Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
|
Acoustics
The emergence and development of aggregation induced emission (AIE) have attracted worldwide attention due to its unique photophysical phenomenon and for removing the obstacle of aggregation-caused quenching (ACQ) which is the most detrimental process thereby making AIE an important and promising aspect in various fields of fluorescent material, sensing, bioimaging, optoelectronics, drug delivery system, and theranostics.
- aggregation induced emission (AIE)
- chemosensors
- anion sensing
- biological cell imaging
- drug delivery system
1. Introduction
The recent development in the field of supramolecular chemistry, especially in chemo-/biosensing, biological cell imaging, and drug delivery systems, has gained a lot of attention due to its high quantum yield and good photostability [1,2,3]. Hence, numerous fluorescent materials have been developed and have attracted the attention of researchers towards the use of the florescent material such as tetraphenylethyne (TPE) derivatives Schiff’s bases, naphthalenediimide (NDI), pyrene, conjugated polymer, and various metal-organic framework (MOFs) and carbon dots (CDs) as a good platform for sensing contaminants and its application in cell imaging and drug delivery systems [4,5,6,7,8].
Luminescence is a spontaneous light emission process that comes from excited electronic states upon absorption of UV-vis light which has received great attention in various fields in chemistry, physics, material science, medicine and biology [9]. Most of the organic luminophores show this property and have been widely used in sensing applications. However, many organic luminophores are studied in dilute solutions, thus, exhibiting very different photophysical phenomena compared with concentrated solutions. This is a common phenomenon where the luminescence is either weakened or quenched at high concentration this effect is known as “concentration quenching” which is caused due to formation of aggregate which is considered as the detrimental process known as aggregation caused quenching (ACQ) [10,11]. The most common conventional luminophore presenting the ACQ phenomenon is N,N-dicyclohexyl-1,7-dibromo-3,4,9,10-perylenetetracarboxylic diimide (DDPD) as shown in Figure 1,which emits strong fluorescence in dilute solutions and suffers from ACQ at high concentration or in the aggregated state. An organic luminophore that shows ACQ is considered detrimental and shows very low sensitivity and hence its use is limited [12,13,14]. However, another phenomenon was discovered by Tang’s group in 2001 group showing overcoming of the ACQ process by the so called aggregation induced emission (AIE) phenomenon [15,16,17] which is exactly opposite to that of ACQ, wherein a molecule is initially non-emissive but becomes highly emissive in an aggregated state, as shown in Figure 2. There are other non-radiative decay processes such as restricted intramolecular vibration and rotational relaxation responsible in the AIE process. Many research groups have reported AIE-active molecules that can be used for chemical sensors [18,19,20]. Among them, hexaphenylsilole (HPS) is the common example of the AIE active molecule which exhibits enhancement in fluorescence in an aggregate state. The motions involved, such as restriction of intramolecular motion along with rotation and vibration mechanisms in the AIE active phenomenon, are well explained and accepted [21,22]. The AIE luminogens have high photostability, large stoke shift, a photobleaching resistance property, and show high sensing reproducibility [23]. This characteristic makes luminogens a promising candidate for sensing application. Nevertheless, the AIE active luminogens find wide application in various fields acting as an excellent platform for sensing of food contaminants such as toxic cations and anions, veterinary drugs, pesticides, fertilizers, pathogens such as Gram-positive and Gram-negative bacteria, food additives and so on. In addition, the AIE-active luminogens have various applications such as mechanofluorochromism, optical light-emitting device (OLED) application, solar cells, cell imaging, biosensing, and drug delivery applications. The abnormal AIE phenomenon involves different mechanisms. Interestingly, due to the unique photophysical phenomenon of AIE activity, the AIE active luminogens shows different “turn-on” sensing mechanism via various interactions involving electrostatic interactions, hydrogen bonding, van der Waals interactions, and metal-ligand interactions. Another approach is through recognition of analyte with “turn-on” fluorescence via restriction of intramolecular rotation. Other different electron transfer processes involved in sensing mechanism are photoinduced electron (PET), intramolecular charge transfer (ICT) and Forster resonance electron transfer (FRET). The potential proposed mechanism responsible for sensing occurs through different pathways such as the restriction of intramolecular rotation (RIR), hydrogen bond interaction, J-aggregation, molecular planarization, and twisted intramolecular charge transfer (TICT) [24,25,26,27,28,29]. Restricted intramolecular rotation (RIR) and restricted intramolecular vibration (RIV) were merged as restricted intramolecular motion (RIM) regarded as the important mechanism involved for the AIE effect, Figure 3.
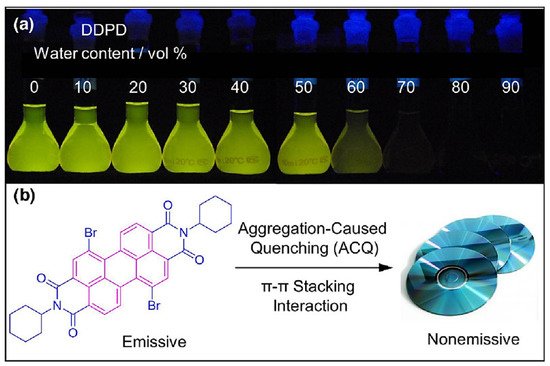
Figure 1. (a) Fluorescence photograph of the (N,N-dicyclohexyl-1,7-dibromo-3,4,9,10-perylenetetracarboxylic diimide) molecule with increasing water content and (b) showing the aggregation-caused quenching (ACQ) phenomenon. Reprinted from reference [13] with the permission of the Royal Chemical Society.

Figure 2. Photograph of hexaphenylsilole (HPS) representing aggregation induced emission (AIE) phenomenon. Reprinted from reference [12] with the permission of the Royal Society of Chemistry.
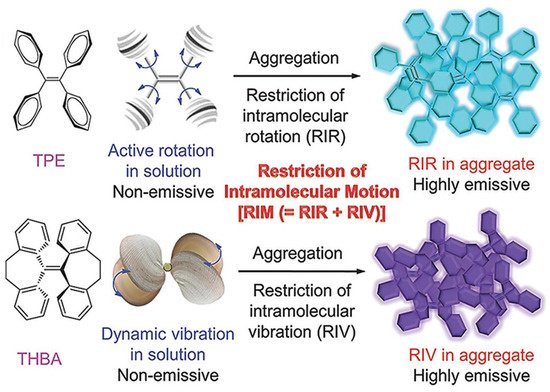
Figure 3. Representation of proposed mechanism for AIE effect. Reprinted from reference [29] with the permission of Wiley-VCH.
2. Aggregation Induced Emission (AIE) Active Molecules for Sensing Application
Metal ions play a very important role in various biological and physiological processes occurring in the body and are required by all life forms. Most of the metal ions participate in many biochemical processes, such as material transportation, energy conversion, information transmission, and metabolic regulation [30]. The presence of excess metal ions may lead to serious health issues and may hamper the various metabolic processes. Therefore, there is a need for monitoring, detection, and study of their distribution in individual systems including tissues, cells, as well as organisms. Several methods have been reported for metal ion detection, but few of these methods suffer from drawbacks such as tedious synthesis, cost and non-selectivity. Nevertheless, small organic molecules with high fluorescence properties found to be more advantageous over other reported chromophores due to high sensitivity, selectivity, high quantum yield, simple synthetic routes, easy operation, and real-time detection [31]. There are several essential (Na+, K+, Ca2+, Zn2+ and Mg2+, Fe2+) and non-essential/toxic metals (Hg2+, Pd2+, Pb2+, Cu2+, As3+ Cr3+) and anions (F−, Cl−, CN−, Br−). Detection with a lower detection limit with quantification is very important for both the essential and non-essential metal ions due to their presencefrom a biological and environmental point of view. Among the wide range of applications of fluorescence techniques using fluorescent molecules of various cations and anions, is the most important and found to be a promising active platform in various areas.
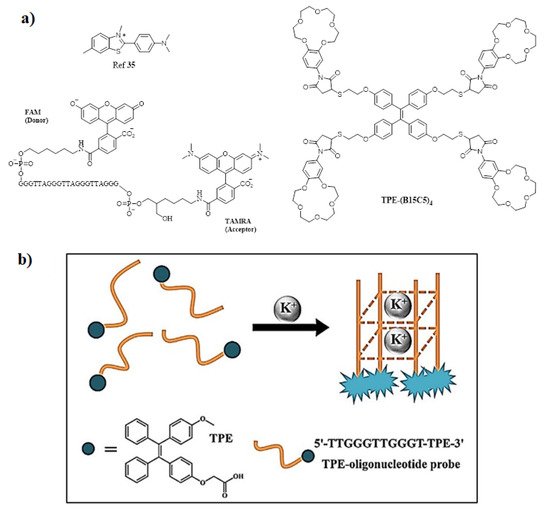
Among cations, potassium ion is an essential component of the human body that plays a very important role in monitoring and regulating the various physiological functions, such as heart beat, muscle strength, regulates nervous and renal functions [30,32]. The normal concentration of K+ in plasma ranges from 3.5 to 5.5 mM; moreover, if the concentration increases to 7.0 mM then it may result in a complication [33]. To quantify the amount of K+ ion, Liu et al. synthesized molecular rotors for detection of K+ ion [34] bearing G-quadruplex derived from guanine (G)-rich DNA sequences is used as a structural motif. Initially, the crown ethers and cryptands were frequently used as host moieties for sensing K+ ions. There are very few AIE active fluorescent probes that have been reported until now. In this regard, Wang and co-workers utilized an AIE strategy and constructed a highly sensitive and selective fluorescent OFF-ON probe via host-guest molecular recognition by functionalizing a novel crown ether on TPE derivative by the thiol via click reaction, yielding TPE-(SH)4 and maleimide-functionalized benzo-15-crown-5(B15C5). The probe TPE-(B15C5)4, consisted of TPE as the core moiety with four substituted B15C5 units. Its optical properties were studied via the AIE mechanism and found to be highly selective and sensitive towards K+ ions [35]. In addition, Lu and coworkers designed TPE modified with a DNA oligonucleotide-based fluorescent probe exhibiting excellent AIE active behavior and a probe showing excellent sensing behavior towards the K+ ion. Furthermore, the probe was used for biological cell imaging [36]. Moreover, another novel potassium sensing oligonucleotide derivative via fluorescence resonance electron transfer (FRET) was studied and used for detecting K+ in water [37]. The structure of the most commonly synthesized AIE active fluorescent probe is illustrated in Figure 4.
Calcium is another essential metal ion for various physiological functions such as muscle contraction, blood pressure, heart beats, vascular and nerve functioning at the same time calcium major component of the bone structure in the body [38]. The intracellular calcium is stored in mitochondria and the endoplasmic reticulum while most of the calcium is found in bones and teeth. The change in concentration in Ca2+ may result in various diseases resulting in obesity and Alzheimer’s disease [39]. The increase in level of Ca2+ in the body may lead to several diseases’ cardiac arrhythmias, sarcoidosis, and tuberculosis. Therefore, sensing and monitoring extracellular and intracellular Ca2+ is important for diagnosis. In this regard, Gao and co-workers designed an AIE active probe for in situ detection of calcium. The probe SA-4CO2Na can distinguish efficiently between the normal and hypercalcemic calcium in the cell. The probe was synthesized by reacting 5-(chloromethyl)-2-hydroxybenzaldehyde with diethyl imino diacetate in the presence of hydrazine monohydrate to give SA-4CO2Et which was further reacted in presence of sodium methoxide affords to form SA-4CO2Na. The exact mechanism involved in the detection of Ca2+ is by the formation of highly emissive fibrillar aggregate via electrostatic and chelating interaction between the iminodiacetate groups and Ca2+ ion. Initially in the absence of calcium the probe shows very weak emission in aqueous solution while in the presence of calcium the formation of fibrillar takes place resulting in the enhancement in fluorescence, as shown in Figure 5 [40].
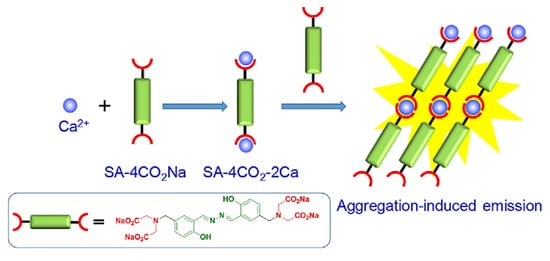
Figure 5. Schematic illustration of the AIE active probe SA-4CO2Na for intracellular detection of Ca2+. Reprinted from reference [40] with the permission of the American Chemical Society.
In another report, Wang and coworkers designed AIE active polyarylene ether nitrile fluorescent nanoparticles acting as an excellent probe for intracellular imaging. The synthesized nanosphere showed excellent biocompatibility in presence of calcium with a 21% quantum yield [41]. Moreover, another TPE-based AIE active fluorescent probe was synthesized by Zhang’s group for sensing Ca2+ using the criteria of AIE behavior. The probe consisted of a TPE moiety with a bidentate pyridine carboxylate unit. The probe showed a “turn-on” fluorescence response to Ca2+ with a lower detection limit of 51.2 nM. Importantly, the probe can also be recycled by addition of ethylenediamine tetra-acetic acid (EDTA) [42] and further reused. Ishiwari’s group demonstrated use of AIE-active TPE-based solid state gel sensor fluorescent molecule for extracellular Ca2+ imaging. This gel consisted of polyacrylic acid (PAA) functionalized with TPE produces PAA-TPE, in which TPE acted as a pendant with an AIE-active property. Interestingly, crosslinking with gel (g-PAA-TPE) showed good selectivity towards Ca2+ [43]. Figure 6 shows various AIE active fluorophores based on TPE for detection of Ca2+.
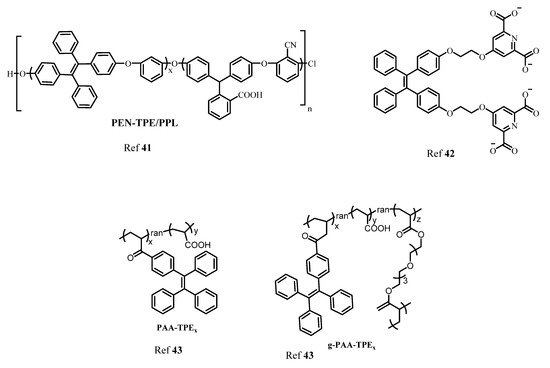
Figure 6. The structures of different AIE active TPE derivatives for sensing of Ca2+.
In the human body it is well known that after iron, zinc is the second most common transition metal ion. Along with Na+, K+ and Ca2+, Zn2+, plays a very important role in various biological and physiological functions such as the nervous system, gene transcription, immune functions, and mammalian reproduction. In fact, one of the most important applications is that the Zn2+ acts as an excellent catalytic cofactor and structural center in various enzymes, DNA, and proteins. Therefore, the need for studying and monitoring the Zn2+ is a major aspect in sensing applications. By taking advantage of AIE-activity of molecules, Sun and coworkers designed a TPE-based “turn-on” fluorescent probe for detection of Zn2+ ion in an aqueous medium. In this, co-ordination of Zn2+ to -N(CH2COO−)2 and intermolecular coordination of Zn2+ lead to aggregation resulting in an enhancement in fluorescence [44]. In another report, Wei and group synthesized AIE active multifunctional metal-organic vesicles with triarylamine carboxylate (TPA-1) for specific detection of Zn2+ ions. This fluorescent probe can be employed for biological cell imaging and drug delivery systems [45]. Another multifunctional TPE-based AIE active fluorescent probe was designed by Tang and coworkers for selective and sensitive detection of Zn2+ and Hg2+ ion with 1.24 × 10−6 molL−1 and 2.55 × 10−9 mol L−1, respectively. The AIE activity of the fluorescent probe was analyzed using the THF-water fraction [46]. More recently, Maity et al. developed an antipyrine fluorescent probe 4-[(2-hydroxy-3-methoxy-benzylidene)-amino-1,5-dimethyl-2-phenyl-1,2-dihydro-pyrazole-3-one (OVAP) for selective detection of Al3+ and Zn2+ detection [47]. Diana and Panunzi in their review described several AIE active fluorescent probes for sensing of Zn2+ ions [48]. Moreover, Sun et al. developed a multifunctional AIE active Schiff base fluorescent probe (TPESB) combined with AIE and ESIPT. TPESB was employed for dual-channel sensing of Zn2+ with high selectivity and sensitivity exhibiting a low limit of detection of 38.9 nM. Thus due to its low cytotoxicity, the probe is applied for sensing of Zn2+ in live cells [49]. In this work, He et al. designed a “turn-on” fluorescent probe with TPE as AIE active fluorophore combined with the peptide chain. The self-assembled complex was formed between the Zn2+ and three histidine residues. The probe showed strong fluorescence emission in presence of Zn2+. The observed limit of detection was 18.56 nM and it can be employed for biological cell imaging and intracellular detection of Zn2+ having low cytotoxicity and good stability [50]. Figure 7 shows the various fluorophores used for detection of Zn2+ ion.
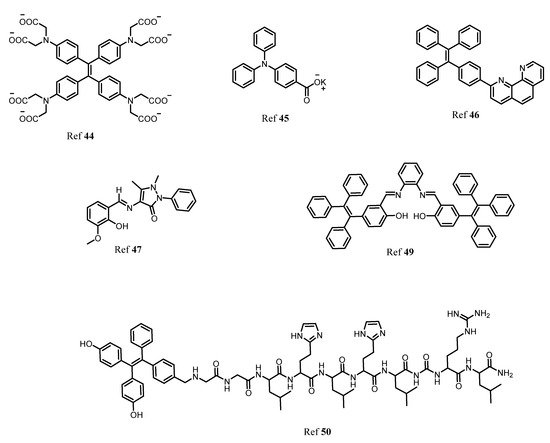
Figure 7. The various AIE active fluorophores illustrated for detection of Zn2+ ion in solution.
There are several non-essential metal ions or heavy metal ions including anions that are very well known for their toxicity to human health causing severe diseases. The major factor responsible for the entry of these toxic metal ions into the environment is due to industrialization, modern agricultural practices, and the release of untreated waste directly into water bodies resulting in contamination and destruction of natural resources indirectly affecting human health [51,52,53,54]. Different toxic ions that have toxic effect are Al3+, Cr3+, Cd2+, Co2+, Mg2+, Hg2+, Sb3+, CN−, F−, Br−, Cl−, As3+, As5+, Cu2+. Therefore, there are several analytical tools have been employed for the detection of this metal ion and anion in the system such as atomic absorption spectroscopy, inductively coupled plasma mass spectrometry, and colorimetric methods. However, fluorescent chemosensors have gained a lot of advantages over all these methods for several reasons such as fast, cost-effective, simple and easy to handle, naked-eye detection, and low cost of instrumentation. However, few organic fluorescents suffer from aggregation-caused quenching thanks to the AIE phenomenon developed to overcome the difficulties faced due to ACQ. Therefore, here different AIE active fluorescent probes (Figure 8) for detection of toxic metal ions and anions are described [55,56].
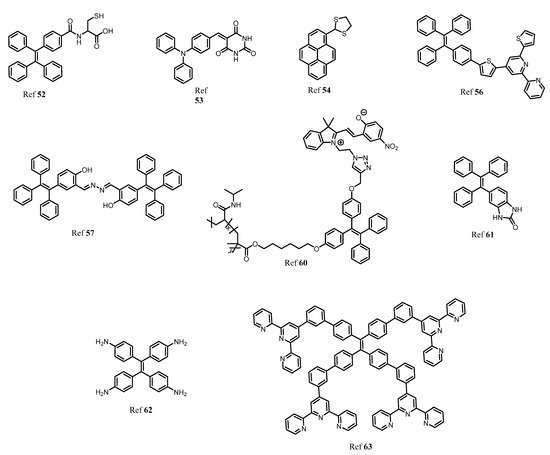
Figure 8. The structural representation and examples of several substituted AIE active fluorescent materials for sensing non-essential metal ions and anions.
Beglan et al. synthesized a derivative of TPE functionalized with cysteine as the fluorescent AIE active dye for the detection of Arsenic (As3+) in aqueous media [57]. Further investigation of the probe revealed that the thiol group of cysteine acts as a binding site for arsenic via the As–S bond. The three binding units of cysteine combines to sense As3+ to give a symmetrical As–(Cys-TPE)3 complex. Wen and group synthesized a novel AIE triphenylamine fluorophore used for sensing of Hg2+ and CN−. The probe showed excellent AIE active behavior and has potential application in biological cell imaging. However, the probe can be recycled and reused without any loss [58]. In another report, a highly selective and sensitive pyrene-based AIE active ratiometrics “turn-on” fluorescent probe (pyrene-DT) was designed by Ma co-workers for detection of Hg2+ in aqueous media. The probe has good practical applicability in the preparation of test strips and detection of Hg2+ in a water sample [59]. Our group recently described different AIE active luminogens that are designed for selective and sensitive detection of various toxic metal ions [60]. Elemental copper plays a very important role in various physiological processes in the environment. However, excess and deficiency of copper may lead to various neurological disorders, mostly kidney and liver damage. More recently, our group synthesized TPE-based AIE active comprising a thiophenylbipyridine receptor for selective and sensitive detection of Cu2+. The probe showed a very low limit of detection up to 7.93 nM and the probe was further used for test strip preparation, which is one of the best advantages for practical applicability [61]. In a similar direction, Jiang and coworkers designed double detecting hydrazono-bis-tetraphenylethylene (Bis-TPE) based AIE active fluorescent probe for Cu2+ and Al3+. Here, the probe showed quenching of fluorescence to Cu2+ and red-orange fluorescence for Zn2+ with 1:1 stoichiometry. However, the strong fluorescence of Bis-TPE+Cu2+ can be recovered by adding adenosine triphosphate (ATP) giving “turn-on” fluorescence similar to Bis-TPE+Zn2+ that can be quenched by adding Cu2+ which is then further recovered by adding ATP. The probe Bis-TPE can be employed for test strips sensing and biological cell imaging [62]. Zhang and colleagues synthesized click triazole bridged cyclodextrin (CD) based AIE active molecules for sensing Cd2+. The probe possesses a good AIE active property and shows a “turn-on” fluorescence response towards Cd2+ ions. The limit of detection for the probe was found to be 0.01 μM. Interference studies in the presence of only Cd2+ showed a good response [63].
Similar to cations, anions sensing has been in more demand due to its wide application in a biological and chemical processes, even though the anions are also considered detrimental and toxic to the environment and human health. The fluorescence method has proved to be the best approach for detection, monitoring, and remediation of anions such as SO42−, CN−, F−, NO3−, Cl−, Br−. Most recently, our group has designed and synthesized a TPE-based AIE active fluorescent probe which is highly selective and sensitive towards cyanide. It can be seen clearly that the probe can be efficiently utilized for naked-eye detection Figure 9 test strips, and importantly the fluorescent probe, were used for the detection of CN− ion in biological food samples. Moreover, the probe was utilized for a biological cell imaging application [64].

Figure 9. A photograph representing the sensing performance of the probe towards CN− ion in an aqueous solution in the presence of other anions under: (a) visible light and (b) UV light (365 nm). Reprinted from reference [60] with the permission of the American Chemical Society.
Nhein and coworkers designed a novel amphiphilic AIE active polymeric fluorescent material, poly(NIPAM-co-TPE-SP) prepared by NIPAM as a hydrophilic unit and tetraphenylethylene-spiropyran monomer (TPE-SP) employed for detection of cyanide in aqueous media. The unique property of this fluorescent probe is that in the presence of UV light the closed spiropyran (non-emissive) poly(NIPAM-co-TPE-SP) opens to give merocyanine (MC) (poly(NIPAM-co-TPE-MC) in an aqueous solution [65]. In addition, our group has synthesized another AIE-active TPE-based cyclic urea-based receptor for selective detection of F− ions. The probe showed good selectivity in the presence of other anions such as Cl−, Br−, I−, HCO3− CO3-, NO2−, NO3−, SO32−, SO42−, AcO−, S2−, ClO4−, HPO4−, CN−. Absorption and fluorescence studies revealed that only F− ion showed excellent selectivity towards the cyclic urea probe [66]. Similarly, using tetraphenylethylene as a moiety to monitor optical as well as calorimetric changes, Anuradha et al. synthesized an amino-functionalized fluorescent probe i.e., tetraamino-TPE (TA-TPE) for the selective detection of nitrite ion in aqueous media. The probe showed a “turn-on” fluorescence response which was considered an advantage over other receptors [67]. In another example, TPE containing AIE active metal-organic supramolecular nanobelt was developed by Li et al., and in their work the TPE was functionalized with terpyridine moiety to give tetrapodal TPE-terpyridine ligand which self assembles to give a metal-organic nanobelt which shows high selectivity towards S2− [68]. AIE active luminogens did not only limit its application to sensing of cations and anions but also showed wide application in detection of pesticides [69], explosives [70,71,72], biomolecules [73,74], pathogens [75], food additives [76] and so on.
3. AIE Active Molecules for Biological Cell Imaging
The various fundamental processes taking place in life can be very well studied and understood which can be achieved effortlessly using fluorescence technique. Fluorescence microscopy is an important tool that has now become most advantageous and launched in biological research for advance and a better understanding of various intracellular processes and dynamics at a cellular level. The field of fluorescence imaging is actively developing as this fluorescent tool plays a major role in monitoring the different analytes and helps in studying the biological events occurring in the intracellular environment. Fluorescence microscopy is one of the best techniques to have been used in the last few decades. Nowadays, there is great progress in instrumentation techniques which makes fluorescence imaging simple and also overcomes the drawbacks and challenges suffered in the past. Doing so, various advantages may be tackled such as minimal photodamage, high resolution, and penetration deep into tissues. Nevertheless, in fluorescence microscopy, two-photon fluorescence imaging has a greater advantage over one-photon fluorescence imaging [77].
In this regard, Li and co-workers designed organic far-red/near-infrared AIE active TPE substituted dots such as TPE-TPAFN and TPA-FN with excellent utility in long-term cellular tracing. Due to its excellent characteristics of high emission efficiency, large absorptivity, excellent biocompatibility, and also its photobleaching resistance, the fluorescent probe is a good candidate for in vitro as well as in vivo cellular tracing. The matrix used for fabrication is the mixture of polyethylene glycol (PEG) and lipid-PEG-NH2. Interestingly, the Tat (transactivator of transpiration)-AIE dots demonstrated excellent imaging properties [78,79].
Wang et al. used “turn-on” fluorescence based on AIE active TPE in conjugation with chitosan to produce chitosan-TPE bioconjugate which can be used as “turn-on” fluorescence for long-term cellular tracing, which is very important for monitoring biological and therapeutic processes. This bioconjugate exhibits excellent AIE active property followed by internalization of aggregate take place in HeLa cells for further biological cell imaging. The HeLa culture was used and incubated for 24 h and the fluorescence images were recorded, and the cell imaging process was carried out until the 15th passage, Figure 10 [80].
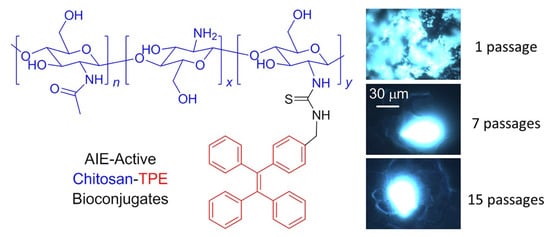
Figure 10. Structural illustration and utilization of TPE-CS as an excellent fluorescent marker in biological cell imaging in HeLa cells. Reprinted from reference [76] with the permission of the Journal of the American Chemical Society.
In the fluorescent materials, far-red/near-infrared (FR/NIR) emissions higher than 640 nm are regarded as an excellent and promising candidate for fluorescence imaging. Using similar criteria, the Qin group synthesized selenium containing FR/NIR AIE active luminogen (TTSe dots) which is very rare; however, there are very few reports available on selenium-containing fluorescent probes for bioimaging application. The synthesized probe has excellent applications in imaging of the sentinel lymph node (SLN) mapping and finally in tumor imaging. In addition, this fluorescent luminogen is utilized for imaging the brain blood vessels of mice using a high-resolution two-photon imaging technique as shown in Figure 11. Hence this highly emissive selenium-containing TPE-based fluorescent luminogen is used for in vivo biological applications [81].
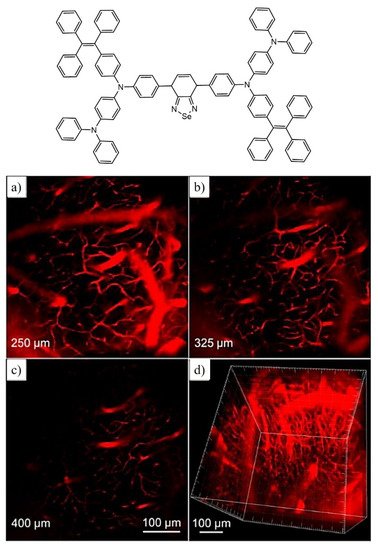
Figure 11. Representative molecular structure with fluorescent images captured under two-photon imaging technique for brain blood vessels upon incubation of mouse cells for 0.5 h with TTSe dots (a–c). (d) represents a high-resolution 3-D image of blood vessels at 100 μM scale. Reprinted from reference [81] with the permission of The Royal Society of Chemistry.
Similarly, Gao and coworkers developed AIE functionalized with Tat-peptide (AIE-Tat NPs) PITBT-TPE for tracing bone marrow mesenchymal stem cells (BMSCs). The fluorogens consisted of a mixture of DSPE-PEG2000 and DSPE-PEG2000 maleimide matrix. Furthermore, the obtained nanoparticle was modified with cysteine Tat-peptide (RKKRRQRRRC). The fluorescence quantum yield of the compound was found to be 23.5% with a lifetime of 5.37ns. Thus, the red emission made the AIE-Tat NPs a promising candidate for bioimaging of BMSCs cells; however, intense red emissions were observed in a confocal microscope. The results indicated 100% labeling efficiency and the concentration of AIE Tat does not affect the cell viability of BMCs cells [82]. Huang et al. reported AIE-active fluorescent luminogen for detecting and intracellular imaging of ClO− in cells. The TPE unit being hydrophobic forms aggregate nanoparticles into micelle that emits red fluorescence. Therefore, the probe can be utilized for endogenous ClO− detection in live cells. To study the endogenous imaging of ClO−, the zebrafish was used as a sample because of their excellent transparency at the embryonic and larval stages. Zebrafishes were incubated with the fluorescent compound for 60min, and green fluorescence was observed however the green fluorescence enhanced with increasing incubation time. This result reveals that the probe can be employed well for in vivo ClO− detection and imaging applications [83]. The Ma group synthesised AIE-active TPE polymer cross liked using N-isopropylacrylamide fluorescent material for long-term cellular tracing. This fluorescent polymer was designed with hydrophilic N-isoprpylacrylamide polymer and hydrophobic TPE subunits with cross-linkers 4,4′-(2,2-dibromoethene-1,1-diyl)bis(vinylbenzene)DDBV. The synthesized TPE-PNIPAM (P6) had good compatibility and long-term imaging properties. In addition, the probe showed temperature-responsive behavior with fluorescence change. Its excellent biocompatibility makes the fluorescent probe a highly promising material for a biological imaging application. Thus, it was observed that the probe was utilized for examination of living A549 human lung adenocarcinoma cells as shown in Figure 12, in which the deep blue color of the cytoplasmic cells suggests the P6 molecule aggregates and internalizes deep into the cells. The P6 molecule shows good AIE activity, low toxicity, and leakage-free staining. Hence it can be concluded that the P6 molecule acts as an excellent fluorescent marker for a biological long-term cell imaging application [84].
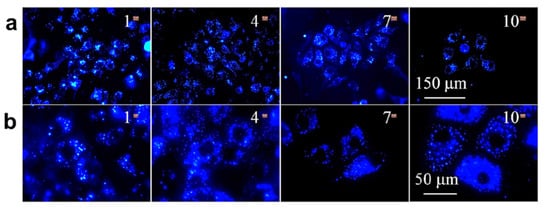
Figure 12. The fluorescence images of A549 cells upon incubation with a P6 probe (100 μg/mL) were captured at magnification (a) ×40 and (b) ×100. Reprinted from reference [84] with the permission of the American Chemical Society.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27010150
This entry is offline, you can click here to edit this entry!
