
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Attila Frigy | + 2119 word(s) | 2119 | 2020-08-20 04:04:20 | | | |
| 2 | Nicole Yin | Meta information modification | 2119 | 2020-09-09 03:43:16 | | |
Video Upload Options
Interatrial block associated with atrial arrhythmias, mainly atrial fibrillation, define the Bayés’ Syndrome (called after Bayés de Luna, a proeminent cardiologist, who published the seminal paper about the problem of P-wave anomalies and interatrial blocks). Finding the signs of interatrial block on ECG represents a call for active screening of atrial fibrillation and starting of prophylactic anticoagulation in selected patients.
1. History
In 1979, Bayés de Luna published a paper about atrial conduction abnormalities, which were classified as intra- and inter-atrial blocks, the latter being further divided into partial and advanced type[1][2]. In 1985, Bayés de Luna and his team determined that the prevalence of advanced IAB was 0.1%, increasing up to 2% when patients with heart valve disease or cardiomyopathy were considered[2][3]. In 1988, the same author published a new study on patients undergoing 24-h Holter monitoring, showing that patients with IAB had higher rates of supraventricular tachycardia compared to those without advanced IAB; furthermore, advanced IAB was associated with a higher incidence of supraventricular premature beats[2][4]. Agarwal et al. observed a higher prevalence of IAB in new onset AF cases versus a group with sinus rhythm[2][5]; Ariyarajah et al. demonstrated that in patients with probable diagnosis of cardioembolic stroke, those patients with IAB on the ECG had a greater probability of presenting left atrial enlargement or a thrombus in the left atrium[2][6]. The first international consensus report on IAB was published in 2012[7].
IAB represents a marker and electro-anatomical substrate for the development of supraventricular (atrial) arrhythmias. The association of IAB and supraventricular arrhythmias, particularly atypical atrial flutter and AF, was named (after its first descriptor) Bayés’ Syndrome[2].
2. Anatomo-Electrical Background
Intra- and interatrial electrical pathways are represented by four bundles: (1) the Bachmann’s bundle (BB), which is the anterior internodal pathway, having also a (2) branch which connects the right atrium to the left atrium, (3) the Wenckebach’s bundle, which is the middle internodal tract, and (4) the Thorel’s bundle, which represents the posterior internodal pathway[8].
During normal sinus rhythm, the largest, most common and preferential anatomical route for interatrial conduction is through the BB and, consequently, IAB is the result of a conduction delay or complete block in this pathway[9]. It has to be mentioned, that other, more slowly conducting structures also electrically bind the two atria. These are located at the coronary sinus, in the antero-superior interatrial septum and in the postero-inferior interatrial septum[10]. The electrophysiological background of advanced IAB is thought to be a situation when a sinus impulse can no longer pass via the Bachmann region, instead, it propagates towards the AV node depolarizing the right atrium, then the left atrium is depolarized in a caudocranial direction starting from the inferior left atrium, near the atrioventricular node (most frequently the coronary sinus, and in a small proportion the fossa ovalis). This superior–inferior–superior activation pattern is responsible for the biphasic (+/−) aspect of the P waves in the inferior ECG leads (II, III, aVF). The IAB types and their pathophysiological and ECG features are presented in Table 1[7].
Table 1. Interatrial conduction block (IAB) types, their electrophysiological background and the corresponding ECG features.
|
IAB Type |
Electrophysiological Background |
ECG Features |
|
first-degree (partial) |
delayed conduction in the zone of BB |
P-wave duration >120 ms |
|
third-degree (advanced) |
blocked conduction in the zone of BB: caudocranial, retrograde activation of the left atrium from the low right atrium (coronary sinus and to a lesser degree, the fossa ovalis) |
P wave duration >120 ms with biphasic morphology (a positive initial component and a terminal negative component) in the inferior leads (II, III, aVF) |
|
second-degree |
delayed or blocked conduction in the zone of BB |
transient appearance of first-degree and/or third-degree IAB pattern on the same ECG recording (atrial aberrancy)—related or not to an atrial premature beat |
Figures 1–3 illustrate the characteristic ECG patterns of first- and third-degree IAB.
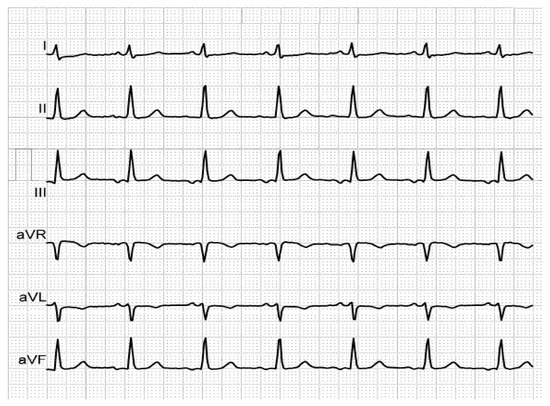
Figure 1. Biphasic P-waves in III and aVF, revealing third-degree IAB, in a patient with mitral prosthesis and episodes of paroxysmal AF.
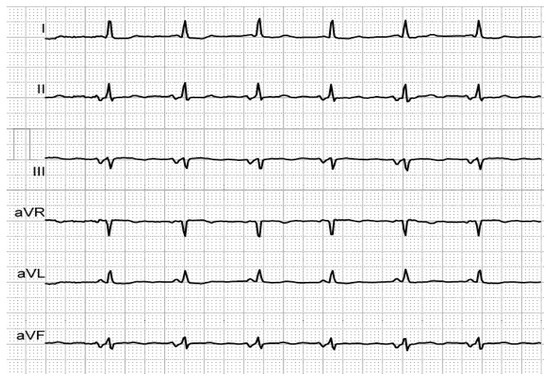
Figure 2. Biphasic P-waves in II, III and aVF, revealing third-degree IAB, in an elderly, hypertensive patient with history of AF.
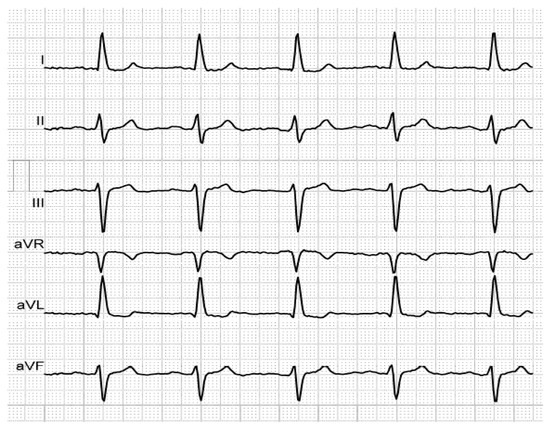
Figure 3. P-wave duration of 140 ms, revealing first-degree IAB, in an elderly, hypertensive patient with history of AF.
Interestingly, procedures such as pulmonary vein antrum isolation could modify the electrocardiographic IAB pattern, up to its disappearance, because of the loss of a part of left atrial signals[11].
3. Pathophysiology and Morpho-Functional Substrate
Atrial morpho-functional remodeling, consisting of fibrosis, atrial enlargement and consecutive alteration of atrial function, is responsible for and related to the development of IAB. Atrial fibrosis is the common end-pathway for various cardiac injuries and is able to produce a delay and/or blockage in cardiac electrical conduction. Although the exact role of atrial fibrosis in the development of IAB is still under investigation, it seems that it represents an important structural substrate[9].
It was observed that IAB shows a degree of reversibility: in several studies a reduction of the P-wave duration over time has been noted, indicating the dynamic character of the pathological processes leading to IAB. This phenomenon could be related to reverse atrial remodeling[9].
Atrial cardiomyopathy has been defined as “any complex of structural, architectural, contractile or electrophysiological changes affecting the atria with the potential to produce clinically-relevant manifestations”[12]. It is well known that the development of atrial cardiomyopathy in the setting of atrial fibrillation depends mainly on AF duration: very short-term AF produces no ultrastructural alterations, AF lasting several weeks causes cardiomyocyte changes, while long-term persistent AF produces combined cardiomyocyte and fibrotic changes. Atrial fibrosis plays an important role in the progression of long-term persistent AF to permanent form. Atrial fibrillation-induced complex atrial remodeling is the substrate of the maintenance, progression and stabilization of AF[12].
Left atrial enlargement (LAE) is often associated with IAB, however its presence is not mandatory for the development of IAB[7]. The presence of IAB in an already enlarged LA determines (because of the electrical delay in the activation of the atria) an increased alteration of the LA systolic indices over time. IAB has been proposed as a marker of the structurally altered and electromechanically dysfunctional LA, and, at the same time, as an electrophysiological risk factor and substrate for AF and conditions related to AF, such as congestive heart failure[13][14]. It is important to mention that interatrial dyssynchrony caused by IAB, beyond generating atrial remodeling (increased atrial pressure, atrial dilation and atrial fibrosis), produces endothelial dysfunction, contributing via both factors to enhanced local thrombogenesis, responsible for cardioembolic events[15].
4. Imaging Correlations
Attempts to correlate inter-atrial septum thickness (measured by computed tomography) to IAB, in order to use it as a predictor for AF recurrence in patients undergoing catheter ablation for paroxysmal AF, have proven unsuccessful[16]. Rather than assessing the thickness of atrial walls, determining the degree of atrial fibrosis seems to be more beneficial. The atrial fibrotic process in the LA can be assessed by various methods, the gold standard being cardiac magnetic resonance imaging (CMR). Additionally, new echocardiographic techniques, like myocardial deformation imaging (strain, strain rate) using speckle tracking can deliver indirect information about the fibrotic process[17].
Classical transthoracic echocardiography can assess the LA both anatomically and functionally. The representative of the LA size is not its antero-posterior diameter, but its volume, measured by two-dimensional, or—recently—three-dimensional methods. Determining the reservoir function, reflected by the difference between the maximum (before the mitral valve opens) and the minimum volume (when the mitral valve closes), is the most important in this regard[17].
Tissue Doppler Imaging (TDI) can assess the atrial electromechanical delay (EMD), helping to identify patients at risk of developing IAB and Bayés’ Syndrome. EMD is defined as the time interval between the onset of atrial electrical activity (onset of P wave on ECG) and the atrial contraction (onset of the A’ wave on TDI). Atrial strain and strain rate, obtained using speckle tracking, are reduced in the case of fibrosis, revealing a decreased atrial compliance and an altered reservoir function, even before the LA starts to dilate [19]. In a 2018 study, IAB has been shown to correlate directly with the structural remodeling and with the decrease in absolute values of LA strain rate[18].
CMR provides multiple anatomical and functional data such as parietal thickness, volumes, and, most importantly, the degree of fibrosis. The latter is obtained by the use of gadolinium, a contrast agent which accumulates in the interstitial space. As a result, conditions that modify the interstitial space, such as fibrosis, can be identified by delayed myocardial enhancement sequences: myocardial fibrotic areas are hyperintense, while healthy regions have a hypointense or null aspect. At the level of atria, fibrotic changes can be seen as fine areas of delayed enhancement, and can be quantified using the Utah classification system (from I to IV)[17]. Another CMR-derived technique that could be used to determine fibrosis is the T1 sequence, which calculates the myocardial T1 relaxation time during a 10-s voluntary apnea. This method has the disadvantage of high cost, the need for a high level of experience and the low availability for current clinical practice[17]. 3D reconstruction of 3T late gadolinium enhancement CMR allows visualization of atrial fibrosis in patients with IAB, showing the involvement of the upper part of the septum, where the BB is situated[15].
The amount of fibrosis identified by these techniques is strongly associated with AF recurrence after pulmonary vein isolation. On the other hand, a larger amount of fibrosis induced at the ablation sites equals a better isolation of the AF foci and less chance of recurrence[15].
5. Clinical Relevance
First degree IAB is relatively common, is associated with a higher risk of atrial fibrillation, causing and is related to higher global and cardiovascular mortality; third degree IAB, although less frequent, represents a strong marker of LAE and paroxysmal supraventricular tachyarrhythmias including AF[7].
The importance of Bayés’ Syndrome and the value of IAB in predicting the occurrence of AF (new onset or recurrent) has been investigated in different clinical scenarios and in large population studies. The main data are presented in Table 2[9][19][20][21][22][23][24][25][26][27].
Table 2. The predictive value of IAB for AF occurrence in different clinical settings and in population studies.
|
Predicting Value |
Clinical Setting/Study |
Reference |
Observations |
|
IAB as a predictor of new onset AF |
- patients with Chagas cardiomyopathy and ICDsa |
[19] |
|
|
- patients with NSTEMIb |
[20] |
|
|
|
- post-transcatheter aortic valve replacement |
[21] |
|
|
|
- patients with severe heart failure undergoing cardiac resynchronization device implantation and no AF history |
[22] |
IAB proved to be an independent predictor of AF |
|
|
IAB as a predictor of AF recurrence |
- post-cardioversion |
[23] |
|
|
- post-pulmonary vein isolation |
[24] |
|
|
|
- post-atrial flutter ablation (cavotricuspid isthmus ablation) |
[25] |
|
|
|
IAB as a predictor of AF occurrence in the general population |
- the Atherosclerosis Risk in Communities (ARIC) study |
[26] |
risk factors associated with advanced IAB development identified by the ARIC study were age, white race, male gender, body mass index, systolic blood pressure, use of antihypertensive medication, low-density lipoprotein cholesterol |
|
- the Copenhagen ECG Study |
[27] |
|
ICD = implantable cardioverter defibrillator; NSTEMI = non ST-segment elevation myocardial infarction.
IAB is common in patients with heart failure and these patients have more frequently new-onset AF, ischemic stroke, higher hospitalization and mortality rates. In patients with valvular heart disease requiring surgery, advanced IAB was independently associated with postoperative AF[15].
Severe obstructive sleep apnea is also a predictor of IAB, which in this case could be reversed in patients receiving treatment with continuous positive airway pressure (CPAP)[17][28].
P-wave duration is positively correlated with age[9]. Aging is associated with the appearance of structural changes in the myocardium, predominantly fibrosis. IAB appears frequently in elderly persons, e.g., being present in half of centenarians, as a result of progressive conduction system degeneration. This phenomenon is associated with an increased prevalence of AF/atrial flutter. Both AF and IAB are recognized markers of worse prognosis, with a reduction in life expectancy, higher occurrence of dementia, and worse perceived health status[29]. Among the risk factors for incident AF (age, sex, heart failure, hypertension, diabetes mellitus, coronary artery disease, chronic kidney disease, sleep apnea), age was the single most influential factor—AF is more prevalent in elderly patients[30].
For a long time, the increased risk of ischemic stroke in patients with IAB was thought to be a consequence of AF episodes. Recent research has shown that IAB is an independent risk factor for stroke, even in patients without supraventricular arrhythmias. IAB has been independently associated with an increased risk of non-lacunar ischemic stroke when considering sex, age or race, or the presence of previous episodes of AF[15].
The centenarians with IAB had higher mortality and higher incidence of dementia[31].
Derived from the thromboembolic risk related to IAB, it has been suggested that IAB may also represent a novel risk factor for acute occlusive mesenteric ischemia, regardless of the occurrence of AF[32].
References
- De Luna, A.J.B. Block at the auricular level. Rev. Esp. Cardiol. 1979, 32, 5–10.
- Conde, D.; Baranchuk, A. What a Cardiologist must know about the Bayes’ Syndrome. Rev. Argent. Cardiol. 2014, 82, 237–239.
- A. Bayes De Luna; R. Fort De Ribot; E. Trilla; J. Julia; J. Garcia; J. Sadurni; J. Riba; F. Sagués; Electrocardiographic and vectorcardiographic study of interatrial conduction disturbances with Left Atrial Retrograde activation. Journal of Electrocardiology 1985, 18, 1-13, 10.1016/s0022-0736(85)80029-7.
- A Bayés De Luna; M Cladellas; R Oter; P Torner; J Guindo; V Martí; I Rivera; P Iturralde; Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia.. European Heart Journal 1988, 9, 1112–1118.
- Yogesh K. Agarwal; Wilbert S. Aronow; James A. Levy; David H. Spodick; Association of interatrial block with development of atrial fibrillation.. The American Journal of Cardiology 2003, 91, 882, 10.1016/s0002-9149(03)00027-4.
- Vignendra Ariyarajah; Puneet Puri; Sirin Apiyasawat; David H. Spodick; Interatrial Block: A Novel Risk Factor for Embolic Stroke?. Annals of Noninvasive Electrocardiology 2007, 12, 15-20, 10.1111/j.1542-474X.2007.00133.x.
- Antoni Bayes-Genís; Pyotr G. Platonov; Francisco G. Cosio; Iwona Cygankiewicz; Carlos Alberto Pastore; Rafa Baranowski; Antoni Bayes-Genís; Josep Guindo; Xavier Viñolas; Javier Garcia-Niebla; et al.Raimundo BarbosaShlomo SternDavid Spodick Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. Journal of Electrocardiology 2012, 45, 445-451, 10.1016/j.jelectrocard.2012.06.029.
- Alfredo De Micheli; Pedro Iturralde Torres; Alberto Aranda-Fraustro; About the specialized myocardial conducting tissue. Archivos de Cardiología de México 2013, 83, 278-281, 10.1016/j.acmx.2013.03.002.
- Adrian Baranchuk; Bryce Alexander; Goksel Cinier; Manuel Martinez-Selles; Roberto Elousa; Antoni Bayes De Luna; Ahmet İlker Tekkesin; Bayes’ Syndrome and the Opportunities for Early Anticoagulation. Northern Clinics of Istanbul 2017, 5, 370-378, 10.14744/nci.2017.60251.
- Shun-Ichiro Sakamoto; Takashi Nitta; Yosuke Ishii; Yasuo Miyagi; Hiroya Ohmori; Kazuo Shimizu; Interatrial Electrical Connections: The Precise Location and Preferential Conduction. Journal of Cardiovascular Electrophysiology 2005, 16, 1077-1086, 10.1111/j.1540-8167.2005.40659.x.
- Sebastien Janin; Maciej Wójcik; Malte Kuniss; Alexander Berkowitsch; Damir Erkapic; Sergey Zaltsberg; Fiona Ecarnot; Christian W. Hamm; Heinz F. Pitschner; Thomas Neumann; et al. Pulmonary Vein Antrum Isolation and Terminal Part of the P Wave. Pacing and Clinical Electrophysiology 2010, 33, 784-789, 10.1111/j.1540-8159.2010.02754.x.
- Andreas Goette; Jonathan M. Kalman; Luis Aguinaga; Joseph Akar; Jose Angel Cabrera; Shih Ann Chen; Sumeet S. Chugh; Domenico Corradi; Andre D'avila; Bromir Dobrev; et al.Guilherme FenelonMario GonzalezStephane N. HatemRobert HelmGerhard HindricksSiew Yen HoBrian HoitJosé JalifeYoung-Hoon KimGregory Y.H. LipChang-Sheng MaGregory M. MarcusKatherine MurrayAkihiko NogamiPrashanthan SandersWilliam UribeDavid R. Van WagonerStanley NattelOsmar A. CenturionAlena Shantsila EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016, 18, 1455-1490, 10.1093/europace/euw161.
- Goyal, S.B.; Spodick, D.H. Electromechanical dysfunction of the left atrium associated with interatrial block. Am. Hear. J. 2001, 142, 823–827.
- Michelucci, A.; Bagliani, G.; Colella, A.; Pieragnoli, P.; Porciani, M.C.; Gensini, G.; Padeletti, L. P wave assessment: State of the art update. Card. Electrophysiol. Rev. 2002, 6, 215–220.
- Leonardo Seoane; Marcia Cortés; Diego Conde; Update on Bayés’ syndrome: the association between an interatrial block and supraventricular arrhythmias. Expert Review of Cardiovascular Therapy 2019, 17, 225-235, 10.1080/14779072.2019.1577137.
- Enes E. Gul; Raveen Pal; Jane Caldwell; Usama Boles; Wilma M. Hopman; Benedict Glover; Kevin A. Michael; Damian Redfearn; Chris Simpson; Hoshiar Abdollah; et al.Adrian Baranchuk Interatrial block and interatrial septal thickness in patients with paroxysmal atrial fibrillation undergoing catheter ablation: Long‐term follow‐up study. Annals of Noninvasive Electrocardiology 2016, 22, e12428, 10.1111/anec.12428.
- Ivan Hernandez-Betancor; Maria Manuela Izquierdo-Gomez; Javier Garcia-Niebla; Ignacio Laynez-Cerdena; Martin Jesus Garcia-Gonzalez; Jose Luis Irribarren- Sarria; Juan Jose Jimenez-Rivera; Juan Lacalzada-Almeida; Bayes Syndrome and Imaging Techniques. Current Cardiology Reviews 2017, 13, 263-273, 10.2174/1573403X13666170713122600.
- Juan Lacalzada-Almeida; María Manuela Izquierdo-Gómez; Carima Belleyo-Belkasem; Patricia Barrio-Martínez; Javier Garcia-Niebla; Roberto Elosua; Alejandro Jiménez; Luis Alberto Escobar-Robledo; Antoni Bayés De Luna; Interatrial block and atrial remodeling assessed using speckle tracking echocardiography. BMC Cardiovascular Disorders 2018, 18, 1-9, 10.1186/s12872-018-0776-6.
- Enriquez, A.; Conde, D.; Femenía, F.; De Luna, A.B.; Ribeiro, A.; Muratore, C.; Valentino, M.; Retyk, E.; Galizio, N.; Hopman, W.M.; et al. Relation of Interatrial Block to New-Onset Atrial Fibrillation in Patients With Chagas Cardiomyopathy and Implantable Cardioverter-Defibrillators. Am. J. Cardiol. 2014, 113, 1740–1743.
- Alexander, B.; MacHaalany, J.; Lam, B.; Van Rooy, H.; Haseeb, S.; Kuchtaruk, A.; Glover, B.; De Luna, A.B.; Baranchuk, A. Comparison of the Extent of Coronary Artery Disease in Patients With Versus Without Interatrial Block and Implications for New-Onset Atrial Fibrillation. Am. J. Cardiol. 2017, 119, 1162–1165.
- Alexander, B.; Rodriguez, C.; De La Isla, L.P.; Islas, F.; Quevedo, P.J.; Nombela-Franco, L.; Hopman, W.; Malik, P.; Baranchuk, A.; Nombela, L. The impact of advanced Interatrial block on new-onset atrial fibrillation following TAVR procedure. Int. J. Cardiol. 2016, 223, 672–673.
- Ali, F.S.; Enriquez, A.; Conde, D.; Redfearn, D.; Michael, K.; Simpson, C.; Abdollah, H.; De Luna, A.B.; Hopman, W.; Baranchuk, A. Advanced Interatrial Block Predicts New Onset Atrial Fibrillation in Patients with Severe Heart Failure and Cardiac Resynchronization Therapy. Ann. Noninvasive Electrocardiol. 2015, 20, 586–591.
- Enriquez, A.; Conde, D.; Hopman, W.; Mondragón, I.; Chiale, P.A.; De Luna, A.B.; Baranchuk, A. Advanced Interatrial Block is Associated with Recurrence of Atrial Fibrillation Post Pharmacological Cardioversion. Cardiovasc. Ther. 2014, 32, 52–56.
- Caldwell, J.; Koppikar, S.; Barake, W.; Redfearn, D.; Michael, K.; Simpson, C.; Hopman, W.; Baranchuk, A. Prolonged P-wave duration is associated with atrial fibrillation recurrence after successful pulmonary vein isolation for paroxysmal atrial fibrillation. J. Interv. Card. Electrophysiol. 2014, 39, 131–138.
- Enriquez, A.; Sarrias, A.; Villuendas, R.; Ali, F.S.; Conde, D.; Hopman, W.M.; Redfearn, D.P.; Michael, K.; Simpson, C.; De Luna, A.B.; et al. New-onset atrial fibrillation after cavotricuspid isthmus ablation: Identification of advanced interatrial block is key. Europace 2015, 17, 1289–1293.
- O’Neal, W.T.; Zhang, Z.-M.; Loehr, L.R.; Chen, L.Y.; Alonso, A.; Soliman, E.Z. Electrocardiographic Advanced Interatrial Block and Atrial Fibrillation Risk in the General Population. Am. J. Cardiol. 2016, 117, 1755–1759.
- Nielsen, J.B.; Kühl, J.T.; Pietersen, A.; Graff, C.; Lind, B.; Struijk, J.J.; Olesen, M.S.; Sinner, M.F.; Bachmann, T.N.; Haunsø, S.; et al. P-wave duration and the risk of atrial fibrillation: Results from the Copenhagen ECG Study. Hear. Rhythm. 2015, 12, 1887–1895
- Adrian Baranchuk; Helen Pang; Geoffrey E. J. Seaborn; Payam Yazdan-Ashoori; Damian P. Redfearn; Christopher S. Simpson; Kevin A. Michael; Michael Fitzpatrick; Reverse atrial electrical remodelling induced by continuous positive airway pressure in patients with severe obstructive sleep apnoea. Journal of Interventional Cardiac Electrophysiology 2012, 36, 247-253, 10.1007/s10840-012-9749-3.
- Lourdes Vicent; Manuel Martínez-Sellés; Electrocardiogeriatrics: ECG in advanced age. Journal of Electrocardiology 2017, 50, 698-700, 10.1016/j.jelectrocard.2017.06.003.
- Tommaso Sanna; Paul D. Ziegler; Filippo Crea; Detection and management of atrial fibrillation after cryptogenic stroke or embolic stroke of undetermined source. Clinical Cardiology 2018, 41, 426-432, 10.1002/clc.22876.
- Manuel Martínez-Sellés; Albert Massó-Van Roessel; Jesús Álvarez-García; Bernardo García De La Villa; A. J. Cruz-Jentoft; María Teresa Vidán; Javier López Díaz; Francisco Javier Felix Redondo; Juan Manuel Durán Guerrero; Antoni Bayes-Genís; et al.Antoni Bayes-Genís Interatrial block and atrial arrhythmias in centenarians: Prevalence, associations, and clinical implications. Heart Rhythm 2016, 13, 645-651, 10.1016/j.hrthm.2015.10.034.
- Lovely Chhabra; Indu Srinivasan; Pooja Sareen; Curuchi Anand; David H. Spodick; Interatrial block - a novel risk factor for acute mesenteric ischemia. Indian Journal of Gastroenterology 2012, 31, 191-194, 10.1007/s12664-012-0194-0.




