Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sumith Ranil Wickramasinghe | + 1979 word(s) | 1979 | 2022-01-04 07:45:51 | | | |
| 2 | Catherine Yang | Meta information modification | 1979 | 2022-02-17 03:57:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wickramasinghe, S. Removal Mechanisms for Emerging Contaminants. Encyclopedia. Available online: https://encyclopedia.pub/entry/19545 (accessed on 07 February 2026).
Wickramasinghe S. Removal Mechanisms for Emerging Contaminants. Encyclopedia. Available at: https://encyclopedia.pub/entry/19545. Accessed February 07, 2026.
Wickramasinghe, Sumith. "Removal Mechanisms for Emerging Contaminants" Encyclopedia, https://encyclopedia.pub/entry/19545 (accessed February 07, 2026).
Wickramasinghe, S. (2022, February 16). Removal Mechanisms for Emerging Contaminants. In Encyclopedia. https://encyclopedia.pub/entry/19545
Wickramasinghe, Sumith. "Removal Mechanisms for Emerging Contaminants." Encyclopedia. Web. 16 February, 2022.
Copy Citation
Emerging contaminants (ECs) can refer to many types of chemicals such as endocrine disrupting compounds (EDCs), fire retardants, therapeutics, personal care or household cleaning products, lawn care and agricultural products. These compounds can bioaccumulate in the food web and can adversely affect human health and the environment.
adsorption
emerging contaminants
1. Biodegradation
Biodegradation is known as a green technique to control the exposure of ECs [1]. However, the nature of ECs plays a significant role in ascertaining its complete biodegradability. This can be explained in terms of the rate constants of the biodegradation of the corresponding materials. Caffeine, acetaminophen, estradiol, and ibuprofen are some of the examples of ECs, which possess high biodegradation rate constants and hence can be degraded easily into the corresponding elemental precursors losing their bioactivity [2]. However, tetracycline, carbamazepine, and iopamidol possess very low biodegradation constants, thereby leading to incomplete or slow degradation of such compounds [3].
The factors influencing the degradation are redox potential, structural features, microbial diversity, temperature, pH, toxicity of the ECs and primary substrates [4]. Generally, biodegradation/bioremediation of these hazardous materials proceeds through highly specific enzymes [5]. The catalytic amount of such bioactive enzymes is very specific and efficient for transformation of these hazardous materials into their non-active precursors and this transformation can be viable for commercial adaptation, which is known as one of the ‘green’ bioremediations. These enzymes are mostly from oxidoreductase families. Some of the important enzymes are as follows [6]:
-
Lignin peroxidase (1,2-bis(3,4-dimethoxyphenyl) propane-1,3-diol;
-
Manganese peroxidase (Mn (II): hydrogen-peroxide oxidoreductase;
-
Laccases;
-
Tyrosinases: o-diphenol;
-
Horseradish peroxidase.
1.1. Lignin Peroxidase
The oxidative cleavage of the lignin bond in the presence of hydrogen peroxide is the main chemical reaction associated with this enzyme [7]. A wide range of phenolic as well as non-phenolic substances were found to be cleaved by this highly efficient relatively non-specific enzyme [8]. Phanerochaete chrysosporium fungus was the first source of this enzyme however, today it is found to be present in a variety of microorganism including basidiomycetes [9]. Attachment through covalent bond formation, physical entrapment in porous matrices, physisorption/chemisorption and cross linking are some of the modes of immobilization of this enzyme on solid inactive surfaces for biocatalysts [10].
Oliveira et al. [11] and Ran et al. [12] have demonstrated the immobilization of lignin peroxidase through covalent bonding on carbon nanotubes and chitosan with a degradation efficiency of more than 50% and 80%, respectively. Chitosan beads were found to be very good crosslinking support for immobilization of lignin peroxidase obtained from S. commune used for the degradation of sandal fix and dyes with efficiencies in the range of ~70–90% [13]. Lignin peroxidase obtained from G. lucidum entrapped in Ca-alginate was shown to degrade sandal fix in a highly efficient manner (degradation efficiency ~70–95%) [14]. Nanoporous gold and microporous silica were found to be very good sorbent materials for the lignin peroxidase obtained from P. chrysosporium for degradation of dyes like rhodamine blue [15][16][17]. The schematic of the mechanism of biodegradation of methyl orange using lignin peroxidase is shown in Figure 1.
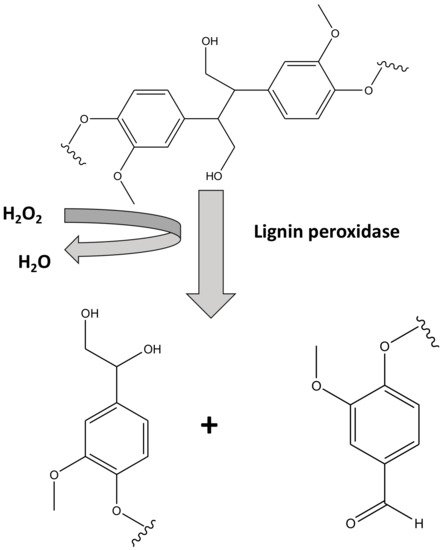
Figure 1. Simplified mechanism of action for lignin peroxidase.
1.2. Manganese Peroxidase
A series of irreversible oxidation-reduction reactions in a ping-pong mode has been demonstrated to be the mechanism for manganese peroxidase predominantly following second order rate kinetics. The subsequent electron transfer results in cleavage of the peroxi bonds and formation of H2O and the Fe (IV) oxo-porphyrin radical. The next step involves radical quenching through participation of Mn2+/Mn3+ redox equilibrium releasing a water molecule. Figure 2 schematically represents the mechanism of action of this enzyme.
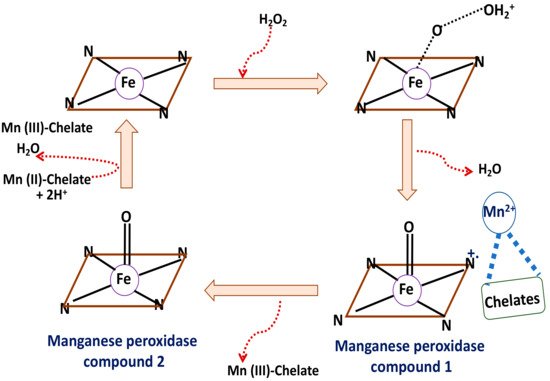
Figure 2. Schematic representation of the simplified mechanism of manganese peroxidase.
This enzyme was first reported to be found in P. chrysosporium. Toxic, carcinogenic, and mutagenic dyes and monomeric, dimeric as well as polymeric phenolic compounds are the main targets for this enzyme for biodegradation. Bilal et al. [18] have reported the encapsulation of G. lucidum to obtain manganese peroxidase on a sol-gel matrix for the biodegradation of the textile effluent from Arzoo, Ayesha, Kalash, Itmad and Crescent with an efficiency of 82–95%. The Ca alginate entrapment of manganese peroxidase has also shown efficient degradation of textile wastes including carcinogenic dyes and their derived compounds [19][20][21][22]. Nano clay was demonstrated as a suitable sorbent for manganese peroxidase immobilization in order to degrade potential aromatic hazards; anthracene, phenanthrene and pyrene [23].
1.3. Laccases
These are multi-copper, extranuclear, one electron transfer oxidoreductase divided broadly into three classes, which are found in different bacteria, plant and even varieties of fungus including Trametes versicolor, T. vilosa or Cerrena unicolor. Monophenols, diphenols, polyphenols, monoamines, diamines, N-heterocycles, and phenothiazines are some of the targets for this enzyme [24][25]. Food polymers in the form of proteins and non-starch polysaccharides were found to be crosslinked in the presence of laccases [1]. Formation of covalent bonds, cross-linking, dopamine assisted self-polymerization, physical entrapment into a pore, and adsorption are some of the modes for immobilization of laccases. These laccases were immobilized onto a variety of matrices including copper alginate beads, chitosan, magnetic nanoparticles, chitosan-CeO2 microsphere, fibrous polymer, hairy polymer grafted materials, sol-gel matrix, calcium alginate-chitosan beads, TiO2-ZrO2, TiO2-ZrO2-SiO2 mixed oxide matrices, and multichannel ceramic membranes for degradation of dyes and other textile ECs [26][27][28].
1.4. Tyrosinases
These are copper-containing oxidases for melanin production by hydroxylation of mono-phenol to o-diphenol to quinone followed by a series of reactions to melanin [29]. Tyrosinases obtained from different plants, humans, other mammals, and fungi have different structural properties, tissue distribution, and cellular location. The oxidation of phenolic compounds by tyrosinase can lead to the formation of different intermediates having a variety of physio-chemical properties. Crosslinked tyrosinase and laccase aggregates in a hybrid bioreactor were reported to degrade a large number of pharmaceutical products (acetaminophen, naproxen, mefenamic acid, ibuprofen, ketoprofen, indomethacin, tri-methoprim, ciprofloxacin, ofloxacin, caffeine, carbamazepine, bezafibrate, fenofibrate, and atenolol) from municipal wastewater streams in five days [30][31][32]. Edible/non-edible mushrooms have been largely exploited as the source of this enzyme. These enzymes have been immobilized on magnetic iron nano composites, zeolite derivatives, and polyacrylonitrile microspheres to degrade phenols and their derivatives [31][32].
1.5. Horseradish Peroxidase
This heme containing enzyme was obtained from the roots of horseradish and extensively used for the oxidation of many phenolic compounds, amines, phenolic acids containing pharmaceuticals, households, dyes, and other industrial ECs [33][34][35][36][37][38]. Immobilization of horseradish peroxidase on Fe3O4/nanotubes was found to improve the degradation of phenolic compounds [39]. Immobilization on graphene oxide showed almost quantitative removal of phenolic contaminants [40]. Immobilization on glutaraldehyde modified carbon nanosphere showed better pH and temperature stability compared to the free enzyme [36].
2. Absorption onto the Sludge
The porous structure of the sludge or the biomass present in biotic or abiotic sludge resulted either in entrapment of the hazardous materials through physical adsorption or chemisorption followed by biodegradation. This approach to remove ECs is attractive.
Kamaz et al. [41] have reported the adsorption of Congo red, Remazol Brilliant Blue R and Eriochrome Black Ton activated municipal sludge. A Freundlich isotherm and pseudo-second-order kinetics were reported to be predominating during the sorption of the dyes. A thermodynamic analysis of the sorption process revealed that the processes were spontaneous. Enhancement in entropy leading to spontaneity of sorption was reported for Remazol Brilliant Blue R and Eriochrome Black T. The activated and deactivated sludge under aerobic and anaerobic conditions exhibited different sorption capacities indicating the involvement of different microorganism [41].
Streit et al. [42] have reported the adsorption of ibuprofen, ketoprofen, and paracetamol on effluent treatment plant sludge in the beverage industry. The porous structure with high surface roughness, surface area (642 m2 g−1), and total pore volume (0.485 cm3 g−1) was responsible for achieving 145, 105, and 57 mg g−1 sorption capacity for these pharmaceutical ECs. Coimbra et al. [43] have reported the adsorption of pharmaceuticals (diclofenac, salicylic acid, ibuprofen and acetaminophen) from municipal wastewater streams using a pulp mill sludge. Although 200 min was required to attain complete equilibrium sorption for all the ECs, their pseudo second-order rate constants followed the trend: salicylic acid > diclofenac > ibuprofen > acetaminophen.
The Sip isotherm was reported to be suitable for explaining the sorption processes with the trend in sorption capacity: diclofenac > ibuprofen ~ acetaminophen > salicylic acid. Removal of 17α-ethinylestradiol, 4-nonylphenol, and carbamazepine in wastewater using an aerobic granular sludge was found to initiate through adsorption followed by degradation with sorption capacity of 16.09 μg/g and 20.05 μg/g, for 17α-ethinylestradiol, and 4-nonylphenol, respectively [44]. Both Langmuir and Freundlich isotherm model has been used to describe the sorption processes. Chiavola et al. [45] reported the adsorption followed by biodegradation of EDCs: BPA, 17α-ethinylestradiol (EE2), estrone(E1) and 17β-estradiol (E2) using activated and inactivated sludge. Pseudo second-order kinetics were reported to be predominate. Temporary inhibition of the biological process was observed at an initial higher concentration of EDCs resulting in a reduction in the mineralization process till the non-inhibiting value of their concentration was reached. They reported the removal of EDCs from wastewater along with simultaneous nitrification.
Recently, activated sludge has been used for 98–99% removal of ibuprofen and paracetamol by adsorption. The kinetics of sorption were found to follow pseudo-first and pseudo-second order models at all concentrations of the pharmaceuticals. Mesoporous biochar obtained from textile mill sludge has been exploited for the removal of ofloxacin pharmaceutical ECs with a sorption capacity of 9.74 mg g−1 following a π-π electron donor-acceptor and H bonding mechanism [46]. The sorption processes were spontaneous and exothermic in nature, best described by a pseudo second-order kinetics model and Redlich–Peterson and Freundlich isotherm models through a multilayer sorption process.
Although adsorption of ECs by the sludge is very common and a widely used cost-effective method, several other sorbents have also been evaluated. Some of them are porous materials obtained naturally, some are modified with specific surface functionalities to capture the ECs. Hence, not only efficient separation but also selective separation can be achieved. More than 90% separation of hormones (17α- dihydrouridine, 17α-Estradiol, 17α-ethinylestradiol, progesterone, estriol, and estrone) can be achieved using cyclodextrin coated silica [47]. However, the same sorbent showed only ~75% efficiency for norgestrel hormone.
The antidepressant, fluoxetine, was found to be adsorbed on zeolite, olive stone, sunflower, and walnut shell with sorption capacity in the range of 10 to 44 mg g−1 [48]. The same sorbents were also reported to separate nicotinic acid and pharmaceutical compounds, with higher sorption capacity in the range of 57 to 92 mg g−1. Avocado seed activated carbon was also reported to be very sorbent materials for sodium diclofenac analgesics with a capacity of 395 mg g−1 [49].
Zn based metal-organic frameworks have been reported to adsorb amodiaquine, whereas carbon nanotubes, graphene and its derivatives were also utilized for adsorption of diclofenac, carbamazepine, and ciprofloxacin [50].
3. Retention by the Membrane
The retention of ECs by the membrane could be by adsorption or size exclusion. Retention by the membrane depends on the membrane pore size and pore size distribution, surface hydrophilicity, morphology, and roughness which in turn are affected by the membrane polymer [51]. Since the ECs possess a wide range of physio-chemical properties, the level of retention by the membrane can be highly variable depending on the specific EC. As indicated in Figure 3, if size exclusion is the main mechanism of retention, then the size of the EC relative to the membrane pore size is very important. If surface adsorption is the main mechanism of retention, the membrane surface interaction with the EC will be more important. Some general observations are as follows.
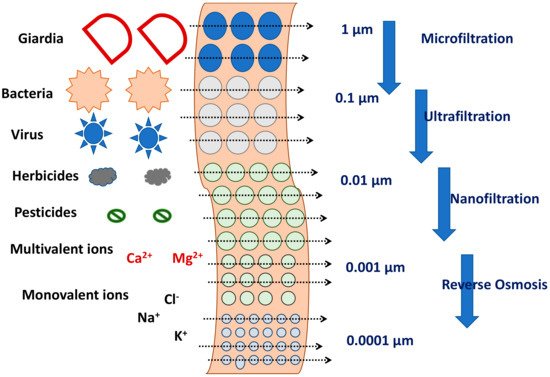
Figure 3. Sieving of ECs through membranes with different pore sizes.
-
Physical sieving can be used for the retention of non-ionic hydrophilic ECs (e.g., paracetamol, caeine, methylparaben);
-
Surface interaction and initial adsorption is the major phenomena during retention of hydrophobic non-ionic ECs (e.g., carbamazepine, estrone). It was also reported that, there is a reduction in ECs rejection after the absorption saturation;
-
For ECs with charged surface (either positive: propranolol, metoprolol or negative: ibuprofen, naproxen, diclofenac), the retention depends on electrostatic interaction between ECs and membrane materials in combination with sieving.
NF membranes were used for the retention of steroids [52]. Size exclusion and surface adsorption were reported to be the main mechanism of retention. Reverse osmosis (RO) has also been used for the removal of ECs [53]. Depending upon the nature of ECs, 40–100% rejection of ECs can be achieved by NF and RO processes [54][55][56].
References
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation—A Review. Environ. Int. 2019, 124, 336–353.
- Richardson, S.D.; Kimura, S.Y. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2020, 92, 473–505.
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment Technologies for Emerging Contaminants in Wastewater Treatment Plants: A Review. Sci. Total Environ. 2021, 753, 141990.
- Ning, B.; Graham, N.; Zhang, Y.; Nakonechny, M.; El-Din, M.G. Degradation of Endocrine Disrupting Chemicals by Ozone/AOPs. Ozone Sci. Eng. 2007, 29, 153–176.
- Kurade, M.B.; Awasthi, M.K.; Govindwar, S.P.; Jeon, B.-H.; Kalyani, D. Microbiotechnology Tools for Wastewater Cleanup and Organic Solids Reduction. Front. Microbiol. 2021, 12, 279.
- Muñoz, R.; Arriaga, S.; Hernández, S.; Guieysse, B.; Revah, S. Enhanced Hexane Biodegradation in a Two Phase Partitioning Bioreactor: Overcoming Pollutant Transport Limitations. Process Biochem. 2006, 41, 1614–1619.
- Singh, A.K.; Bilal, M.; Iqbal, H.M.N.; Raj, A. Lignin Peroxidase in Focus for Catalytic Elimination of Contaminants—A Critical Review on Recent Progress and Perspectives. Int. J. Biol. Macromol. 2021, 177, 58–82.
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial Sources, Production, Purification, and Potential Biotechnological Applications. Enzym. Res. 2011, 2011, 217861.
- Shi, J.; Chinn, M.S.; Sharma-Shivappa, R.R. Microbial Pretreatment of Cotton Stalks by Solid State Cultivation of Phanerochaete Chrysosporium. Bioresour. Technol. 2008, 99, 6556–6564.
- Radi, A.-E. Recent Updates of Chemically Modified Electrodes in Pharmaceutical Analysis. Comb. Chem. High Throughput Screen. 2010, 13, 728–752.
- Oliveira, S.F.; da Luz, J.M.R.; Kasuya, M.C.M.; Ladeira, L.O.; Correa Junior, A. Enzymatic Extract Containing Lignin Peroxidase Immobilized on Carbon Nanotubes: Potential Biocatalyst in Dye Decolourization. Saudi J. Biol. Sci. 2018, 25, 651–659.
- Ran, Y.; Zhifeiche; Chen, W. Co-Immobilized Lignin Peroxidase and Manganese Peroxidase from Coriolus Versicolor Capable of Decolorizing Molasses Waste Water. Appl. Mech. Mater. 2012, 138–139, 1067–1071.
- Sofia, P.; Asgher, M.; Shahid, M.; Randhawa, M.A. Chitosan beads immobilized schizophyllum commune ibl-06 lignin peroxidase with novel thermo stability, catalytic and dye removal properties. J. Anim. Plant Sci. 2016, 26, 1451–1463.
- Shaheen, R.; Asgher, M.; Hussain, F.; Bhatti, H.N. Immobilized Lignin Peroxidase from Ganoderma Lucidum IBL-05 with Improved Dye Decolorization and Cytotoxicity Reduction Properties. Int. J. Biol. Macromol. 2017, 103, 57–64.
- Hu, Z.; Xu, L.; Wen, X. Mesoporous Silicas Synthesis and Application for Lignin Peroxidase Immobilization by Covalent Binding Method. J. Environ. Sci. 2013, 25, 181–187.
- Xu, L.-Q.; Wen, X.-H.; Ding, H.-J. Immobilization of Lignin Peroxidase on Spherical Mesoporous Material. Huan Jing Ke Xue Huanjing Kexue 2010, 31, 2493–2499.
- Qiu, H.; Li, Y.; Ji, G.; Zhou, G.; Huang, X.; Qu, Y.; Gao, P. Immobilization of Lignin Peroxidase on Nanoporous Gold: Enzymatic Properties and in Situ Release of H2O2 by Co-Immobilized Glucose Oxidase. Bioresour. Technol. 2009, 100, 3837–3842.
- Bilal, M.; Asgher, M. Enhanced Catalytic Potentiality of Ganoderma Lucidum IBL-05 Manganese Peroxidase Immobilized on Sol-Gel Matrix. J. Mol. Catal. B: Enzym. 2016, 128, 82–93.
- Bilal, M.; Asgher, M.; Shahid, M.; Bhatti, H.N. Characteristic Features and Dye Degrading Capability of Agar-Agar Gel Immobilized Manganese Peroxidase. Int. J. Biol. Macromol. 2016, 86, 728–740.
- Bilal, M.; Asgher, M.; Hu, H.; Zhang, X. Kinetic Characterization, Thermo-Stability and Reactive Red 195A Dye Detoxifying Properties of Manganese Peroxidase-Coupled Gelatin Hydrogel. Water Sci. Technol. 2016, 74, 1809–1820.
- Bilal, M.; Asgher, M. Dye Decolorization and Detoxification Potential of Ca-Alginate Beads Immobilized Manganese Peroxidase. BMC Biotechnol. 2015, 15, 111.
- Bilal, M.; Asgher, M.; Iqbal, M.; Hu, H.; Zhang, X. Chitosan Beads Immobilized Manganese Peroxidase Catalytic Potential for Detoxification and Decolorization of Textile Effluent. Int. J. Biol. Macromol. 2016, 89, 181–189.
- Acevedo, F.; Pizzul, L.; Castillo, M.; González, M.E.; Cea, M.; Gianfreda, L.; Diez, M.C. Degradation of Polycyclic Aromatic Hydrocarbons by Free and Nanoclay-Immobilized Manganese Peroxidase from Anthracophyllum Discolor. Chemosphere 2010, 80, 271–278.
- Bronikowski, A.; Hagedoorn, P.L.; Koschorreck, K.; Urlacher, V.B. Expression of a New Laccase from Moniliophthora Roreri at High Levels in Pichia Pastoris and Its Potential Application in Micropollutant Degradation. AMB Express 2017, 7, 73.
- Cha, J.Y.; Kim, T.W.; Choi, J.H.; Jang, K.S.; Khaleda, L.; Kim, W.Y.; Jeon, J.R. Fungal Laccase-Catalyzed Oxidation of Naturally Occurring Phenols for Enhanced Germination and Salt Tolerance of Arabidopsis Thaliana: A Green Route for Synthesizing Humic-like Fertilizers. J. Agric. Food Chem. 2017, 65, 1167–1177.
- Brugnari, T.; Pereira, M.G.; Bubna, G.A.; de Freitas, E.N.; Contato, A.G.; Corrêa, R.C.G.; Castoldi, R.; de Souza, C.G.M.; de Moraes, M.D.L.T.; Bracht, A.; et al. A Highly Reusable MANAE-Agarose-Immobilized Pleurotus Ostreatus Laccase for Degradation of Bisphenol A. Sci. Total Environ. 2018, 634, 1346–1351.
- Bayramoglu, G.; Karagoz, B.; Arica, M.Y. Cyclic-Carbonate Functionalized Polymer Brushes on Polymeric Microspheres: Immobilized Laccase for Degradation of Endocrine Disturbing Compounds. J. Ind. Eng. Chem. 2018, 60, 407–417.
- Antecka, K.; Zdarta, J.; Siwińska-Stefańska, K.; Sztuk, G.; Jankowska, E.; Oleskowicz-Popiel, P.; Jesionowski, T. Synergistic Degradation of Dye Wastewaters Using Binary or Ternary Oxide Systems with Immobilized Laccase. Catalysts 2018, 8, 402.
- Ba, S.; Haroune, L.; Soumano, L.; Bellenger, J.P.; Jones, J.P.; Cabana, H. A Hybrid Bioreactor Based on Insolubilized Tyrosinase and Laccase Catalysis and Microfiltration Membrane Remove Pharmaceuticals from Wastewater. Chemosphere 2018, 201, 749–755.
- Faccio, G.; Kruus, K.; Saloheimo, M.; Thöny-Meyer, L. Bacterial Tyrosinases and Their Applications. Process Biochem. 2012, 47, 1749–1760.
- Land, E.J.; Ramsden, C.A.; Riley, P.A. Tyrosinase Autoactivation and the Chemistry of Ortho-Quinone Amines. Acc. Chem. Res. 2003, 36, 300–308.
- Selinheimo, E.; NiEidhin, D.; Steffensen, C.; Nielsen, J.; Lomascolo, A.; Halaouli, S.; Record, E.; O’Beirne, D.; Buchert, J.; Kruus, K. Comparison of the Characteristics of Fungal and Plant Tyrosinases. J. Biotechnol. 2007, 130, 471–480.
- Zhang, F.; Zhang, W.; Zhao, L.F.; Liu, H. Degradation of Phenol with Horseradish Peroxidase Immobilized on ZnO Nanocrystals under Combined Irradiation of Microwaves and Ultrasound. Desalination Water Treat. 2016, 57, 24406–24416.
- Sun, H.; Jin, X.; Jiang, F.; Zhang, R. Immobilization of Horseradish Peroxidase on ZnO Nanowires/Macroporous SiO2 Composites for the Complete Decolorization of Anthraquinone Dyes. Biotechnol. Appl. Biochem. 2018, 65, 220–229.
- Šekuljica, N.; Prlainović, N.; Jovanović, J.R.; Stefanović, A.B.; Djokić, V.R.; Mijin, D.; Knezevic-Jugovic, Z.D. Immobilization of Horseradish Peroxidase onto Kaolin. Bioprocess Biosyst. Eng. 2016, 39, 461–472.
- Lu, Y.M.; Yang, Q.Y.; Wang, L.M.; Zhang, M.Z.; Guo, W.Q.; Cai, Z.N.; Wang, D.D.; Yang, W.W.; Chen, Y. Enhanced Activity of Immobilized Horseradish Peroxidase by Carbon Nanospheres for Phenols Removal. Clean—Soil Air Water 2017, 45, 1600077.
- Chang, Q.; Jiang, G.; Tang, H.; Li, N.; Huang, J.; Wu, L. Enzymatic Removal of Chlorophenols Using Horseradish Peroxidase Immobilized on Superparamagnetic Fe3O4/Graphene Oxide Nanocomposite. Chin. J. Catal. 2015, 36, 961–968.
- Bilal, M.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Enhanced Bio-Catalytic Performance and Dye Degradation Potential of Chitosan-Encapsulated Horseradish Peroxidase in a Packed Bed Reactor System. Sci. Total Environ. 2017, 575, 1352–1360.
- Zhang, C.; Cai, X. Immobilization of Horseradish Peroxidase on Fe3O4/Nanotubes Composites for Biocatalysis-Degradation of Phenol. Compos. Interfaces 2019, 26, 379–396.
- Zhang, F.; Zheng, B.; Zhang, J.; Huang, X.; Liu, H.; Guo, S.; Zhang, J. Horseradish Peroxidase Immobilized on Graphene Oxide: Physical Properties and Applications in Phenolic Compound Removal. J. Phys. Chem. C 2010, 114, 8469–8473.
- Kamaz, M.; Rocha, P.; Sengupta, A.; Qian, X.; Wickramasinghe, R.S. Efficient Removal of Chemically Toxic Dyes Using Microorganism from Activated Sludge: Understanding Sorption Mechanism, Kinetics, and Associated Thermodynamics. Sep. Sci. Technol. 2018, 53, 1760–1776.
- Streit, A.F.M.; Collazzo, G.C.; Druzian, S.P.; Verdi, R.S.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. Adsorption of Ibuprofen, Ketoprofen, and Paracetamol onto Activated Carbon Prepared from Effluent Treatment Plant Sludge of the Beverage Industry. Chemosphere 2021, 262, 128322.
- Coimbra, R.N.; Calisto, V.; Ferreira, C.I.A.; Esteves, V.I.; Otero, M. Removal of Pharmaceuticals from Municipal Wastewater by Adsorption onto Pyrolyzed Pulp Mill Sludge. Arab. J. Chem. 2019, 12, 3611–3620.
- Kent, J.; Tay, J.H. Treatment of 17α-ethinylestradiol, 4-nonylphenol, and Carbamazepine in Wastewater Using an Aerobic Granular Sludge Sequencing Batch Reactor. Sci. Total Environ. 2019, 652, 1270–1278.
- Chiavola, A.; Tedesco, P.; Boni, M.R. Fate of Some Endocrine Disruptors in Batch Experiments Using Activated and Inactivated Sludge. Water Air Soil Pollut. 2016, 227, 424.
- Singh, V.; Srivastava, V.C. Self-Engineered Iron Oxide Nanoparticle Incorporated on Mesoporous Biochar Derived from Textile Mill Sludge for the Removal of an Emerging Pharmaceutical Pollutant. Environ. Pollut. 2020, 259, 113822.
- Bhattarai, B.; Muruganandham, M.; Suri, R.P.S. Development of High Efficiency Silica Coated β-Cyclodextrin Polymeric Adsorbent for the Removal of Emerging Contaminants of Concern from Water. J. Hazard. Mater. 2014, 273, 146–154.
- Román, S.; Nabais, J.M.V.; Ledesma, B.; Laginhas, C.; Titirici, M.M. Surface Interactions during the Removal of Emerging Contaminants by Hydrochar-Based Adsorbents. Molecules 2020, 25, 2264.
- Leite, A.J.B.; Sophia, A.C.; Thue, P.S.; dos Reis, G.S.; Dias, S.L.P.; Lima, E.C.; Vaghetti, J.C.P.; Pavan, F.A.; de Alencar, W.S. Activated Carbon from Avocado Seeds for the Removal of Phenolic Compounds from Aqueous Solutions. Desalination Water Treat. 2017, 71, 168–181.
- Lin, K.-Y.A.; Yang, H.; Hsu, F.-K. Zr-Metal Organic Framework and Derivatives for Adsorptive and Photocatalytic Removal of Acid Dyes. Water Environ. Res. 2018, 90, 144–154.
- Li, C.; Feng, G.; Song, C.; Zhong, G.; Tao, P.; Wang, T.; Shao, M. Improved Oil Removal Ability by the Integrated Electrocoagulation (EC)-Carbon Membrane Coupling with Electrochemical Anodic Oxidation (CM/EAO) System. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 305–313.
- Schäfer, A.I.; Nghiem, L.D.; Meier, A.; Neale, P.A. Impact of Organic Matrix Compounds on the Retention of Steroid Hormone Estrone by a “loose” Nanofiltration Membrane. Sep. Purif. Technol. 2010, 73, 179–187.
- Torii, S.; Hashimoto, T.; Do, A.T.; Furumai, H.; Katayama, H. Impact of Repeated Pressurization on Virus Removal by Reverse Osmosis Membranes for Household Water Treatment. Environ. Sci. Water Res. Technol. 2019, 5, 910–919.
- Naidu, L.D.; Saravanan, S.; Chidambaram, M.; Goel, M.; Das, A.; Babu, J.S.C. Nanofiltration in Transforming Surface Water into Healthy Water: Comparison with Reverse Osmosis. J. Chem. 2015, 2015, 326869.
- Cséfalvay, E.; Imre, P.M.; Mizsey, P. Applicability of Nanofiltration and Reverse Osmosis for the Treatment of Wastewater of Different Origin. Cent. Eur. J. Chem. 2008, 6, 277–283.
- Zazouli, M.A.; Kalankesh, L.R. Removal of Precursors and Disinfection Byproducts (DBPs) by Membrane Filtration from Water; a Review. J. Environ. Health Sci. Eng. 2017, 15, 25.
More
Information
Subjects:
Agricultural Engineering
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
17 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




