOCA is approximately 10% of all type 1 myocardial infarction (MI), more common in women and in patients presenting with Non-ST-elevation myocardial infarction (NSTEMI)[73][74] [108,109]. CMD, expressed by coronary microvascular spasm, accounts for about 20% of MINOCA patients[67][73][75]. A seasonal pattern of occurrence with a peak in summer and autumn has been observed in patients with MINOCA[76]. In contrast, it is still uncertain whether MI due to CAD is equally distributed across seasons or more frequent in winter, in association with respiratory tract infections. These differences may be related to different pathophysiologic triggers leading to MI, e.g., microvascular spasm in patients with MINOCA or atherosclerotic plaque rupture in MI due to CAD.
Figure 1. A hypertensive, smoker, young male patient was admitted for inferior ST-elevation myocardial infarction (STEMI). Coronary angiography was performed with no coronary lesions on the left (panel A, 1,2, and 3) and right (panel B, 4) coronary artery. Subsequentially, cardiac magnetic resonance (CMR) was performed. Delayed enhancement sequences, from apex to base, show sub-endocardial lesions (white arrows, image 1 and 4) with transmural extension (white arrows 2 and 3), confirming the ischemic lesions of the inferior-lateral wall.
CMD may occur in asymptomatic individuals and may be diagnosed incidentally. Symptoms of myocardial ischemia are indistinguishable from those caused by epicardial stenosis. One to two-thirds of patients present typical exertional angina, more common in postmenopausal women than in men[77]. Atypical symptoms, including retrosternal chest pain at rest or angina-equivalent, such as dyspnea on exertion, are also common. Effort-induced symptoms tend to occur in the post exercise recovery period, due to persistence of imbalance between metabolic demand and oxygen delivery. Nitrates are less effective in relieving symptoms because their vasodilator effect is more pronounced in the epicardial arteries compared to microvascular circulation. Standardized criteria for clinical diagnosis of MVA have been developed by the “Coronary Vasomotion Disorders International Study Group” (COVADIS), based on symptoms, absence of obstructive CAD, evidence of myocardial ischemia, and evidence of microvascular dysfunction (Table 1)[62].
Table 1. Clinical criteria for the diagnosis of microvascular angina (MVA).
|
1. Symptoms of myocardial ischemia |
|
-Effort and/or rest angina |
|
-Angina equivalents (exertional dyspnea) |
|
2. Absence of obstructive CAD (<50% diameter reduction or FFR > 0.80) by coronary CTA or invasive angiography |
|
|
|
3. Objective evidence of myocardial ischemia |
|
-Ischemic ECG changes during chest pain |
|
-Stress-induced chest pain and/or ischemic ECG changes with or without transient |
|
or reversible abnormal myocardial perfusion and/or wall motion abnormality |
|
4. Evidence of impaired coronary microvascular function |
|
-Impaired coronary flow reserve ≤ 2.0 |
|
-Coronary microvascular spasm, defined as symptoms and ischemic ECG changes but not epicardial spasm during acetylcholine testing |
|
-Abnormal coronary microvascular resistance indices (IMR > 25) |
|
-Coronary slow flow phenomenon, defined as TIMI frame count > 25 |
Definitive MVA if all 4 criteria are present. Suspected MVA if symptoms of ischemia with no obstructive coronary artery disease are present (criteria 1 and 2) but only objective evidence of myocardial ischemia (criteria 3) or evidence of impaired coronary microvascular function (criteria 4) alone. CAD = coronary artery disease; ECG = electrocardiogram; CTA computed tomographic angiography; FFR = fractional flow reserve; IMR = index of microcirculatory resistance; TIMI = thrombolysis in myocardial infarction. For details, please refer to: https://www.mdpi.com/authors/rights Changed.
3.1. Gender Differences in CMD
Several studies have shown that females have a lesser extent of obstructive CAD compared with males[9][74]. In contrast, they have higher prevalence of angina without obstructive CAD[78][79][80]. In the presence of non-obstructive CAD, myocardial ischemia may be due to microvascular endothelial dysfunction, epicardial and microvascular spasm, or conduit artery stiffening. Moreover, in patients without angiographically obstructive CAD, intracoronary imaging studies using IVUS (intravascular ultrasound), which allows direct cross-sectional visualization of the arterial wall, have shown high prevalence of positive remodeling and preserved lumen size[13][14]. This may explain the absence of flow-limiting lesions which can only outline contrast-filled coronary lumen. These features are more frequent in women and are associated with increased risk of CV events[81][82]. Additionally, women have higher prevalence of MINOCA—10.5% compared to 3.4% for men[67][83].
Symptomatic women are generally older than men and often have greater number and severity of CV risk factors. Moreover, most patients are in the peri- or post-menopausal stage, suggesting that reduced levels of estrogen have a role in the development of CMD[84]. Angina in the absence of obstructive CAD is also psychologically relevant, as recognition of the ischemic heart disease may be delayed. This may induce anxiety in the patient because there is no clear diagnosis, thus reducing the patient’s quality of life.
3.2. Clinical Classification
CMD includes a wide spectrum of conditions variously associated with atherosclerosis. Accordingly, the traditional CMD classification takes into account the clinical and pathophysiological setting in which it may occur[5][85].This classification has been recently updated in relation to the severity of CAD [20] (Table 2).
Table 2. Clinical classifications of coronary microvascular dysfunction (CMD).
|
A. CMD in chronic coronary syndrome |
|
with non-obstructive chronic coronary syndrome |
|
with obstructive chronic coronary syndrome |
|
CMD in acute coronary syndrome (ACS) |
|
with non-obstructive ACS |
|
with obstructive ACS |
|
with coronary no-reflow phenomenon |
|
CMD following successful revascularization after MI |
|
|
|
B. Group 1. CMD in the absence of obstructive CAD and myocardial disease |
|
Group 2. CMD in the presence of myocardial disease |
|
Group 3. CMD in the presence of obstructive CAD |
|
Group 4. CMD after successful percutaneous coronary intervention |
|
|
|
C. CMD without atherosclerosis |
|
CMD with non-obstructive atherosclerosis |
|
CMD with obstructive atherosclerosis |
In addition, because CMD may be associated with clinical conditions in which atherosclerosis is minimal or absent or associated with obstructive or non-obstructive coronary atherosclerosis, a simplified classification has been proposed, including three main CMD phenotypes: without atherosclerosis, with non-obstructive atherosclerosis, and with obstructive atherosclerosis[39].
References
- Erika R. Gehrie; Harmony R. Reynolds; Anita Y. Chen; Brian H. Neelon; Matthew T. Roe; W. Brian Gibler; E. Magnus Ohman; L. Kristin Newby; Eric D. Peterson; Judith S. Hochman; et al. Characterization and outcomes of women and men with non–ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: Results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) Quality Improvement Initiative. American Heart Journal 2009, 158, 688-694, 10.1016/j.ahj.2009.08.004.
- Padro, T.; Manfrini, O.; Bugiardini, R.; Canty, J.; Cenko, E.; De Luca, G.; Duncker, D.J.; Eringa, E.C.; Koller, A.; Tousoulis, D.; et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on ‘coronary microvascular dysfunction in cardiovascular disease’. Cardiovasc. Res. 2020, 116, 741–755.
- Bugiardini, R.; Manfrini, O.; De Ferrari, G.M. Unanswered Questions for Management of Acute Coronary Syndrome: Risk Stratification of Patients With Minimal Disease or Normal Findings on Coronary Angiography. Arch. Intern. Med. 2006, 166, 1391–1395.
- Planer, D.; Mehran, R.; Ohman, E.M.; White, H.D.; Newman, J.D.; Xu, K.; Stone, G.W. Prognosis of Patients With Non-ST-Segment-Elevation Myocardial Infarction and Nonobstructive Coronary Artery Disease. Circ. Cardiovasc. Interv. 2014, 7, 285–293.
- Camici, P.G.; Crea, F. Coronary Microvascular Dysfunction. N. Engl. J. Med. 2007, 356, 830–840.
- Lasse Jespersen; Anders Hvelplund; Steen Z. Abildstrøm; Frants Pedersen; Søren Galatius; Jan K. Madsen; Erik Jørgensen; H. Kelbaek; Eva Prescott; Henning Kelbæk; et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. European Heart Journal 2011, 33, 734-744, 10.1093/eurheartj/ehr331.
- Thomas M. Maddox; Maggie A. Stanislawski; Gary K. Grunwald; Steven M. Bradley; P. Michael Ho; Thomas T. Tsai; Manesh R. Patel; Amneet Sandhu; Javier Valle; David J. Magid; et al.Benjamin LeonDeepak L. BhattStephan D. FihnJohn S. Rumsfeld Nonobstructive coronary artery disease and risk of myocardial infarction.. JAMA 2014, 312, 1754-1763, 10.1001/jama.2014.14681.
- John, W.P.; Johnson, B.D.; Kevin, E.K.; Anderson, R.D.; Eileen, M.H.; Barry, S.; Puja, K.M.; Sheryl, F.K.; Merz, C.N.B.; Carl, J.P. TIMI frame count and adverse events in women with no obstructive coronary disease: A pilot study from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). PLoS ONE 2014, 9, e96630.
- Gulati, M.; Cooper-DeHoff, R.M.; McClure, C.; Johnson, B.D.; Shaw, L.J.; Handberg, E.M.; Zineh, I.; Kelsey, S.F.; Arnsdorf, M.F.; Black, H.R.; et al. Adverse Cardiovascular Outcomes in Women With Nonobstructive Coronary Artery Disease: A Report From the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch. Intern. Med. 2009, 169, 843–850.
- Brainin, P.; Frestad, D.; Prescott, E. The prognostic value of coronary endothelial and microvascular dysfunction in subjects with normal or non-obstructive coronary artery disease: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 254, 1–9.
- Herscovici, R.; Sedlak, T.; Wei, J.; Pepine, C.J.; Handberg, E.; Merz, C.N.B. Ischemia and No Obstructive Coronary Artery Disease (INOCA): What Is the Risk? J. Am. Heart Assoc. 2018, 7, e008868.
- Lind, L.; Berglund, L.; Larsson, A.; Sundström, J. Endothelial Function in Resistance and Conduit Arteries and 5-Year Risk of Cardiovascular Disease. Circulation 2011, 123, 1545–1551
- Lee, F.B.-K.; Lim, S.H.-S.; Fearon, P.W.; Yong, C.A.; Yamada, A.R.; Tanaka, A.S.; Lee, A.D.; Yeung, A.A.; Tremmel, A.J. Invasive Evaluation of Patients With Angina in the Absence of Obstructive Coronary Artery Disease. Circulation 2015, 131, 1054–1060.
- Khuddus, M.A.; Pepine, C.J.; Handberg, E.M.; Bairey Merz, C.N.; Sopko, G.; Bavry, A.A.; Denardo, S.J.; McGorray, S.P.; Smith, K.M.; Sharaf, B.L.; et al. An Intravascular Ultrasound Analysis in Women Experiencing Chest Pain in the Absence of Obstructive Coronary Artery Disease: A Substudy from the National Heart, Lung and Blood Institute–Sponsored Women’s Ischemia Syndrome Evaluation (WISE). J. Interv. Cardiol. 2010, 23, 511–519
- Tim P. Van De Hoef; Martijn A. Van Lavieren; Peter Damman; Ronak Delewi; Martijn A. Piek; Steven A.J. Chamuleau; Michiel Voskuil; José P.S. Henriques; Karel T. Koch; Robbert J. De Winter; et al.J. A. E. SpaanMaria SiebesJ. G. P. TijssenMartijn MeuwissenJan J. Piek Physiological Basis and Long-Term Clinical Outcome of Discordance Between Fractional Flow Reserve and Coronary Flow Velocity Reserve in Coronary Stenoses of Intermediate Severity. Circulation: Cardiovascular Interventions 2014, 7, 301-311, 10.1161/circinterventions.113.001049.
- Regina E Konst; Tomasz J Guzik; Juan Carlos Kaski; Angela H E M Maas; Suzette E Elias-Smale; The pathogenic role of coronary microvascular dysfunction in the setting of other cardiac or systemic conditions. Cardiovascular Research 2020, 116, 817-828, 10.1093/cvr/cvaa009.
- Gaetano A. Lanza; Filippo Crea; Primary Coronary Microvascular Dysfunction. Circulation 2010, 121, 2317-2325, 10.1161/circulationaha.109.900191.
- William C. Aird; Phenotypic Heterogeneity of the Endothelium. Circulation Research 2007, 100, 174-190, 10.1161/01.res.0000255690.03436.ae.
- Dirk L Brutsaert; Cardiac Endothelial-Myocardial Signaling: Its Role in Cardiac Growth, Contractile Performance, and Rhythmicity. Physiological Reviews 2003, 83, 59-115, 10.1152/physrev.00017.2002.
- Gutiérrez, E.; Flammer, A.J.; Lerman, L.O.; Elízaga, J.; Lerman, A.; Fernández-Avilés, F. Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 2013, 34, 3175–3181.
- Kinlay, S.; Libby, P.; Ganz, P. Endothelial function and coronary artery disease. Curr. Opin. Lipidol. 2001, 12, 383–389.
- Huige, L.; Ulrich, F. Prevention of Atherosclerosis by Interference with the Vascular Nitric Oxide System. Curr. Pharm. Des. 2009, 15, 3133–3145.
- Rubinshtein, R.; Yang, E.H.; Rihal, C.S.; Prasad, A.; Lennon, R.J.; Best, P.J.; Lerman, L.O.; Lerman, A. Coronary microcirculatory vasodilator function in relation to risk factors among patients without obstructive coronary disease and low to intermediate Framingham score. Eur. Heart J. 2010, 31, 936–942.
- Granger, D.N.; Rodrigues, S.F.; Yildirim, A.; Senchenkova, E.Y. Microvascular Responses to Cardiovascular Risk Factors. Microcirculation 2010, 17, 192–205.
- Wessel, T.R.; Arant, C.B.; McGorray, S.P.; Sharaf, B.L.; Reis, S.E.; Kerensky, R.A.; von Mering, G.O.; Smith, K.M.; Pauly, D.F.; Handberg, E.M.; et al. Coronary microvascular reactivity is only partially predicted by atherosclerosis risk factors or coronary artery disease in women evaluated for suspected ischemia: Results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE). Clin. Cardiol. 2007, 30, 69–74.
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175.
- Aird, C.W. Endothelium as an organ system. Crit. Care Med. 2004, 32, S271–S279.
- Tomai, F.; Ribichini, F.; Ghini, A.S.; Ferrero, V.; Andò, G.; Vassanelli, C.; Romeo, F.; Crea, F.; Chiariello, L. Elevated C-reactive protein levels and coronary microvascular dysfunction in patients with coronary artery disease. Eur. Heart J. 2005, 26, 2099–2105.
- Recio-Mayoral, A.; Rimoldi, O.E.; Camici, P.G.; Kaski, J.C. Inflammation and Microvascular Dysfunction in Cardiac Syndrome X Patients Without Conventional Risk Factors for Coronary Artery Disease. JACC Cardiovasc. Imaging 2013, 6, 660–667.
- Meng-Yu, W.; Chia-Jung, L.; Ming-Feng, H.; Pei-Yi, C. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int J. Mol. Sci. 2017, 18, 2034.
- Adukauskienė, D.; Čiginskienė, A.; Adukauskaitė, A.; Pentiokinienė, D.; Šlapikas, R.; Čeponienė, I. Clinical relevance of high sensitivity C-reactive protein in cardiology. Medicina 2016, 52, 1–10.
- Singh, T.P.; Morris, D.R.; Smith, S.; Moxon, J.V.; Golledge, J. Systematic Review and Meta-Analysis of the Association Between C-Reactive Protein and Major Cardiovascular Events in Patients with Peripheral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 220–233.
- Twine, C.P. The Relationship Between CRP and MACE: Controversial and Confounded. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 234.
- Kwak, B.R.; Bäck, M.; Bochaton-Piallat, M.-L.; Caligiuri, G.; Daemen, M.J.A.P.; Davies, P.F.; Hoefer, I.E.; Holvoet, P.; Jo, H.; Krams, R.; et al. Biomechanical factors in atherosclerosis: Mechanisms and clinical implications†. Eur. Heart J. 2014, 35, 3013–3020.
- Helderman, F.; Segers, D.; de Crom, R.; Hierck, B.P.; Poelmann, R.E.; Evans, P.C.; Krams, R. Effect of shear stress on vascular inflammation and plaque development. Curr. Opin. Lipidol. 2007, 18, 527–533.
- Michail I. Papafaklis Md; Saeko Takahashi; Antonios P. Antoniadis; Ahmet U. Coskun; Masaya Tsuda; Shingo Mizuno; Ioannis Andreou; Shigeru Nakamura; Yasuhiro Makita; Atsushi Hirohata; et al.Shigeru SaitoCharles L. FeldmanPeter H. Stone Effect of the local hemodynamic environment on the de novo development and progression of eccentric coronary atherosclerosis in humans: Insights from PREDICTION. Atherosclerosis 2015, 240, 205-211, 10.1016/j.atherosclerosis.2015.03.017.
- Tesauro, M.; Mauriello, A.; Rovella, V.; Annicchiarico-Petruzzelli, M.; Cardillo, C.; Melino, G.; Di Daniele, N. Arterial ageing: From endothelial dysfunction to vascular calcification. J. Intern. Med. 2017, 281, 471–482.
- Stone, P.H.; Maehara, A.; Coskun, A.U.; Maynard, C.C.; Zaromytidou, M.; Siasos, G.; Andreou, I.; Fotiadis, D.; Stefanou, K.; Papafaklis, M.; et al. Role of Low Endothelial Shear Stress and Plaque Characteristics in the Prediction of Nonculprit Major Adverse Cardiac Events: The PROSPECT Study. JACC Cardiovasc. Imaging 2018, 11, 462–471.
- Viviany R. Taqueti; Marcelo F. Di Carli; Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options. Journal of the American College of Cardiology 2018, 72, 2625-2641, 10.1016/j.jacc.2018.09.042.
- Peter Ong; Anastasios Athanasiadis; Gabor Borgulya; Heiko Mahrholdt; Juan Carlos Kaski; Udo Sechtem; High Prevalence of a Pathological Response to Acetylcholine Testing in Patients With Stable Angina Pectoris and Unobstructed Coronary Arteries. Journal of the American College of Cardiology 2012, 59, 655-662, 10.1016/j.jacc.2011.11.015.
- Goligorsky, M.S. Microvascular rarefaction. Organogenesis 2010, 6, 1–10.
- O’Riordan, E.; Mendelev, N.; Patschan, S.; Patschan, D.; Eskander, J.; Cohen-Gould, L.; Chander, P.; Goligorsky, M.S. Chronic NOS inhibition actuates endothelial-mesenchymal transformation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H285–H294.
- Vita, J.A. Endothelial Function. Circulation 2011, 124, e906–e912.
- Huang, A.L.; Vita, J.A. Effects of Systemic Inflammation on Endothelium-Dependent Vasodilation. Trends Cardiovasc. Med. 2006, 16, 15–20
- Zannad, F.; Radauceanu, A. Effect of MR Blockade on Collagen Formation and Cardiovascular Disease with a Specific Emphasis on Heart Failure. Heart Fail. Rev. 2005, 10, 71–78.
- González, A.; Ravassa, S.; Beaumont, J.; López, B.; Díez, J. New Targets to Treat the Structural Remodeling of the Myocardium. J. Am. Coll. Cardiol. 2011, 58, 1833–1843.
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; Schlippenbach, J.v.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac Inflammation Contributes to Changes in the Extracellular Matrix in Patients With Heart Failure and Normal Ejection Fraction. Circ. Heart Fail. 2011, 4, 44–52.
- Lindemann, H.; Petrovic, I.; Hill, S.; Athanasiadis, A.; Mahrholdt, H.; Schaufele, T.; Klingel, K.; Sechtem, U.; Ong, P. Biopsy-confirmed endothelial cell activation in patients with coronary microvascular dysfunction. Coron. Artery Dis. 2018, 29, 216–222.
- Suzuki, H.; Takeyama, Y.; Koba, S.; Suwa, Y.; Katagiri, T. Small vessel pathology and coronary hemodynamics in patients with microvascular angina. Int. J. Cardiol. 1994, 43, 139–150.
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary Microvascular Rarefaction and Myocardial Fibrosis in Heart Failure With Preserved Ejection Fraction. Circulation 2015, 131, 550–559.
- Tsagalou, E.P.; Anastasiou-Nana, M.; Agapitos, E.; Gika, A.; Drakos, S.G.; Terrovitis, J.V.; Ntalianis, A.; Nanas, J.N. Depressed Coronary Flow Reserve Is Associated With Decreased Myocardial Capillary Density in Patients With Heart Failure Due to Idiopathic Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2008, 52, 1391–1398.
- Escaned, J.; Flores, A.; García-Pavía, P.; Segovia, J.; Jimenez, J.; Aragoncillo, P.; Salas, C.; Alfonso, F.; Hernández, R.; Angiolillo, D.J.; et al. Assessment of Microcirculatory Remodeling With Intracoronary Flow Velocity and Pressure Measurements. Circulation 2009, 120, 1561–1568.
- Hong, H.; Aksenov, S.; Guan, X.; Fallon, J.T.; Waters, D.; Chen, C. Remodeling of Small Intramyocardial Coronary Arteries Distal to a Severe Epicardial Coronary Artery Stenosis. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 2059–2065.
- Heusch, G.; Skyschally, A.; Kleinbongard, P. Coronary microembolization and microvascular dysfunction. Int. J. Cardiol. 2018, 258, 17–23.
- Kleinbongard, P.; Konorza, T.; Böse, D.; Baars, T.; Haude, M.; Erbel, R.; Heusch, G. Lessons from human coronary aspirate. J. Mol. Cell. Cardiol. 2012, 52, 890–896.
- Dirk J. Duncker; Akos Koller; Daphne Merkus; John M. Canty; Regulation of coronary blood flow in health and ischemic heart disease.. Progress in Cardiovascular Diseases 2014, 57, 409-422, 10.1016/j.pcad.2014.12.002.
- Dirk J. Duncker; Robert J. Bache; Daphne Merkus; Regulation of coronary resistance vessel tone in response to exercise. Journal of Molecular and Cellular Cardiology 2012, 52, 802-813, 10.1016/j.yjmcc.2011.10.007.
- Heusch, G.; Baumgart, D.; Camici, P.; Chilian, W.; Gregorini, L.; Hess, O.; Indolfi, C.; Rimoldi, O. α-Adrenergic Coronary Vasoconstriction and Myocardial Ischemia in Humans. Circulation 2000, 101, 689–694.
- Heusch, G. The paradox of α-adrenergic coronary vasoconstriction revisited. J. Mol. Cell. Cardiol. 2011, 51, 16–23.
- Levy, B.I.; Heusch, G.; Camici, P.G. The many faces of myocardial ischaemia and angina. Cardiovasc. Res. 2019, 115, 1460–1470.
- Merz, C.N.B.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L.; Camici, P.G.; Chilian, W.M.; Clayton, J.A.; Cooper, L.S.; Crea, F.; Carli, M.D.; et al. Ischemia and No Obstructive Coronary Artery Disease (INOCA). Circulation 2017, 135, 1075–1092.
- Peter Ong; Paolo G. Camici; John F. Beltrame; Filippo Crea; Hiroaki Shimokawa; Udo Sechtem; Juan Carlos Kaski; Noel Bairey Merz; International standardization of diagnostic criteria for microvascular angina. International Journal of Cardiology 2018, 250, 16-20, 10.1016/j.ijcard.2017.08.068.
- Vijay Kunadian; Alaide Chieffo; Paolo G Camici; Colin Berry; Javier Escaned; Angela H E M Maas; Eva Prescott; Nicole Karam; Yolande Appelman; Chiara Fraccaro; et al.Gill Louise BuchananStephane Manzo-SilbermanRasha Al-LameeEvelyn RegarAlexandra LanskyJ Dawn AbbottLina BadimonDirk J DunckerRoxana MehranDavide CapodannoAndreas Baumbach OUP accepted manuscript. European Heart Journal 2020, null, null, 10.1093/eurheartj/ehaa503.
- Ford, T.J.; Berry, C. How to Diagnose and Manage Angina Without Obstructive Coronary Artery Disease: Lessons from the British Heart Foundation CorMicA Trial. Interv. Cardiol. 2019, 14, 76–82.
- Ford, T.J.; Yii, E.; Sidik, N.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; et al. Ischemia and No Obstructive Coronary Artery Disease. Circ. Cardiovasc. Interv. 2019, 12, e008126.
- Kristian Thygesen; Joseph S. Alpert; Allan S Jaffe; Bernard R Chaitman; Jeroen J Bax; David A Morrow; Harvey D. White; Hans Mickley; Filippo Crea; Frans Van De Werf; et al.Chiara Bucciarelli-DucciHugo A KatusFausto PintoElliott M AntmanChristian W HammRaffaele De CaterinaJames L. JanuzziFred S AppleMaria Angeles Alonso GarciaS Richard UnderwoodJohn M CantyAlexander R LyonP J DevereauxJose Luis ZamoranoBertil LindahlWilliam S WeintraubL Kristin NewbyRenu VirmaniPascal VranckxDon CutlipRaymond J GibbonsSidney C SmithDan AtarRussell V LuepkerRose Marie RobertsonRobert O BonowP Gabriel StegPatrick T O’GaraKeith A A FoxDavid HasdaiVictor AboyansStephan AchenbachStefan AgewallThomas AlexanderAlvaro AvezumEmanuele BarbatoJean-Pierre BassandEric BatesJohn A. BittlGüenter BreithardtHéctor BuenoRaffaele BugiardiniMauricio G. CohenGeorge DangasJames A De LemosVictoria DelgadoGerasimos FilippatosEdward FryChristopher B GrangerSigrun HalvorsenMark A HlatkyBorja IbáñezStefan JamesAdnan KastratiChristophe LeclercqKenneth W MahaffeyLaxmi MehtaChristian MüllerCarlo PatronoMassimo Francesco PiepoliDaniel PiñeiroMarco RoffiAndrea RubboliSamin SharmaIain A SimpsonMichael TenderaMarco ValgimigliAllard C Van Der WalStephan WindeckerMohamed ChettibiHamlet HayrapetyanFranz Xaver RoithingerFarid AliyevVolha SujayevaMarc ClaeysElnur SmajićPetr KalaKasper Karmak IversenEhab El HefnyToomas MarandiPekka PorelaSlobodan AntovMartine GilardStefan BlankenbergPeriklis DavlourosThorarinn GudnasonRonny AlcalaiFurio ColivicchiShpend EleziGulmira BaitovaIlja ZakkeOlivija GustieneJean BeisselPhilip DingliAurel GrosuPeter DammanVibeke JuliebøJacek LegutkoJoäo MoraisGabriel Tatu-ChitoiuAlexey YakovlevMarco ZavattaMilan NedeljkovicPeter RadselAlessandro SionisTomas JembergLeila AbidAdnan AbaciAlexandr ParkhomenkoSimon CorbettEsc Scientific Document Group Fourth universal definition of myocardial infarction (2018). European Heart Journal 2018, 40, 237-269, 10.1093/eurheartj/ehy462.
- Stefan Agewall; John F. Beltrame; Harmony R. Reynolds; Alexander Niessner; Giuseppe Rosano; Alida L. P. Caforio; Raffaele De Caterina; Marco Zimarino; Marco Roffi; Keld Kjeldsen; et al.Dan AtarJuan C. KaskiUdo SechtemPer Tornvall ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. European Heart Journal 2016, 38, 143–153, 10.1093/eurheartj/ehw149.
- Scalone, G.; Niccoli, G.; Crea, F. Editor’s Choice- Pathophysiology, diagnosis and management of MINOCA: An update. Eur. Heart J. Acute Cardiovasc. Care 2018, 8, 54–62.
- Niccoli, G.; Camici, P.G. Myocardial infarction with non-obstructive coronary arteries: What is the prognosis? Eur. Heart J. Suppl. 2020, 22, E40–E45.
- Pasupathy, S.; Tavella, R.; Beltrame, J.F. Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA). Circulation 2017, 135, 1490–1493.
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2016, 37, 1024–1033.
- Collste, O.; Sörensson, P.; Frick, M.; Agewall, S.; Daniel, M.; Henareh, L.; Ekenbäck, C.; Eurenius, L.; Guiron, C.; Jernberg, T.; et al. Myocardial infarction with normal coronary arteries is common and associated with normal findings on cardiovascular magnetic resonance imaging: Results from the Stockholm Myocardial Infarction with Normal Coronaries study. J. Intern. Med. 2013, 273, 189–196.
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic Review of Patients Presenting With Suspected Myocardial Infarction and Nonobstructive Coronary Arteries. Circulation 2015, 131, 861–870.
- Pacheco Claudio, C.; Quesada, O.; Pepine, C.J.; Noel Bairey Merz, C. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease). Clin. Cardiol. 2018, 41, 185–193.
- Rocco A. Montone; Giampaolo Niccoli; Francesco Fracassi; Michele Russo; Filippo Gurgoglione; Giulia Cammà; Gaetano A Lanza; Filippo Crea; Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. European Heart Journal 2017, 39, 91–98, 10.1093/eurheartj/ehx667.
- Asha M. Mahajan; Himali Gandhi; Nathaniel R. Smilowitz; Matthew T Roe; Anne S. Hellkamp; Karen Chiswell; Martha Gulati; Harmony R. Reynolds; Seasonal and circadian patterns of myocardial infarction by coronary artery disease status and sex in the ACTION Registry-GWTG.. International Journal of Cardiology 2018, 274, 16-20, 10.1016/j.ijcard.2018.08.103.
- Ahmed Aziz; Henrik Steen Hansen; Udo Sechtem; Eva Prescott; Peter Ong; Sex-Related Differences in Vasomotor Function in Patients With Angina and Unobstructed Coronary Arteries. Journal of the American College of Cardiology 2017, 70, 2349-2358, 10.1016/j.jacc.2017.09.016.
- Carl J. Pepine; Keith C. Ferdinand; Leslee J. Shaw; Kelly Ann Light-McGroary; Rashmee U. Shah; Martha Gulati; Claire Duvernoy; Mary Norine Walsh; Noel Bairey Merz; ACC CVD in Women Committee; et al. Emergence of Nonobstructive Coronary Artery Disease. Journal of the American College of Cardiology 2015, 66, 1918-1933, 10.1016/j.jacc.2015.08.876.
- Taqueti, V.R.; Shaw, L.J.; Cook, N.R.; Murthy, V.L.; Shah, N.R.; Foster, C.R.; Hainer, J.; Blankstein, R.; Dorbala, S.; Carli, M.F.D. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation 2017, 135, 566–577.
- Smilowitz, N.R.; Sampson, B.A.; Abrecht, C.R.; Siegfried, J.S.; Hochman, J.S.; Reynolds, H.R. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: An autopsy study. Am. Heart J. 2011, 161, 681–688
- Waheed, N.; Elias-Smale, S.; Malas, W.; Maas, A.H.; Sedlak, T.L.; Tremmel, J.; Mehta, P.K. Sex differences in non-obstructive coronary artery disease. Cardiovasc. Res. 2020, 116, 829–840.
- Murthy, V.L.; Naya, M.; Foster, C.R.; Gaber, M.; Hainer, J.; Klein, J.; Dorbala, S.; Blankstein, R.; Carli, M.F.D. Association Between Coronary Vascular Dysfunction and Cardiac Mortality in Patients With and Without Diabetes Mellitus. Circulation 2012, 126, 1858–1868
- Jacqueline E. Tamis-Holland; Hani Jneid; Harmony R. Reynolds; Stefan Agewall; Emmanouil S. Brilakis; Todd M. Brown; Amir Lerman; Mary Cushman; Dharam J. Kumbhani; Cynthia Arslanian-Engoren; et al.Ann F. BolgerJohn F. BeltrameAmerican Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical CardiologyCouncil on Cardiovascular and Stroke NursingCouncil on Epidemiology and Preventionand Council on Quality of Care and Outcomes ResearchOn behalf of the American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical CardiologyCouncil on Cardiovascular and Stroke NursingCouncil on Epidemiology and Preventionand Council on Quality of Care and Outcom Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association.. Circulation 2019, 139, e891-e908, 10.1161/CIR.0000000000000670.
- Elif Tunc; Alicia Arredondo Eve; Zeynep Madak-Erdogan; Coronary Microvascular Dysfunction and Estrogen Receptor Signaling. Trends in Endocrinology & Metabolism 2020, 31, 228-238, 10.1016/j.tem.2019.11.001.
- Filippo Crea; Paolo G. Camici; Cathleen Noel Bairey Merz; Coronary microvascular dysfunction: an update.. European Heart Journal 2013, 35, 1101-1111, 10.1093/eurheartj/eht513.