
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zaleha Abdullah Mahdy | + 2465 word(s) | 2465 | 2022-02-14 04:04:54 | | | |
| 2 | Zaleha Abdullah Mahdy | Meta information modification | 2465 | 2022-02-17 15:47:16 | | | | |
| 3 | Bruce Ren | Meta information modification | 2465 | 2022-02-18 02:25:24 | | |
Video Upload Options
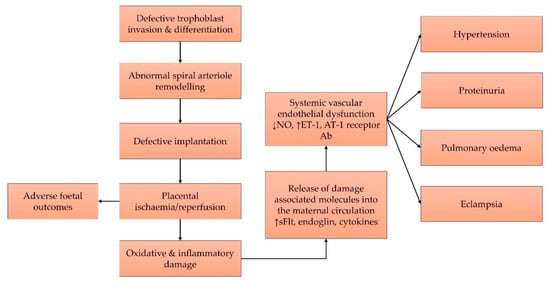
The pathophysiology of pre-eclampsia involves two major pathways, namely systemic oxidative stress and subsequent generalised inflammatory response, which eventually culminates in endothelial cell injury and the syndrome of pre-eclampsia with multi-organ dysfunction. Aspirin has been used to reduce the risk of pre-eclampsia, but it only possesses anti-inflammatory properties without any antioxidant effect. Hence, it can only partially alleviate the problem. Tocotrienols are a unique form of vitamin E with strong antioxidant and anti-inflammatory properties that can be exploited as a preventive agent for pre-eclampsia. Many preclinical models showed that tocotrienol can also prevent hypertension and ischaemic/reperfusion injury, which are the two main features in pre-eclampsia.
1. Introduction
2. Current Concept on the Pathophysiology of Pre-Eclampsia
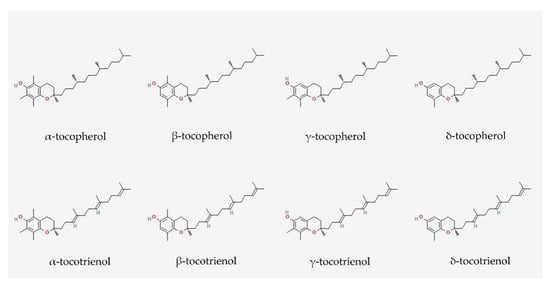
3. Vitamin E: Tocopherol and Tocotrienol
4. Antioxidant Effects of Tocotrienol
5. Anti-Inflammatory Effects of Tocotrienol
References
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43.
- Say, L.; Chou, D.; Gemmill, A.; Tuncalp, O.; Moller, A.B.; Daniels, J.; Gulmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333.
- Wang, W.; Xie, X.; Yuan, T.; Wang, Y.; Zhao, F.; Zhou, Z.; Zhang, H. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: A population-based study. BMC Pregnancy Childbirth 2021, 21, 364.
- Bartsch, E.; Medcalf, K.E.; Park, A.L.; Ray, J.G.; High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016, 353, i1753.
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112.
- Ronsmans, C.; Campbell, O. Quantifying the fall in mortality associated with interventions related to hypertensive diseases of pregnancy. BMC Public Health 2011, 11 (Suppl. 3), S8.
- U. S. Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Kubik, M.; et al. Aspirin Use to Prevent Preeclampsia and Related Morbidity and Mortality: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 326, 1186–1191.
- Van Doorn, R.; Mukhtarova, N.; Flyke, I.P.; Lasarev, M.; Kim, K.; Hennekens, C.H.; Hoppe, K.K. Dose of aspirin to prevent preterm preeclampsia in women with moderate or high-risk factors: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0247782.
- American College of Obstetricians and Gynecologists ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstet. Gynecol. 2018, 132, e44–e52.
- Atallah, A.; Lecarpentier, E.; Goffinet, F.; Doret-Dion, M.; Gaucherand, P.; Tsatsaris, V. Aspirin for Prevention of Preeclampsia. Drugs 2017, 77, 1819–1831.
- Hofmeyr, G.J.; Lawrie, T.A.; Atallah, A.N.; Torloni, M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 2018, 10, CD001059.
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289.
- Cohen, J.M.; Kramer, M.S.; Platt, R.W.; Basso, O.; Evans, R.W.; Kahn, S.R. The association between maternal antioxidant levels in midpregnancy and preeclampsia. Am. J. Obstet. Gynecol. 2015, 213, 695.e1–695.e13.
- Taravati, A.; Tohidi, F. Comprehensive analysis of oxidative stress markers and antioxidants status in preeclampsia. Taiwan J. Obstet. Gynecol. 2018, 57, 779–790.
- Raijmakers, M.T.; Dechend, R.; Poston, L. Oxidative stress and preeclampsia: Rationale for antioxidant clinical trials. Hypertension 2004, 44, 374–380.
- Marin, R.; Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Sobrevia, L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165961.
- Hobson, S.R.; Gurusinghe, S.; Lim, R.; Alers, N.O.; Miller, S.L.; Kingdom, J.C.; Wallace, E.M. Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early-onset preeclampsia. J. Pineal Res. 2018, 65, e12508.
- Chuffa, L.G.A.; Lupi, L.A.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Seiva, F.R.F. Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms. Int. J. Mol. Sci. 2019, 21, 300.
- Filardi, T.; Varì, R.; Ferretti, E.; Zicari, A.; Morano, S.; Santangelo, C. Curcumin: Could This Compound Be Useful in Pregnancy and Pregnancy-Related Complications? Nutrients 2020, 12, 3179.
- Salles, A.M.; Galvao, T.F.; Silva, M.T.; Motta, L.C.; Pereira, M.G. Antioxidants for preventing preeclampsia: A systematic review. Sci. World J. 2012, 2012, 243476.
- Tenorio, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 865–876.
- Conde-Agudelo, A.; Romero, R.; Kusanovic, J.P.; Hassan, S.S. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2011, 204, 503.e1–503.e12.
- Aminuddin, N.A.; Sutan, R.; Mahdy, Z.A. Role of Palm Oil Vitamin E in Preventing Pre-eclampsia: A Secondary Analysis of a Randomized Clinical Trial Following ISSHP Reclassification. Front. Med. 2020, 7, 596405.
- Tenorio, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; de Oliveira, A.C.M.; Goulart, M.O.F. Cross-Talk between Oxidative Stress and Inflammation in Preeclampsia. Oxidative Med. Cell. Longev. 2019, 2019, 8238727.
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113.
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vazquez, C.M.; Mate, A.; Sobrevia, L.; Marin, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165354.
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496.
- Jena, M.K.; Sharma, N.R.; Petitt, M.; Maulik, D.; Nayak, N.R. Pathogenesis of Preeclampsia and Therapeutic Approaches Targeting the Placenta. Biomolecules 2020, 10, 953.
- Cindrova-Davies, T.; Sanders, D.A.; Burton, G.J.; Charnock-Jones, D.S. Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc. Res. 2011, 89, 671–679.
- Chen, B.; Longtine, M.S.; Sadovsky, Y.; Nelson, D.M. Hypoxia downregulates p53 but induces apoptosis and enhances expression of BAD in cultures of human syncytiotrophoblasts. Am. J. Physiol. Cell Physiol. 2010, 299, C968–C976.
- Ishioka, S.; Ezaka, Y.; Umemura, K.; Hayashi, T.; Endo, T.; Saito, T. Proteomic analysis of mechanisms of hypoxia-induced apoptosis in trophoblastic cells. Int. J. Med. Sci. 2006, 4, 36–44.
- Zhuang, B.; Luo, X.; Rao, H.; Li, Q.; Shan, N.; Liu, X.; Qi, H. Oxidative stress-induced C/EBPbeta inhibits beta-catenin signaling molecule involving in the pathology of preeclampsia. Placenta 2015, 36, 839–846.
- Yang, F.; Zheng, Q.; Jin, L. Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2317.
- Aggarwal, R.; Jain, A.K.; Mittal, P.; Kohli, M.; Jawanjal, P.; Rath, G. Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 2019, 33, e22834.
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-alpha in vascular dysfunction. Clin. Sci. 2009, 116, 219–230.
- Xia, Y.; Kellems, R.E. Angiotensin receptor agonistic autoantibodies and hypertension: Preeclampsia and beyond. Circ. Res. 2013, 113, 78–87.
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90.
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharm. 2010, 80, 1613–1631.
- Wong, S.K.; Kamisah, Y.; Mohamed, N.; Muhammad, N.; Masbah, N.; Fahami, N.A.M.; Mohamed, I.N.; Shuid, A.N.; Saad, Q.M.; Abdullah, A.; et al. Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence. Nutrients 2020, 12, 259.
- Chin, K.Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Dev. Ther. 2015, 9, 2049–2061.
- Chin, K.Y.; Ima-Nirwana, S. The effects of alpha-tocopherol on bone: A double-edged sword? Nutrients 2014, 6, 1424–1441.
- Hosomi, A.; Arita, M.; Sato, Y.; Kiyose, C.; Ueda, T.; Igarashi, O.; Arai, H.; Inoue, K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997, 409, 105–108.
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The Effects of Tocotrienol on Bone Peptides in a Rat Model of Osteoporosis Induced by Metabolic Syndrome: The Possible Communication between Bone Cells. Int. J. Environ. Res. Public Health 2019, 16, 3313.
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The effects of palm tocotrienol on metabolic syndrome and bone loss in male rats induced by high-carbohydrate high-fat diet. J. Funct. Foods 2018, 44, 246–254.
- Hidalgo, M.; Rodriguez, V.; Kreindl, C.; Porras, O. Biological Redox Impact of Tocopherol Isomers Is Mediated by Fast Cytosolic Calcium Increases in Living Caco-2 Cells. Antioxidants 2020, 9, 155.
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275.
- Jiang, Q. Metabolism of natural forms of vitamin E and biological actions of vitamin E metabolites. Free Radic. Biol. Med. 2021, 179, 375–387.
- Pein, H.; Ville, A.; Pace, S.; Temml, V.; Garscha, U.; Raasch, M.; Alsabil, K.; Viault, G.; Dinh, C.P.; Guilet, D.; et al. Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase. Nat. Commun. 2018, 9, 3834.
- Qureshi, A.A.; Reis, J.C.; Papasian, C.J.; Morrison, D.C.; Qureshi, N. Tocotrienols inhibit lipopolysaccharide-induced pro-inflammatory cytokines in macrophages of female mice. Lipids Health Dis. 2010, 9, 143.
- Emami, M.R.; Safabakhsh, M.; Alizadeh, S.; Asbaghi, O.; Khosroshahi, M.Z. Effect of vitamin E supplementation on blood pressure: A systematic review and meta-analysis. J. Hum. Hypertens. 2019, 33, 499–507.
- Rumbold, A.; Ota, E.; Hori, H.; Miyazaki, C.; Crowther, C.A. Vitamin E supplementation in pregnancy. Cochrane Database Syst. Rev. 2015, 18, CD004069.
- Pang, K.L.; Chin, K.Y. The Role of Tocotrienol in Protecting Against Metabolic Diseases. Molecules 2019, 24, 923.
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. Vitamin E As a Potential Interventional Treatment for Metabolic Syndrome: Evidence from Animal and Human Studies. Front. Pharm. 2017, 8, 444.
- Niki, E.; Abe, K. Chapter 1: Vitamin E: Structure, Properties and Functions. In Vitamin E: Chemistry and Nutritional Benefits; Niki, E., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 1–11.
- Suzuki, Y.J.; Tsuchiya, M.; Wassall, S.R.; Choo, Y.M.; Govil, G.; Kagan, V.E.; Packer, L. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: Implication to the molecular mechanism of their antioxidant potency. Biochemistry 1993, 32, 10692–10699.
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733.
- Atia, A.; Alrawaiq, N.S.; Abdullah, A. Tocotrienols Activate Nrf2 Nuclear Translocation and Increase the Antioxidant- Related Hepatoprotective Mechanism in Mice Liver. Curr. Pharm. Biotechnol. 2021, 22, 1085–1098.
- Takano, H.; Momota, Y.; Kani, K.; Aota, K.; Yamamura, Y.; Yamanoi, T.; Azuma, M. gamma-Tocotrienol prevents 5-FU-induced reactive oxygen species production in human oral keratinocytes through the stabilization of 5-FU-induced activation of Nrf2. Int. J. Oncol. 2015, 46, 1453–1460.
- Casati, L.; Pagani, F.; Limonta, P.; Vanetti, C.; Stancari, G.; Sibilia, V. Beneficial effects of delta-tocotrienol against oxidative stress in osteoblastic cells: Studies on the mechanisms of action. Eur. J. Nutr. 2020, 59, 1975–1987.
- Chin, K.Y.; Ima-Nirwana, S. Vitamin E as an Antiosteoporotic Agent via Receptor Activator of Nuclear Factor Kappa-B Ligand Signaling Disruption: Current Evidence and Other Potential Research Areas. Evid. Based Complement. Altern. Med. 2012, 2012, 747020.
- Chin, K.Y.; Mo, H.; Soelaiman, I.N. A review of the possible mechanisms of action of tocotrienol—A potential antiosteoporotic agent. Curr. Drug Targets 2013, 14, 1533–1541.
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Therapeutic potential of annatto tocotrienol with self-emulsifying drug delivery system in a rat model of postmenopausal bone loss. Biomed. Pharm. 2021, 137, 111368.
- Nur Azlina, M.F.; Kamisah, Y.; Chua, K.H.; Ibrahim, I.A.; Qodriyah, H.M. Preventive Effects of Tocotrienol on Stress-Induced Gastric Mucosal Lesions and Its Relation to Oxidative and Inflammatory Biomarkers. PLoS ONE 2015, 10, e0139348.
- Nafeeza, M.I.; Norzana, A.G.; Jalaluddin, H.L.; Gapor, M.T. The effects of a tocotrienol-rich fraction on experimentally induced atherosclerosis in the aorta of rabbits. Malays. J. Pathol. 2001, 23, 17–25.
- Newaz, M.A.; Nawal, N.N. Effect of gamma-tocotrienol on blood pressure, lipid peroxidation and total antioxidant status in spontaneously hypertensive rats (SHR). Clin. Exp. Hypertens. 1999, 21, 1297–1313.
- Goon, J.A.; Nor Azman, N.H.E.; Abdul Ghani, S.M.; Hamid, Z.; Wan Ngah, W.Z. Comparing palm oil tocotrienol rich fraction with alpha-tocopherol supplementation on oxidative stress in healthy older adults. Clin. Nutr. ESPEN 2017, 21, 1–12.
- Khor, B.H.; Tiong, H.C.; Tan, S.C.; Wong, S.K.; Chin, K.Y.; Karupaiah, T.; Ima-Nirwana, S.; Abdul Gafor, A.H. Effects of tocotrienols supplementation on markers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2021, 16, e0255205.
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basilio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-kappaB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85.
- Yang, C.; Jiang, Q. Vitamin E delta-tocotrienol inhibits TNF-alpha-stimulated NF-kappaB activation by up-regulation of anti-inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides. J. Nutr. Biochem. 2019, 64, 101–109.
- Wang, Y.; Jiang, Q. gamma-Tocotrienol inhibits lipopolysaccharide-induced interlukin-6 and granulocyte colony-stimulating factor by suppressing C/EBPbeta and NF-kappaB in macrophages. J. Nutr. Biochem. 2013, 24, 1146–1152.
- Shembade, N.; Harhaj, E.W. Regulation of NF-kappaB signaling by the A20 deubiquitinase. Cell. Mol. Immunol. 2012, 9, 123–130.
- Ahn, K.S.; Sethi, G.; Krishnan, K.; Aggarwal, B.B. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J. Biol. Chem. 2007, 282, 809–820.
- Newton, R.; Kuitert, L.M.; Bergmann, M.; Adcock, I.M.; Barnes, P.J. Evidence for involvement of NF-kappaB in the transcriptional control of COX-2 gene expression by IL-1beta. Biochem. Biophys. Res. Commun. 1997, 237, 28–32.
- Tan, S.W.; Israf Ali, D.A.B.; Khaza’ai, H.; Wong, J.W.; Vidyadaran, S. Cellular uptake and anti-inflammatory effects of palm oil-derived delta (delta)-tocotrienol in microglia. Cell. Immunol. 2020, 357, 104200.
- Zhang, Y.H.; Ma, K.; Liu, J.R.; Wang, H.X.; Tian, W.X.; Tu, Y.H.; Sun, W.G. gamma-tocotrienol inhibits the invasion and migration of human gastric cancer cells through downregulation of cyclooxygenase-2 expression. Oncol. Rep. 2018, 40, 999–1007.
- Yam, M.L.; Abdul Hafid, S.R.; Cheng, H.M.; Nesaretnam, K. Tocotrienols suppress proinflammatory markers and cyclooxygenase-2 expression in RAW264.7 macrophages. Lipids 2009, 44, 787–797.
- Wang, Y.; Moreland, M.; Wagner, J.G.; Ames, B.N.; Illek, B.; Peden, D.B.; Jiang, Q. Vitamin E forms inhibit IL-13/STAT6-induced eotaxin-3 secretion by up-regulation of PAR4, an endogenous inhibitor of atypical PKC in human lung epithelial cells. J. Nutr. Biochem. 2012, 23, 602–608.
- Nafeeza, M.I.; Fauzee, A.M.; Kamsiah, J.; Gapor, M.T. Comparative effects of a tocotrienol-rich fraction and tocopherol in aspirin-induced gastric lesions in rats. Asia Pac. J. Clin. Nutr. 2002, 11, 309–313.
- Azlina, M.F.; Nafeeza, M.I.; Khalid, B.A. A comparison between tocopherol and tocotrienol effects on gastric parameters in rats exposed to stress. Asia Pac. J. Clin. Nutr. 2005, 14, 358–365.
- Nur Azlina, M.F.; Kamisah, Y.; Chua, K.H.; Qodriyah, H.M. Tocotrienol Attenuates Stress-Induced Gastric Lesions via Activation of Prostaglandin and Upregulation of COX-1 mRNA. Evid. Based Complement. Altern. Med. 2013, 2013, 804796.
- Jiang, Q.; Moreland, M.; Ames, B.N.; Yin, X. A combination of aspirin and gamma-tocopherol is superior to that of aspirin and alpha-tocopherol in anti-inflammatory action and attenuation of aspirin-induced adverse effects. J. Nutr. Biochem. 2009, 20, 894–900.




