
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carlos Ocampo-López | + 3541 word(s) | 3541 | 2022-02-14 03:03:56 | | | |
| 2 | Bruce Ren | Meta information modification | 3541 | 2022-02-16 02:29:34 | | |
Video Upload Options
Colombia has a diverse range of marine ecosystems in the coastal and insular areas of the Caribbean Sea and the Pacific Ocean. Seaweed research has focused mainly on the identification and taxonomic distribution of 628 species identified so far, mainly in the Caribbean Sea. Among the most widely cultivated genera of seaweeds in open-sea pilot systems in Colombia are Hydropuntia, Gracilaria, Hypnea, Kappaphycus, and Eucheuma. These genera have shown low yields as a consequence of high tissue fragility, epiphytism, sedimentation, and nitrogen deficiency. In addition, the evaluation of the biological activity of selected seaweed compounds has advanced considerably, focusing on their composition and their use for direct consumption by humans and animals. Despite the diversity of seaweeds, as well as certain technical and scientific advances, Colombia is still lagging behind other countries in seaweed exploitation, both in Latin America and worldwide.
1. Introduction
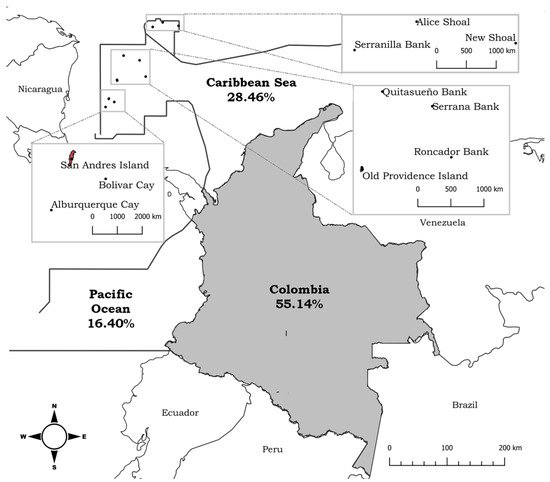
2. Seaweed Cultivation Experiences in Colombia
| Specie | Region | Type of Culture 1 | DGR 2 | Reference | |
|---|---|---|---|---|---|
| Rhodophyta | |||||
| Order Gracilariales | |||||
| Grateloupia sp. | Guajira | B, R | - | [38] | |
| Gracilariopsis tenuifrons | Guajira | B, R | 0.59 | [39] | |
| Gracilaria cervicornis | Guajira | B, R | 0.44 | [38] | |
| Gracilaropsis longissima (as Gracilaria verrucosa) | Magdalena | B | - | [33] | |
| Crassiphycus corneus (as Hydropuntia cornea) Kappaphycus alvarezii * |
Guajira Guajira |
B, R R |
0.97, 0.51 5.1 |
[38][39] [40] |
|
| Order Gigartinales | |||||
| Hypnea musciformis | Guajira-Santa Marta | B, R | 2.66, - | [38][41] | |
| Eucheumatopsis isiformis (as Eucheuma isiforme) | Guajira | B, R | 1.58 | [39] | |
| Order Halymeniales | |||||
| Grateloupia filicina | Magdalena | - | - | [32] | |
| Chlorophyta | |||||
| Order Bryopsidales | |||||
| Caulerpa sertularioides | Nariño | P | 4.82 | [42] | |
3. Applications of Seaweeds in Colombia
3.1. Fertilizers
3.2. Biological Activity
| Species | Biological Activity | Reference | |
|---|---|---|---|
| Rhodophyta | |||
| Order Gelidiales | |||
| Gelidiella acerosa | Cytotoxic | [68] | |
| Order Corallinales | |||
| Amphiroa fragilissima | Cytotoxic | [68] | |
| Order Gracilariales | |||
| Gracilaria mammillaris | Antioxidant | [69] | |
| Order Gigartinales | |||
| Hypnea musciformis | Antibacterial—Phenolic and steroidal compounds | [70][71] | |
| Order Ceramiales | |||
| Digenea simplex | Cytotoxic | [68] | |
| Laurencia sp. | Antioxidant | [72] | |
| Laurencia microcladia | Antibacterial | [73][74] | |
| Chlorophyta | |||
| Order Ulvales | |||
| Ulva sp. (as Enteromorpha sp.) | Antibacterial | [70] | |
| Order Bryopsidales | |||
| Caulerpa mexicana | Antibacterial—Antioxidant | [70][72] | |
| C. sertularioides | Cytotoxic | [68] | |
| Ochrophyta | |||
| Order Dictyotales | |||
| Dictyota bartayresiana | Antibacterial—Feeding inhibitor | [73][74] | |
| Dictyota pulchella | Antibacterial-Cytotoxic—Feeding inhibitor | [73][74] | |
| Dictyota sp. | Antioxidant | [72] | |
| Padina boergesenii | Antibacterial | [73][74] | |
| Order Fucales | |||
| Sargassum cymosum | Antibacterial-Cytotoxic – Feeding inhibitor | [68][72][73] | |
| Sargassum sp. | Antioxidant | [72] | |
| Sargassum schnetteri (as Cladophyllum schnetteri) | Feeding inhibitor | [73] | |
3.3. Polysaccharides and Pigments
| Species | Extract/Compound | Reference | ||
|---|---|---|---|---|
| Rhodophyta | ||||
| Order Gracilariales | ||||
| Gracilariopsis longissima (as Gracilaria verrucosa) | Agar-agar | [81] | ||
| Gracilariopsis tenuifrons | Agar and carrageenan | [82] | ||
| Ochrophyta | ||||
| Order Fucales | ||||
| Sargassum sp. | Alginate | [83] | ||
| S. cymosum | Alginate | [83] | ||
| S. filipendula | Fucoxanthin | [84] | ||
| Turbinaria spp. | Fucoxanthin | [84] | ||
| S. polyceratium | Fucoxanthin | [84] | ||
| Order Dictyotales | ||||
| Dictyota caribaea | Fucoxanthin | [84] | ||
| D. pinnatifida | Fucoxanthin | [84] | ||
References
- Grand View Research. Global Commercial Seaweeds Market Size Report, 2020–2027. Report Overview. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/commercial-seaweed-market (accessed on 20 November 2020).
- Cioš, A.M.; Jerković, I.; Molnar, M.; Šubarić, D.; Jokić, S. New trends for macroalgal natural products applications. Nat. Prod. Res. 2019, 35, 1180–1191.
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77.
- Algaebase: Listing the World’s Algae. Available online: http://www.algaebase.org/ (accessed on 12 November 2021).
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 17.
- Duarte, C.M.; Marbá, N.; Holmer, M. Rapid domestication of marine species. Science 2007, 316, 382–383.
- Pereira, L.; Cotas, J. Historical use of seaweed as an agricultural fertilizer in the european atlantic area. In Seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder; Pereira, L., Bahcevandziev, K., Joshi, N., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–23.
- Chapman, V.J.; Chapman, D.J. Seaweeds and Their Uses, 3rd ed.; Springer: Dordrecht, The Netherlands, 1980.
- Newton, L. Animal and human nutrition from seaweed resources in Europe: Uses and potential. In Seaweed Utilization; Sampson Low: London, UK, 1951; pp. 31–57.
- Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for food and industrial applications. In Food Industry, 1st ed.; Muzzalupo, I., Ed.; IntechOpen: London, UK, 2013.
- Dillehay, T.M.; Ramírez, C.; Pino, M.; Collins, M.B.; Rossen, J.; Pino-Navarro, J.D. Monte verde: Seaweed, food, medicine, and the peopling of South America. Science 2008, 9, 784–786.
- Gao, G.; Burgess, J.G.; Wu, M.; Wang, S.; Gao, K. Using macroalgae as biofuel: Current opportunities and challenges. Bot. Mar. 2020, 63, 355–370.
- Kelly, M.S.; Dworjanyn, S. The Potential of Marine Biomass for Anaerobic Biogas Production. The Crown Estate: London, UK, 2008. Available online: https://arpa-e.energy.gov/sites/default/files/The%20Potential%20of%20Marine%20Biomass%20for%20Anaerobic%20Biogas%20Production%202008.pdf (accessed on 20 August 2020).
- Qin, Y. Production of seaweed-derived food hydrocolloids. In Bioactive Seaweeds for Food Applications: Natural Ingredients for Healthy Diets; Qin, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 53–69.
- Coas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta). Life 2020, 10, 19.
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263.
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577.
- Tanna, B.; Mishra, A. Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831.
- Couteau, C.; Coiffard, L. Seaweed application in cosmetics. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–441.
- Mrais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds compounds: An ecosustainable source of cosmetic ingredients? Cosmetics 2021, 8, 8.
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy-cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68.
- Thomas, N.V.; Kim, S.-K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164.
- Rengasamy, K.R.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013.
- Siahaan, E.A.; Pangestuti, R.; Kim, S.-K. Seaweeds: Valuable ingredients for the pharmaceutical industries. In Grand Challenges in Marine Biotechnology; Rampelotto, P.H., Trincone, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 49–95.
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262.
- Informe del Estado de los Ambientes Marinos y Costeros en Colombia. 2017. Available online: http://www.invemar.org.co/documents/10182/14479/IER_2017_baja_Final.pdf/76690566-f6e1-4610-906f-1c49c610b2c8 (accessed on 20 October 2020).
- Comisión Colombiana del Océano. Reserva de Biósfera Seaflower. Available online: http://www.cco.gov.co/la-reserva.html (accessed on 3 October 2020).
- Seaflower Biosphere Reserve. Available online: https://en.unesco.org/biosphere/lac/seaflower (accessed on 20 March 2020).
- Díaz, J.M.; Acero, A. Marine biodiversity in Colombia: Achievements, status of knowledge, and challenges. Gayana 2003, 67, 261–274.
- Márquez, G. Biodiversidad marina: Aproximación con referencia al Caribe. In Ecosistemas Estratégicos y Otros Estudios de Ecología Ambiental; Fondo FEN: Bogotá, Colombia, 1996; pp. 67–102.
- Rincón-Díaz, M.N.; Gavio, B. Diversidad de Macroalgas Marinas del Caribe Colombiano, v2.8; Instituto de Investigaciones Marinas y Costeras—Invemar: Santa Marta, Colombia, 2020.
- Álvarez, R.; Pardo, C.M.; Trespalacios, A.A. Evaluación y utilización potencial de las macroalgas marinas del caribe y el pacífico de Colombia: Estado actual de su conocimiento. Biosalud 2007, 6, 113–129.
- Molina, J.N.; Álvarez, R. Resultados preliminares del cultivo experimental de Gracilaria Verrucosa (Hudson) Papenfuss (=G. caudata J. Agardh) (Rhodophyta: Gracilariaceae) en la costa caribe de Colombia. Rev. Acad. Colomb. Cienc. 2014, 38, 79–87.
- Peña, E.J.; Álvarez, R. Experiencias en el cultivo experimental de algas rojas en el caribe y pacífico de Colombia. Rev. Luna Azul 2006, 23, 16–20.
- Gallo, H.M.; Rincones, R.E. Factibilidad del Cultivo de Algas Marinas. Proyecto Corpoguajira/IIRBAvH/FAO. Fortalecimiento Para el Desarrollo de Empresas Rurales a Partir de Productos de la Biodiversidad en el Cabo de la Vela, Departamento de La Guajira; Consultoría Fase II: Bogotá, Colombia, 2003.
- Montaña, J.C. Ensayos de Cultivo en Medio Natural de la Macroalga Hypnea Musciformis (Wulfen) Lamoroux en las Areas de Taganga y Puerto Luz (Santa Marta). Bachelor’s Thesis, Universidad Jorge Tadeo Lozano, Cundinamarca, Colombia, 2006.
- Rincones, R.E.; Gallo, H. Proyecto Jimoula: El Cultivo de Algas Marinas Como Alternativa Sustentable Para las Comunidades Costeras de la Península de la Guajira; BIOTACOL Ltda.: Rioacha, Colombia, 2004.
- Delgadillo, O.; Newmark, F. Cultivo piloto de macroalgas rojas (Rhodophyta) en Bahía Portete, La Guajira, Colombia. Bol. Investig. Mar. Cost 2008, 37, 7–26.
- Rincones, R.E.; Moreno, D.A. Aspectos técnicos y económicos para el establecimiento comercial del maricultivo de algas en Colombia: Experiencias en la península de la Guajira. Ambiente Desarro. 2011, 28, 1–52.
- Peña, E.J.; Palacios, M.L. Introducción del alga roja Kappaphycus alvarezzi (Doty) en Colombia y experiencias de cultivo en la península de la guajira, caribe Colombiano. In Guiίa de las Especies Introducidas Marinas y Costeras de Colombia; Gracia, A., Medelliίn, J., Gil, D.L., Puentes, V., Eds.; INVEMAR. Ministerio de Ambiente y Desarrollo Sostenible: Santa Marta, Colombia, 2011; pp. 93–103.
- Camacho, O.; Montaña, J. Cultivo experimental en el mar del alga roja Hypnea musciformis en el area de Santa Marta, Caribe Colombiano. Bol. Investig. Mar. Cost. 2012, 41, 29–46.
- Mosquera, Z.; Peña, E.J. Effect of salinity on growth of the green alga Caulerpa sertularioides (Bryopsidales, Chlorophyta) under laboratory conditions. Hidrobiológica 2016, 26, 277–282.
- Islam, M.; Khan, S.; Hasan, J.; Mallick, D.; Hoq, E. Seaweed Hypnea sp. culture in Cox’s Bazart coast, Bangladesh. Bangladesh J. Zool. 2017, 45, 37–46.
- Pereira, S.; Kimpara, J.; Valentin, W. A simple substrate to produce the tropical epiphytic algae Hypnea pseudomusciformis. Aquac. Eng. 2020, 89, 102066.
- Ganesan, M.; Thiruppathi, S.; Jha, B. Mariculture of Hypnea musciformis (Wulfen) Lamouroux in South east coast of India. Aquaculture 2006, 256, 201–211.
- Gómez, A.; Millán, J. Cultivo experimental de Gracilaria dentata Agardh y de Gracilariopsis tenuifrons (Bird et Oliveira) (Rhodophyta: Gigartinales) en la isla de Margarita, Venezuela. Rev. Biol. Mar. Oceanogr. 1997, 32, 137–144.
- Bermejeo, R.; Cara, C.; Macías, M.; Sánchez-García, J.; Hernández, I. Growth rates of Gracilariopsis longissima, Gracilaria bursa-pastoris and Chondracanthus teedei (Rhodophyta) cultured in ropes: Implication for N biomitigation in Cadiz Bay (Southern Spain). J. Appl. Phycol. 2020, 32, 1879–1891.
- Pérez-Lloréns, J.L.; Brun, F.G.; Andría, J.; Vergara, J.J. Seasonal and tidal variability of environmental carbon related physico-chemical variables and inorganic C acquisition in Gracilariopsis longissima and Enteromorpha intestinalis from Los Toruños salt marsh (Cádiz Bay, Spain). J. Exp. Mar. Biol. Ecol. 2004, 304, 183–201.
- Jiang, H.; Zou, D.; Lou, W.; Chen, W.; Yang, Y. Growth and photosynthesis by Gracilariopsis lemaneiformis (Gracilariales, Rhodophyta) in response to di!erent stocking densities along Nan’ao Island coastal waters. Aquaculture 2019, 501, 279–284.
- Hayashi, L.; Reis, R.P.; Alves, A.; Castelar, B.; Robledo, D.; de Vega, G.B.; Msuya, F.E.; Eswaran, K.; Yasir, S. The cultivation of Kappaphycus and Eucheuma in tropical and sub-tropical waters. In Tropical Seaweed Farming Trends, Problems and Opportunities; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Springer: Cham, Switzerland, 2017; pp. 55–90.
- Hurtado, A.Q.; Reis, R.P.; Loureiro, R.R.; Critchley, A.T. Kappaphycus (Rhodophyta) cultivation: Problems and the impacts of acadian marine plant extract powder. In Marine Algae; Pereira, L., Neto, J.M., Eds.; Taylor & Francis Group LLC: Oxfordshire, UK, 2014; pp. 251–299.
- Hayashi, L.; Hurtado, A.Q.; Msuya, F.E.; Bleicher, G.; Critchley, A.T. A review of Kappaphycus farming: Prospects and constraints. In Seaweeds and Their Role in Globally Changing Enviorments; Israel, A., Einav, R., Seckbach, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 251–283.
- Chandrasekaran, S.; Nagendran, N.A.; Pandiaraja, D.; Krishnankutty, N.; Kamalakannan, B. Bioinvasion of Kappaphycus alvarezii on corals in the Gulf of Mannar, India. Curr. Sci. 2008, 94, 1167–1172. Available online: https://www.jstor.org/stable/24100697 (accessed on 2 December 2021).
- Mulyani, S.; Tuwo, A.; Syamsuddin, R.; Jompa, J. Effect of seaweed Kappaphycus alvarezii aquaculture on growth and survival of coral Acropora Muricata. AACL Bioflux 2018, 11, 1792–1798.
- Rajaram, R.; Rameshumar, S.; Ahmad, B.; Albeshr, M.F. Impacts of cultivation of red algae Kappaphycus alvarezii on planktonic and benthic faunal density in relation to environmental and hydrobiological parameters in tropical coastal ecosystem. Acta Ecol. Sin. 2021, 41, 39–49.
- Reis, R.P.; Castelar, B.; Moura, A.L.; Kirk, R. Invasive potential of Kappaphycus alvarezii off the south coast of Rio de Janeiro state, Brazil: A contribution to environmentally secure cultivation in the tropics. Bot. Mar. 2009, 52, 283–289.
- Chen, S.; Ganapin, D. Polycentric coastal and ocean management in the Caribbean sea large marine ecosystem: Harnessing community-based actions to implement regional frameworks. Environ. Dev. 2016, 17 (Suppl. 1), 264–276.
- Chen, S.; Akhtar, T.; Currea, A.M. Sustainable seaweed production, Belize. In Scaling up Community Actions for International Water Management—Experiences from the GEF Small Grants Programme; The GEF Small Grants Programme: Port Louis, Mauritius, 2016; Available online: https://www.thegef.org/sites/default/files/publications/IW_PublicationCaseStudiesMay-Digital.pdf (accessed on 20 October 2020).
- Gracilarias de Panamá. Available online: https://gracilarias.org (accessed on 10 May 2021).
- Chung, I.K.; Sondak, C.F.A.; Beardall, J. The future of seaweed aquaculture in a rapidly changing world. Eur. J. Phycol. 2017, 52, 495–505.
- INVEMAR. La calidad ambiental marina y costera en Colombia. In Technical Report: Informe de Estado de los Ecosistemas Marinos y Costeros; Invemar: Santa Mata, Colombia, 2004; pp. 39–74.
- Rodríguez, L. Programa Nacional de Investigación, Evaluación, Prevención, Reducción y Control de Fuentes Terrestres y Marinas de Contaminación al Mar—Actualización del Plan de Acción 2014–2019; Comisión Colombiana Del Océano (CCO): Bogotá, Colombia, 2016; p. 70.
- Seakura Sea of Life. Available online: https://www.seakura.co.il/en/ (accessed on 20 October 2020).
- Craigie, J.S.; Cornish, M.L.; Deveau, L.E. Commercialization of Irish moss aquaculture: The Canadian experience. Bot. Mar. 2019, 62, 411–432.
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17.
- Sierra-Vélez, L.; Álvarez-León, R. Comparación bromatológica de las algas nativas (Gracilariopsis tenuifrons, Sargassum filipendula) y exóticas (Kappaphycus alvarezii) del caribe Colombiano. Bol. Cient. Mus. Hist. Nat. Univ. Caldas 2009, 13, 17–25.
- Bula-Meyer, G. Marine macroalgae in the agronomy and potential use of floating Sargassum for manure production in the San Andres and Providencia Archipelago, Colombia. Rev. Intropica 2004, 1, 91–103.
- Ospina, S.P.; López, J.B.; Márquez, M.E. Efecto antimitótico in vitro en el extracto metanólico de macroalgas marinas de la costa caribe Colombiana. Vitae 2007, 14, 84–89.
- Ospina, M.; Castro-Vargas, H.I.; Parada-Alfonso, F. Antioxidant capacity of Colombian seaweeds: 1. extracts obtained from Gracilaria mammillaris by means of supercritical fluid extraction. J. Supercrit. Fluids 2017, 128, 314–322.
- Arteaga, M.; De Silvestri, J. Estudio de las sustancias con propiedades antimicrobianas extraordinarias de algas marinas pertenecientes al litoral atlántico colombiano. Rev. Colomb. Cienc. Químico Farm. 1985, 4, 47–52.
- Rozo, G.; Rozo, C.; Plazas, E.; Patiño, O. Fraccionamiento bioguiado del extracto etanólico de Hypnea musciformis en búsqueda de compuestos con actividad antioxidante. Vitae 2011, 18 (Suppl. 2), S162–S163.
- Echavarría, B.; Franco, A.; Martínez, A. Evaluación de la actividad antioxidante y determinación del contenido de compuestos fenólicos en extractos de macroalgas del caribe Colombiano. Vitae 2009, 16, 126–131.
- Díaz, M.C.; Bula-Meyer, G.; Zea, S.; Martínez, A. Ensayos de actividad biológica y ecología química de extractos orgánicos de macroalgas del caribe Colombiano. Bol. Investig. Mar. Cost 2006, 35, 241–247.
- Martínez, A.; Arias, L.A.; Rueda, J.L.; Diaz, M.C.; Bula-Meyer, G. Antimicrobial activity study for alcohol extracts of some macroalgae from Colombian Caribbean Sea. Vitae 2002, 9, 49–55.
- Nuñez, E.; Arteaga, M.; Castro, M. Investigación de actividad antibacteriana de extractos de algunas algas marinas colombianas. Rev. Colomb. Cienc. Químico Farm. 1972, 2, 123–130.
- Sreenivasa, P.; Parekh, K. Antibacterial activity of Indian seaweed extracts. Bot. Mar. 1981, 24, 577–582.
- Valle, H.; Ospina, S.; Galeano, E.; Martínez, A.; Márquez, M.E.; López, J.B. Obtención de una fracción antimitótica del extracto etanólico de la acroalga Digenia simplex. Bol. Investig. Mar. Cost 2009, 38, 109–117.
- Valle, H.; Ospina, S.; Galeano, E.; Martínez, A.; Marquez, M.E.; López, J.B. Componentes de la fracción antimitótica del extracto etanólico de la macroalga Digenia simplex. Vitae 2008, 15, 141–149.
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Goncąlves, A.M.M.; Jorge Da Silva, G.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384.
- Syad, A.N.; Rajamohamed, B.S.; Shunmugaiah, K.P.; Kasi, P.D. Neuroprotective effect of the marine macroalga Gelidiella acerosa: Identification of active compounds through bioactivity-guided fractionation. Pharm. Biol. 2016, 54, 2073–2081.
- Molina, J.N.; de la Rosa, C.R.; Olivares, L.A.; Parra, X.J.; Serrano, A. Evaluación de las condiciones de extracción de agar-agar a partir de Gracilaria verrucosa en el balneario de Santa Verónica en el departamento del atlántico. Vitae 2011, 18 (Suppl. 2), S219.
- Monsalve-Bustamante, Y.; Rincón-Valencia, S.; Puertas-Mejía, M.; Moreno-Tirado, D.; Mejía-Giraldo, J.; Restrepo-Moná, Y. Potencial material de encapsulación a partir del alga Gracilariopsis tenuifrons del mar caribe Colombiano. Vitae 2018, 25 (Suppl. 1), 153–155.
- Camacho, O.; Hernández-Carmona, G. Phenology and alginates of two Sargassum species from the Caribbean coast of Colombia. Cienc. Mar. 2012, 38, 381–393.
- Restrepo, N. Extracción, Purificación Y Análisis del Contenido de Fucoxantina en Algas Pardas del Caribe Colombiano. Bachelor’s Thesis, Universidad de Bogotá Jorge Tadeo Lozano, Cundinamarca, Colombia, 2015.
- Monsalve-Bustamante, Y.; Rincón-Valencia, S.; Mejía-Giraldo, J.; Moreno-Tirado, D.; Puertas-Mejía, M. Screening of the UV absorption capacity, proximal and chemical characterization of extracts, and polysaccharide fractions of the Gracilariopsis tenuifrons cultivated in Colombia. J. Appl. Pharm. Sci. 2019, 9, 103–109.
- Vargas, P.A. Efectos Fotoprotectores y Humectantes de Formulaciones Cosméticas que Contienen Extractos de Hypnea Musciformis Recolectadas en el Caribe Colombiano. Bachelor’s Thesis, Universidad de Bogotá Jorge Tadeo Lozano, Cundinamarca, Colombia, 2020.
- Ferrara, L. Seaweeds: A food for our future. J. Food Chem. Nanotechnol. 2020, 6, 56–64.
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140.
- Ullmann, J.; Grimm, D. Algae and their potential for a future bioeconomy, landless food production, and the socio-economic impact of an algae industry. Org. Agric. 2021, 11, 261–267.
- Figueroa, V.; Farfán, M.; Aguilera, J.M. Seaweeds as novel foods and source of culinary flavors. Food Rev. Int. 2021, 37, 1–26.
- Mouritsen, O.G.; Rhatigan, P.; Pérez, J.L. World cuisine of seaweeds: Science meets gastronomy. Int. J. Gastron. Food Sci. 2018, 14, 55–65.
- Colombia Potencia Bioceánica Sostenible 2030—Documento CONPES 3990. Consejo Nacional de Politica Economica y Social—Republica de Colombia 91. 2020. Available online: https://www.co.undp.org/content/colombia/es/home/sustainable-development-goals.html (accessed on 30 May 2021).
- Objetivos de Desarrollo Sostenible|El PNUD en Colombia. United Nations Development Programme. 2015. Available online: https://www.co.undp.org/content/colombia/es/home/sustainable-development-goals/background/ (accessed on 30 March 2021).




