
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sheshanath Vishwanath Bhosale | + 4204 word(s) | 4204 | 2022-01-05 07:41:00 | | | |
| 2 | Vivi Li | + 69 word(s) | 4273 | 2022-02-16 06:53:56 | | | | |
| 3 | Vivi Li | + 69 word(s) | 4273 | 2022-02-16 06:58:51 | | |
Video Upload Options
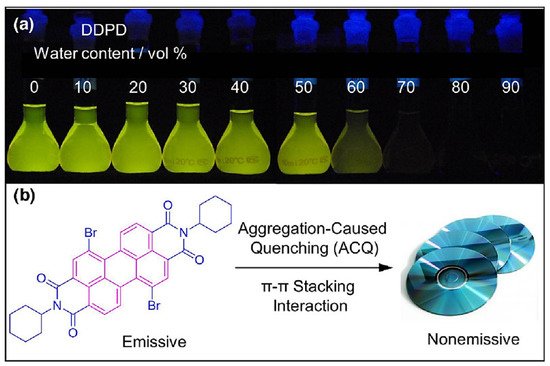
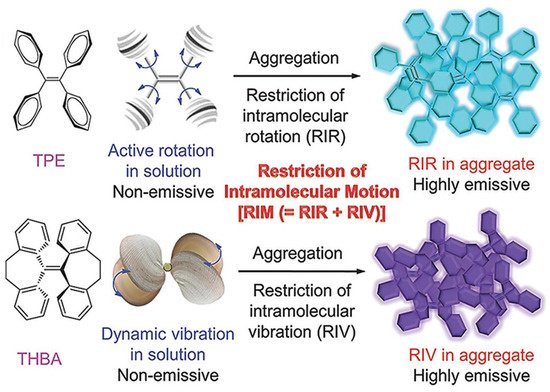
The emergence and development of aggregation induced emission (AIE) have attracted worldwide attention due to its unique photophysical phenomenon and for removing the obstacle of aggregation-caused quenching (ACQ) which is the most detrimental process thereby making AIE an important and promising aspect in various fields of fluorescent material, sensing, bioimaging, optoelectronics, drug delivery system, and theranostics. hexaphenylsilole (HPS) is the common example of the AIE active molecule which exhibits enhancement in fluorescence in an aggregate state. The motions involved, such as restriction of intramolecular motion along with rotation and vibration mechanisms in the AIE active phenomenon, are well explained and accepted. The AIE luminogens have high photostability, large stoke shift, a photobleaching resistance property, and show high sensing reproducibility. This characteristic makes luminogens a promising candidate for sensing application
1. Introduction

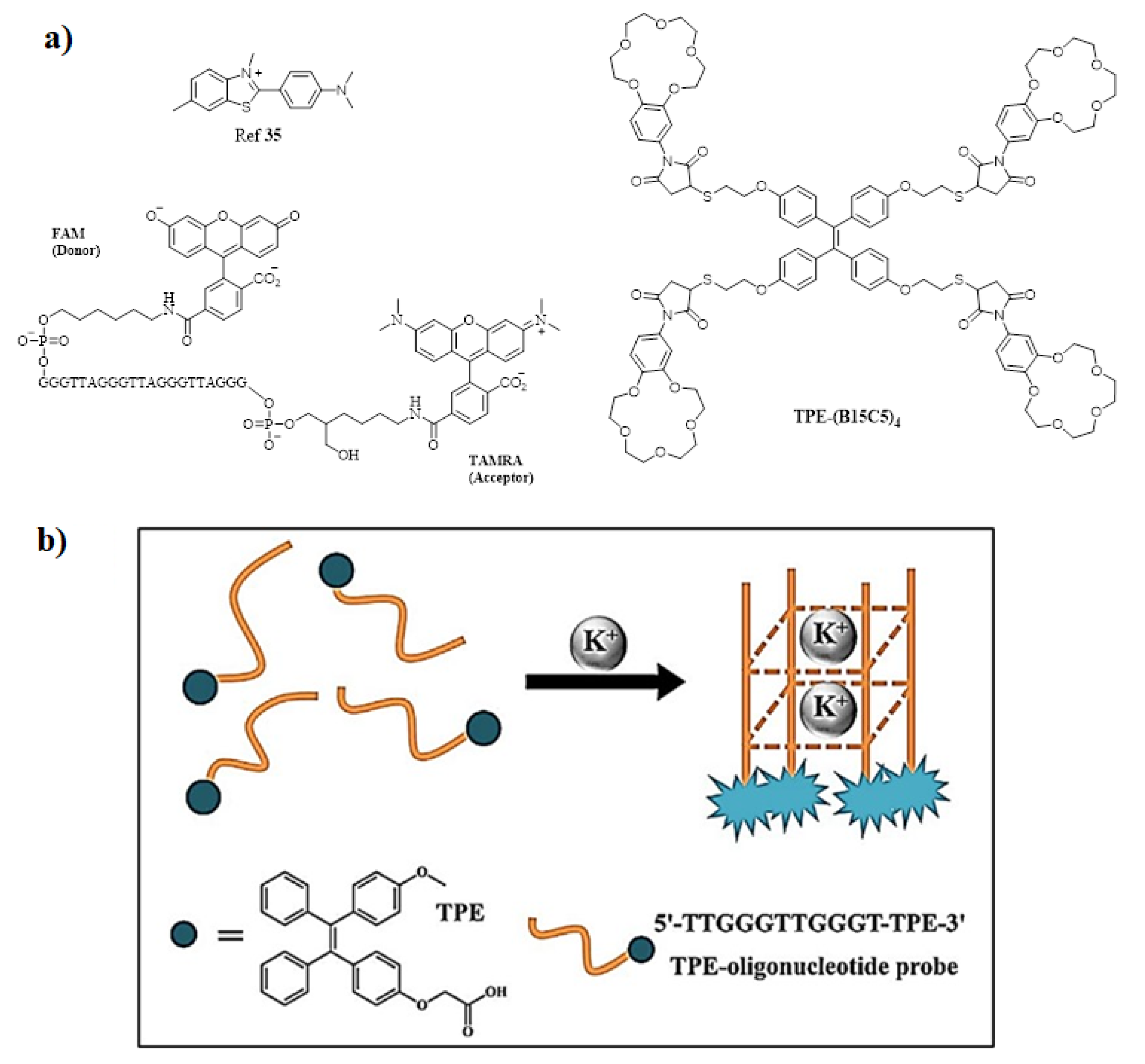
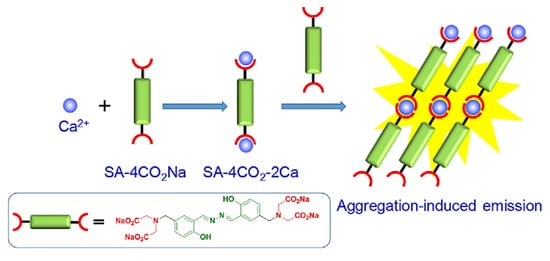
2. Aggregation Induced Emission (AIE) Active Molecules for Sensing Application
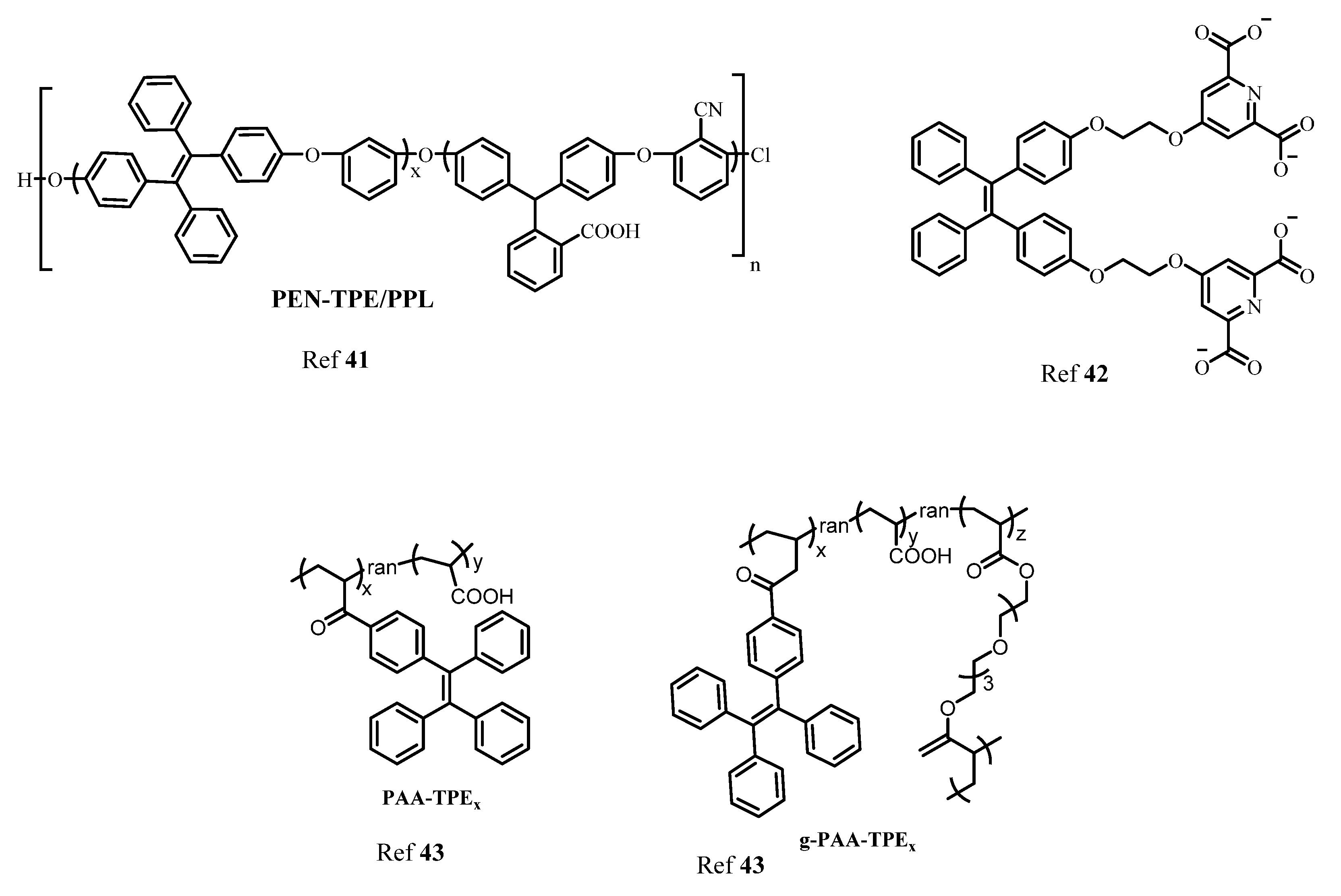
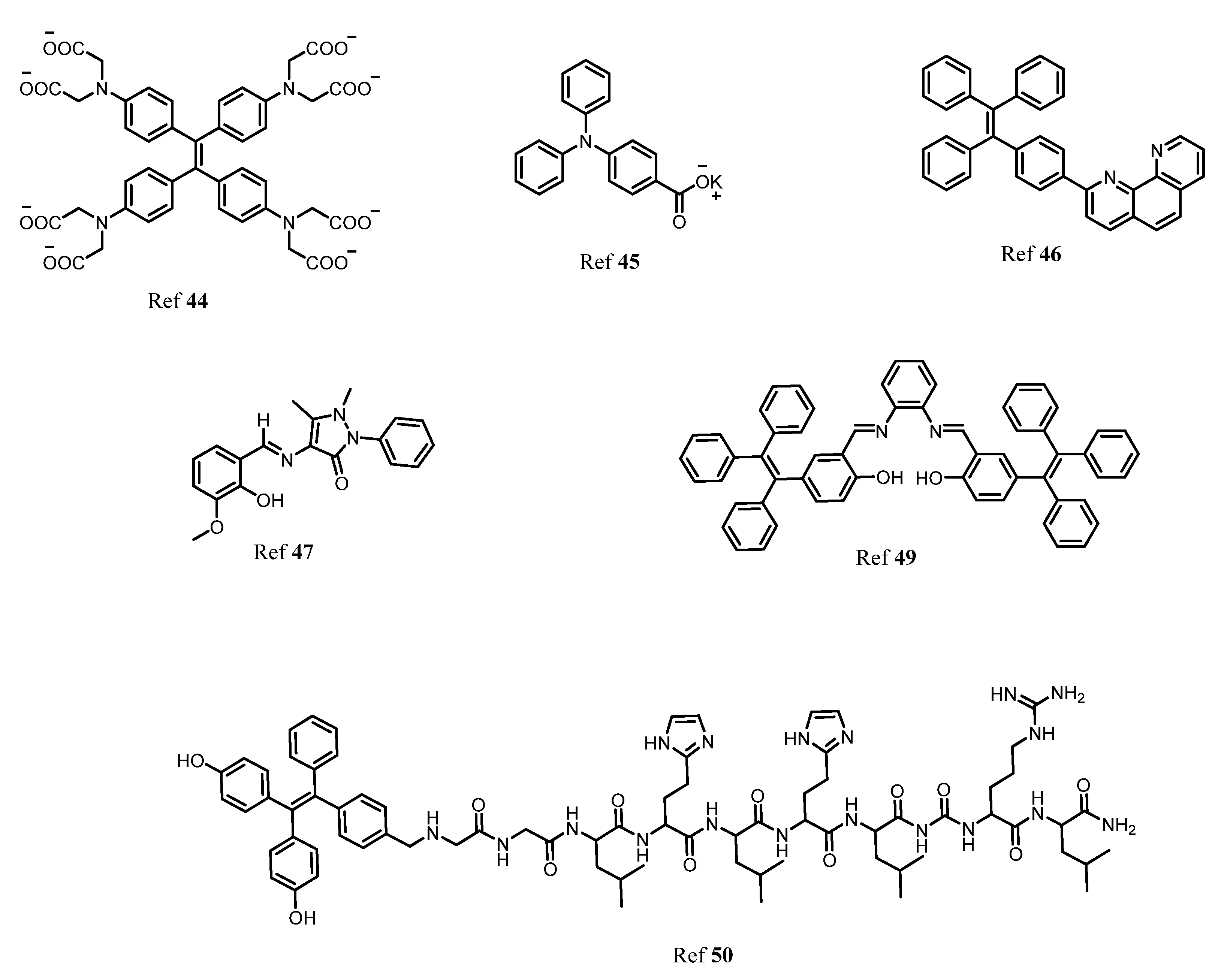
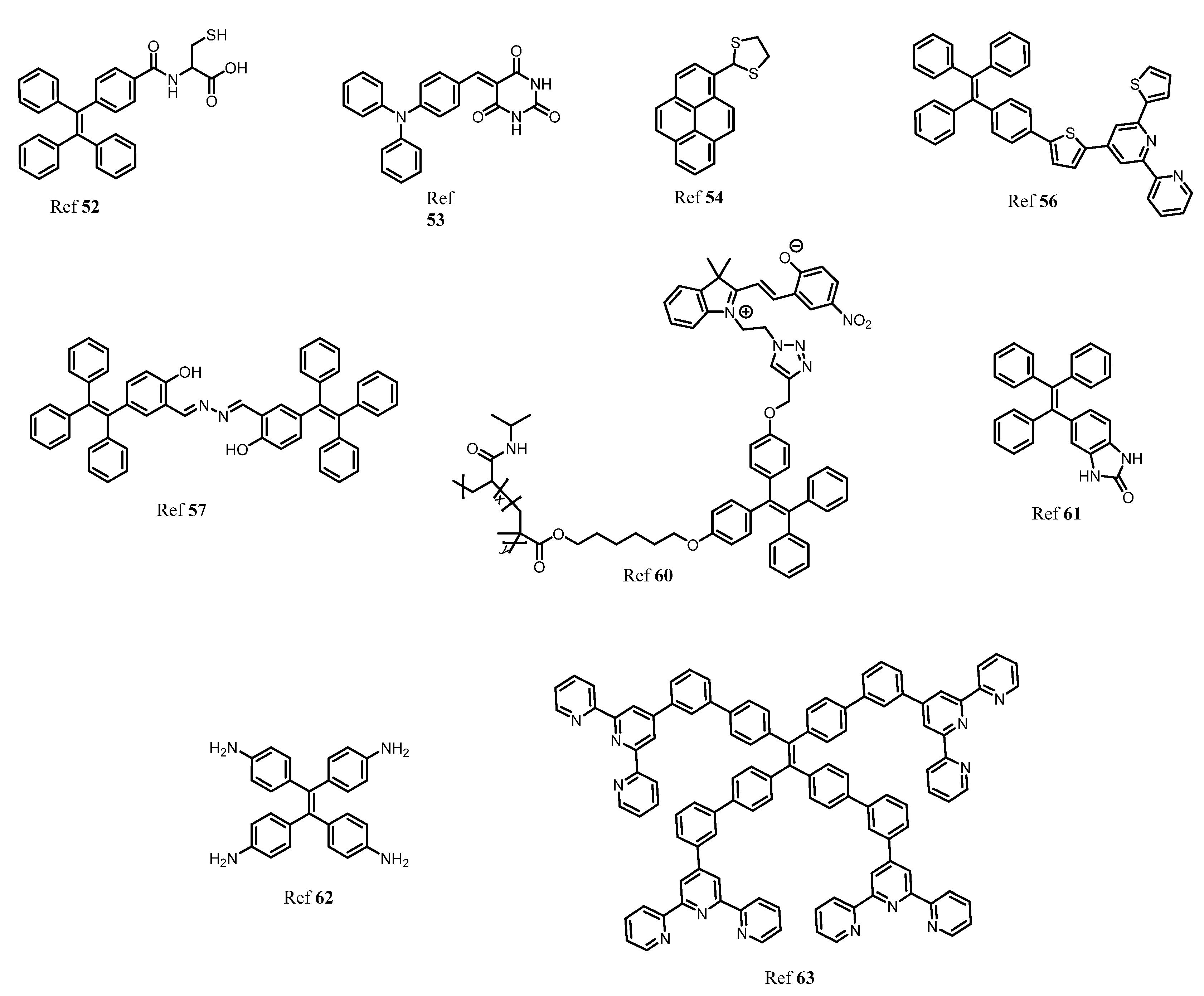

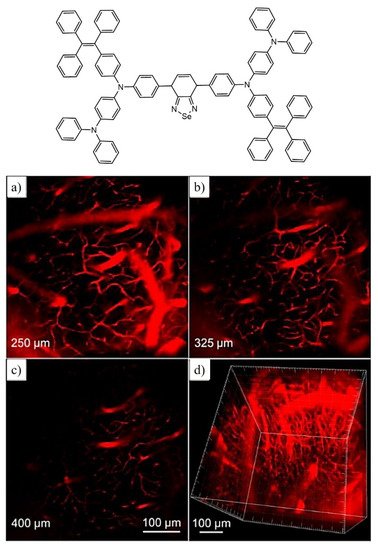
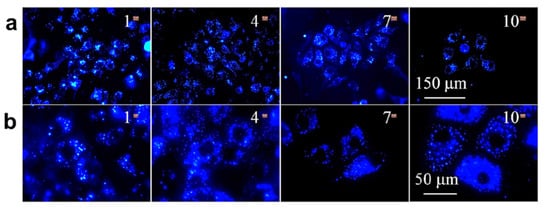
3. AIE Active Molecules for Biological Cell Imaging
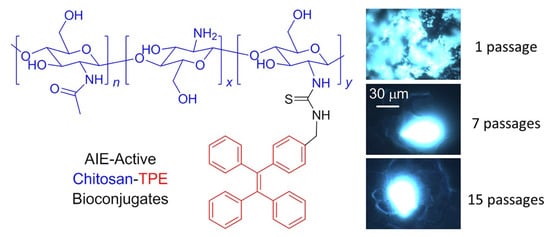
References
- Elahi, N.; Kamali, M.; Baghersad, M.H.; Amini, B. A Fluorescence Nano-Biosensors Immobilization on Iron (MNPs) and Gold (AuNPs) Nanoparticles for Detection of Shigella spp. Mater. Sci. Eng. C 2019, 105, 110113.
- Reineck, P.; Francis, A.; Orth, A.; Lau, D.W.M.; Nixon-Luke, R.D.V.; Rastogi, I.; Razali, W.A.W.; Cordina, N.M.; Parker, L.M.; Sreenivasan, V.K.A.; et al. Brightness and Photostability of Emerging Red and Near-IR Fluorescent Nanomaterials for Bioimaging. Adv. Opt. Mater. 2016, 4, 1549–1557.
- Yuan, Y.; Zhang, C.J.; Liu, B. A Photoactivatable AIE Polymer for Light-Controlled Gene Delivery: Concurrent Endo/Lysosomal Escape and DNA Unpacking. Angew. Chem. Int. Ed. 2015, 54, 11419–11423.
- Qu, J.H.; Wei, Q.; Sun, D.W. Carbon Dots: Principles and Their Applications in Food Quality and Safety Detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475.
- Lange, L.; Meyer, A.S. Potentials and Possible Safety Issues of Using Biorefinery Products in Food Value Chains. Trends Food Sci. Technol. 2019, 84, 7–11.
- Mattarozzi, M.; Bianchi, F.; Maffini, M.; Vescovi, F.; Catellani, D.; Suman, M.; Careri, M. ESEM-EDS-Based Analytical Approach to Assess Nanoparticles for Food Safety and Environmental Control. Talanta 2019, 196, 429–435.
- Huang, L.; Sun, D.W.; Pu, H.; Wei, Q. Development of Nanozymes for Food Quality and Safety Detection: Principles and Recent Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1496–1513.
- Zhang, H.; Yang, S.; De Ruyck, K.; Beloglazova, N.; Eremin, S.A.; De Saeger, S.; Zhang, S.; Shen, J.; Wang, Z. Fluorescence Polarization Assays for Chemical Contaminants in Food and Environmental Analyses. TrAC Trends Anal. Chem. 2019, 114, 293–313.
- Wang, H.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. AIE Luminogens: Emission Brightened by Aggregation. Mater. Today 2015, 18, 365–377.
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453.
- Chen, G.; Li, W.; Zhou, T.; Peng, Q.; Zhai, D.; Li, H.; Yuan, W.Z.; Zhang, Y.; Tang, B.Z. Conjugation-Induced Rigidity in Twisting Molecules: Filling the Gap between Aggregation-Caused Quenching and Aggregation-Induced Emission. Adv. Mater. 2015, 27, 4496–4501.
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Phenomenon, Mechanism and Applications. Chem. Commun. 2009, 29, 4332–4353.
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388.
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479.
- Luo, J.; Xie, Z.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741.
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907.
- Li, X.; Li, M.; Yang, M.; Xiao, H.; Wang, L.; Chen, Z.; Liu, S.; Li, J.; Li, S.; James, T.D. “Irregular” Aggregation-Induced Emission Luminogens. Coord. Chem. Rev. 2020, 418, 213358.
- Gu, M.; Zeng, Z.; Xing, M.; Xiong, Y.; Deng, Z.; Chen, S.; Wang, L. The Biological Applications of Two Aggregation-Induced Emission Luminogens. Biotechnol. J. 2019, 14, e1900212.
- Cai, X.; Liu, B. Aggregation-Induced Emission: Recent Advances in Materials and Biomedical Applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886.
- Ma, J.; Sengupta, M.K.; Yuan, D.; Dasgupta, P.K. Speciation and Detection of Arsenic in Aqueous Samples: A Review of Recent Progress in Non-Atomic Spectrometric Methods. Anal. Chim. Acta 2014, 831, 1–23.
- Hong, Y. Aggregation-Induced Emission-Fluorophores and Applications. Methods Appl. Fluoresc. 2016, 4, 22003.
- Feng, G.; Kwok, R.T.K.; Tang, B.Z.; Liu, B. Functionality and Versatility of Aggregation-Induced Emission Luminogens. Appl. Phys. Rev. 2017, 4, 021307.
- Niu, G.; Zhang, R.; Shi, X.; Park, H.; Xie, S.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE Luminogens as Fluorescent Bioprobes. TrAC Trends Anal. Chem. 2020, 123, 115769.
- Leung, N.L.C.; Xie, N.; Yuan, W.; Liu, Y.; Wu, Q.; Peng, Q.; Miao, Q.; Lam, J.W.Y.; Tang, B.Z. Restriction of Intramolecular Motions: The General Mechanism behind Aggregation-Induced Emission. Chem. A Eur. J. 2014, 20, 15349–15353.
- Yang, J.; Chi, Z.; Zhu, W.; Tang, B.Z.; Li, Z. Aggregation-Induced Emission: A Coming-of-Age Ceremony at the Age of Eighteen. Sci. China Chem. 2019, 62, 1090–1098.
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-Based AIE-Active Probes for Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 12189–12216.
- He, T.; Wang, H.; Chen, Z.; Liu, S.; Li, J.; Li, S. Natural Quercetin AIEgen Composite Film with Antibacterial and Antioxidant Properties for in Situ Sensing of Al3+ Residues in Food, Detecting Food Spoilage, and Extending Food Storage Times. ACS Appl. Bio Mater. 2018, 1, 636–642.
- Khan, I.M.; Niazi, S.; Iqbal Khan, M.K.; Pasha, I.; Mohsin, A.; Haider, J.; Iqbal, M.W.; Rehman, A.; Yue, L.; Wang, Z. Recent Advances and Perspectives of Aggregation-Induced Emission as an Emerging Platform for Detection and Bioimaging. TrAC Trends Anal. Chem. 2019, 119, 115637.
- Bhosale, S.V.; Al Kobaisi, M.; Jadhav, R.W.; Morajakar, P.P.; Jones, L.A.; George, S. Naphthalene Diimides: Perspectives and Promise. Chem. Soc. Rev. 2021, 50, 9845–9998.
- Mente, A.; O’donnell, M.; Yusuf, S. Sodium Intake and Health: What Should We Recommend Based on the Current Evidence? Nutrients 2021, 13, 3232.
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601.
- Gritter, M.; Rotmans, J.I.; Hoorn, E.J. Role of Dietary K+ in Natriuresis, Blood Pressure Reduction, Cardiovascular Protection, and Renoprotection. Hypertension 2019, 73, 15–23.
- Xiong, M.; Zhu, H.; Rong, Q.; Yang, C.; Qiu, L.; Zhang, X.B.; Tan, W. A Membrane-Anchored Fluorescent Probe for Detecting K+ in the Cell Microenvironment. Chem. Commun. 2016, 52, 4679–4682.
- Liu, L.; Shao, Y.; Peng, J.; Huang, C.; Liu, H.; Zhang, L. Molecular Rotor-Based Fluorescent Probe for Selective Recognition of Hybrid G-Quadruplex and as a K+ Sensor. Anal. Chem. 2014, 86, 1622–1631.
- Wang, X.; Hu, J.; Liu, T.; Zhang, G.; Liu, S. Highly Sensitive and Selective Fluorometric Off-on K+ Probe Constructed via Host-Guest Molecular Recognition and Aggregation-Induced Emission. J. Mater. Chem. 2012, 22, 8622–8628.
- Lu, D.; He, L.; Wang, Y.; Xiong, M.; Hu, M.; Liang, H.; Huan, S.; Zhang, X.B.; Tan, W. Tetraphenylethene Derivative Modified DNA Oligonucleotide for in Situ Potassium Ion Detection and Imaging in Living Cells. Talanta 2017, 167, 550–556.
- Ueyama, H.; Takagi, M.; Takenaka, S. A Novel Potassium Sensing in Aqueous Media with a Synthetic Oligonucleotide Derivative. Fluorescence Resonance Energy Transfer Associated with Guanine Quartet-Potassium Ion Complex Formation. J. Am. Chem. Soc. 2002, 124, 14286–14287.
- Breitwieser, G.E. Extracellular Calcium as an Integrator of Tissue Function. Int. J. Biochem. Cell Biol. 2008, 40, 1467–1480.
- Akkaya, E.U.; Turkyilmaz, S. A Squaraine-Based near IR Fluorescent Chemosensor for Calcium. Tetrahedron Lett. 1997, 38, 4513–4516.
- Gao, M.; Li, Y.; Chen, X.; Li, S.; Ren, L.; Tang, B.Z. Aggregation-Induced Emission Probe for Light-Up and in Situ Detection of Calcium Ions at High Concentration. ACS Appl. Mater. Interfaces 2018, 10, 14410–14417.
- Wang, P.; Jia, K.; Zhou, X.; Guan, X.; Wang, L.; Tian, Y.; Wu, C.; Liu, X. Ca2+ Induced Crosslinking of AIE-Active Polyarylene Ether Nitrile into Fluorescent Polymeric Nanoparticles for Cellular Bioimaging. Macromol. Rapid Commun. 2017, 38, 1700360.
- Zhang, J.; Yan, Z.; Wang, S.; She, M.; Zhang, Z.; Cai, W.; Liu, P.; Li, J. Water Soluble Chemosensor for Ca2+ Based on Aggregation-Induced Emission Characteristics and Its Fluorescence Imaging in Living Cells. Dye Pigment. 2018, 150, 112–120.
- Ishiwari, F.; Hasebe, H.; Matsumura, S.; Hajjaj, F.; Horii-Hayashi, N.; Nishi, M.; Someya, T.; Fukushima, T. Bioinspired Design of a Polymer Gel Sensor for the Realization of Extracellular Ca2+ Imaging. Sci. Rep. 2016, 6, 24275.
- Sun, F.; Zhang, G.; Zhang, D.; Xue, L.; Jiang, H. Aqueous Fluorescence “Turn-on” Sensor for Zn2+ with a Tetraphenylethylene Compound. Org. Lett. 2011, 13, 6378–6381.
- Wei, Y.; Wang, L.; Huang, J.; Zhao, J.; Yan, Y. Multifunctional Metallo-Organic Vesicles Displaying Aggregation-Induced Emission: Two-Photon Cell-Imaging, Drug Delivery, and Specific Detection of Zinc Ion. ACS Appl. Nano Mater. 2018, 1, 1819–1827.
- Tang, A.; Yin, Y.; Chen, Z.; Fan, C.; Liu, G.; Pu, S. A Multifunctional Aggregation-Induced Emission (AIE)-Active Fluorescent Chemosensor for Detection of Zn2+ and Hg2+. Tetrahedron 2019, 75, 130489.
- Maity, S.; Shyamal, M.; Maity, R.; Mudi, N.; Hazra, P.; Giri, P.K.; Samanta, S.S.; Pyne, S.; Misra, A. An Antipyrine Based Fluorescent Probe for Distinct Detection of Al3+ and Zn2+ and Its AIEE Behaviour. Photochem. Photobiol. Sci. 2020, 19, 681–694.
- Diana, R.; Panunzi, B. Zinc (Ii) and Aiegens: The “Clip Approach” for a Novel Fluorophore Family: A Review. Molecules 2021, 26, 4176.
- Sun, H.; Jiang, Y.; Nie, J.; Wei, J.; Miao, B.; Zhao, Y.; Zhang, L.; Ni, Z. Multifunctional AIE-ESIPT Dual Mechanism Tetraphenylethene-Based Schiff Base for Inkless Rewritable Paper and a Colorimetric/Fluorescent Dual-Channel Zn2+ sensor. Mater. Chem. Front. 2021, 5, 347–354.
- He, X.; Wang, X.; Zhang, L.; Fang, G.; Liu, J.; Wang, S. Sensing and Intracellular Imaging of Zn2+ Based on Affinity Peptide Using an Aggregation Induced Emission Fluorescence “Switch-on” Probe. Sens. Actuators B Chem. 2018, 271, 289–299.
- FN, C.; MF, M. Factors Affecting Water Pollution: A Review. J. Ecosyst. Ecography 2017, 7, 5–8.
- Vongdala, N.; Tran, H.D.; Xuan, T.D.; Teschke, R.; Khanh, T.D. Heavy Metal Accumulation in Water, Soil, and Plants of Municipal Solid Waste Landfill in Vientiane, Laos. Int. J. Environ. Res. Public Health 2019, 16, 22.
- Ebenstein, A. The Consequences of Industrialization: Evidence From. Rev. Econ. Stat. 2012, 94, 186–201.
- Moss, B. Water Pollution by Agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 659–666.
- Chua, M.H.; Zhou, H.; Zhu, Q.; Tang, B.Z.; Xu, J.W. Recent Advances in Cation Sensing Using Aggregation-Induced Emission. Mater. Chem. Front. 2021, 5, 659–708.
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular Materials Based on AIE Luminogens (AIEgens): Construction and Applications. Chem. Soc. Rev. 2020, 49, 1144–1172.
- Baglan, M.; Atılgan, S. Selective and Sensitive “Turn-on” Fluorescent Sensing of Arsenite Based on Cysteine Fused Tetraphenylethene with AIE Characteristics in Aqueous Media. Chem. Commun. 2013, 49, 5325–5327.
- Wen, X.; Yan, L.; Fan, Z. A Novel AIE Active NIR Fluorophore Based Triphenylamine for Sensing of Hg2+ and CN− and Its Multiple Application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118664.
- Ma, J.; Xiao, Y.; Zhang, C.; Zhang, M.; Wang, Q.; Zheng, W.; Zhang, S. Preparation a Novel Pyrene-Based AIE-Active Ratiometric “Turn-on” Fluorescent Probe for Highly Selective and Sensitive Detection of Hg2+. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 259, 114582.
- Zalmi, G.A.; Gawade, V.K.; Nadimetla, D.N.; Bhosale, S.V. Aggregation Induced Emissive Luminogens for Sensing of Toxic Elements. ChemistryOpen 2021, 10, 681–696.
- Nadimetla, D.N.; Bhosale, S.V. Tetraphenylethylene AIEgen Bearing Thiophenylbipyridine Receptor for Selective Detection of Copper(Ii) Ion. New J. Chem. 2021, 45, 7614–7621.
- Jiang, S.; Qiu, J.; Chen, S.; Guo, H.; Yang, F. Double-Detecting Fluorescent Sensor for ATP Based on Cu2+ and Zn2+ Response of Hydrazono-Bis-Tetraphenylethylene. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117568.
- Zhang, L.; Hu, W.; Yu, L.; Wang, Y. Click Synthesis of a Novel Triazole Bridged AIE Active Cyclodextrin Probe for Specific Detection of Cd2+. Chem. Commun. 2015, 51, 4298–4301.
- Zalmi, G.A.; Nadimetla, D.N.; Kotharkar, P.; Puyad, A.L.; Kowshik, M.; Bhosale, S.V. Aggregation-Induced Emission-Based Material for Selective and Sensitive Recognition of Cyanide Anions in Solution and Biological Assays. ACS Omega 2021, 6, 16704–16713.
- Nhien, P.Q.; Chou, W.L.; Cuc, T.T.K.; Khang, T.M.; Wu, C.H.; Thirumalaivasan, N.; Hue, B.T.B.; Wu, J.I.; Wu, S.P.; Lin, H.C. Multi-Stimuli Responsive FRET Processes of Bifluorophoric AIEgens in an Amphiphilic Copolymer and Its Application to Cyanide Detection in Aqueous Media. ACS Appl. Mater. Interfaces 2020, 12, 10959–10972.
- Nadimetla, D.N.; Zalmi, G.A.; Bhosale, S.V. An AIE-Active Tetraphenylethylene-Based Cyclic Urea as a Selective and Efficient Optical and Colorimetric Chemosensor for Fluoride Ions. ChemistrySelect 2020, 5, 8566–8571.
- Anuradha; Latham, K.; Bhosale, S.V. Selective Detection of Nitrite Ion by an AIE-Active Tetraphenylethene Dye through a Reduction Step in Aqueous Media. RSC Adv. 2016, 6, 45009–45013.
- Li, K.; Li, Z.; Liu, D.; Chen, M.; Wang, S.C.; Chan, Y.T.; Wang, P. Tetraphenylethylene(TPE)-Containing Metal-Organic Nanobelt and Its “Turn-on” Fluorescence for Sulfide (S2−). Inorg. Chem. 2020, 59, 6640–6645.
- Chen, M.; Xiang, S.; Lv, P.; Qi, C.; Feng, H.T.; Tang, B.Z. A Novel Fluorescence Tool for Monitoring Agricultural Industry Chain Based on AIEgens. Chem. Res. Chinese Univ. 2021, 37, 38–51.
- Zhou, H.; Chua, M.H.; Tang, B.Z.; Xu, J. Aggregation-Induced Emission (AIE)-Active Polymers for Explosive Detection. Polym. Chem. 2019, 10, 3822–3840.
- Hou, X.G.; Wu, Y.; Cao, H.T.; Sun, H.Z.; Li, H.B.; Shan, G.G.; Su, Z.M. A Cationic Iridium(Iii) Complex with Aggregation-Induced Emission (AIE) Properties for Highly Selective Detection of Explosives. Chem. Commun. 2014, 50, 6031–6034.
- Jackson, S.L.; Rananaware, A.; Rix, C.; Bhosale, S.V.; Latham, K. Highly Fluorescent Metal-Organic Framework for the Sensing of Volatile Organic Compounds. Cryst. Growth Des. 2016, 16, 3067–3071.
- Chang, J.; Li, H.; Hou, T.; Duan, W.; Li, F. Paper-Based Fluorescent Sensor via Aggregation Induced Emission Fluorogen for Facile and Sensitive Visual Detection of Hydrogen Peroxide and Glucose. Biosens. Bioelectron. 2018, 104, 152–157.
- Hong, Y.; Chen, S.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Water-Soluble Tetraphenylethene Derivatives as Fluorescent “Light-up” Probes for Nucleic Acid Detection and Their Applications in Cell Imaging. Chem. Asian J. 2013, 8, 1806–1812.
- He, X.; Xiong, L.H.; Zhao, Z.; Wang, Z.; Luo, L.; Lam, J.W.Y.; Kwok, R.T.K.; Tang, B.Z. AIE-Based Theranostic Systems for Detection and Killing of Pathogens. Theranostics 2019, 9, 3223–3248.
- Zhuang, X.; Wang, D.; Wang, Z.; Wang, X.; Tian, C.; Luan, F.; Fu, X. Functionalized Copper Nanoclusters-Based Fluorescent Probe with Aggregation-Induced Emission Property for Selective Detection of Sulfide Ions in Food Additives. J. Agric. Food Chem. 2020, 68, 11301–11308.
- Lu, Q.; Wu, C.J.; Liu, Z.; Niu, G.; Yu, X. Fluorescent AIE-Active Materials for Two-Photon Bioimaging Applications. Front. Chem. 2020, 8, 617463.
- Li, K.; Qin, W.; Ding, D.; Tomczak, N.; Geng, J.; Liu, R.; Liu, J.; Zhang, X.; Liu, H.; Liu, B.; et al. Photostable Fluorescent Organic Dots with Aggregation-Induced Emission (AIE Dots) for Noninvasive Long-Term Cell Tracing. Sci. Rep. 2013, 3, srep01150.
- Qi, J.; Chen, C.; Ding, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens: Union Is Strength, Gathering Illuminates Healthcare. Adv. Healthc. Mater. 2018, 7, e1800477.
- Wang, Z.; Chen, S.; Lam, J.W.Y.; Qin, W.; Kwok, R.T.K.; Xie, N.; Hu, Q.; Tang, B.Z. Long-Term Fluorescent Cellular Tracing by the Aggregates of AIE Bioconjugates. J. Am. Chem. Soc. 2013, 135, 8238–8245.
- Qin, W.; Alifu, N.; Cai, Y.; Lam, J.W.Y.; He, X.; Su, H.; Zhang, P.; Qian, J.; Tang, B.Z. Synthesis of an Efficient Far-Red/near-Infrared Luminogen with AIE Characteristics for: In Vivo Bioimaging Applications. Chem. Commun. 2019, 55, 5615–5618.
- Gao, M.; Chen, J.; Lin, G.; Li, S.; Wang, L.; Qin, A.; Zhao, Z.; Ren, L.; Wang, Y.; Tang, B.Z. Long-Term Tracking of the Osteogenic Differentiation of Mouse BMSCs by Aggregation-Induced Emission Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 17878–17884.
- Huang, Y.; Zhang, P.; Gao, M.; Zeng, F.; Qin, A.; Wu, S.; Tang, B.Z. Ratiometric Detection and Imaging of Endogenous Hypochlorite in Live Cells and: In Vivo Achieved by Using an Aggregation Induced Emission (AIE)-Based Nanoprobe. Chem. Commun. 2016, 52, 7288–7291.
- Ma, H.; Qi, C.; Cheng, C.; Yang, Z.; Cao, H.; Yang, Z.; Tong, J.; Yao, X.; Lei, Z. AIE-Active Tetraphenylethylene Cross-Linked N-Isopropylacrylamide Polymer: A Long-Term Fluorescent Cellular Tracker. ACS Appl. Mater. Interfaces 2016, 8, 8341–8348.





