Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Deng-Guang Yu | + 3439 word(s) | 3439 | 2022-01-27 03:48:15 | | | |
| 2 | Jason Zhu | + 34 word(s) | 3473 | 2022-02-11 04:05:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yu, D. Polymer-Based Nanofiber–Nanoparticle Hybrids. Encyclopedia. Available online: https://encyclopedia.pub/entry/19327 (accessed on 07 February 2026).
Yu D. Polymer-Based Nanofiber–Nanoparticle Hybrids. Encyclopedia. Available at: https://encyclopedia.pub/entry/19327. Accessed February 07, 2026.
Yu, Deng-Guang. "Polymer-Based Nanofiber–Nanoparticle Hybrids" Encyclopedia, https://encyclopedia.pub/entry/19327 (accessed February 07, 2026).
Yu, D. (2022, February 10). Polymer-Based Nanofiber–Nanoparticle Hybrids. In Encyclopedia. https://encyclopedia.pub/entry/19327
Yu, Deng-Guang. "Polymer-Based Nanofiber–Nanoparticle Hybrids." Encyclopedia. Web. 10 February, 2022.
Copy Citation
When nanoparticles and nanofibers are combined, the composite material can perform more functions, such as photothermal, magnetic response, biosensing, antibacterial, drug delivery and biosensing. To prepare nanofiber and nanoparticle hybrids (NNHs), there are two primary ways. The electrospinning technology was used to produce NNHs in a single step. An alternate way is to use a self-assembly technique to create nanoparticles in fibers.
polymer composites
nanoparticle
polymer blends
1. Introduction
COVID-19 has contributed to a worldwide healthcare crisis resulting in several hundreds of thousands of deaths over recent years and complications that can lead to severe pneumonia [1][2]. In synergy with Polymer Engineering, Bioengineering, and Advanced Manufacturing, the mRNA vaccine was successfully developed and blocked the further spread of the virus on a large scale [3][4]. The mRNA-1273 vaccine approved for use under urgent circumstances has the potential to be unsafe for delivery, being inherently unstable while the large density of negative charges makes it difficult to cross cell membranes [5][6], and the latest research uses polymer-based composites as carriers for effective vaccine delivery [7].
In medical applications, polymers with superior biocompatibility are often used to not produce antibody reactions or even stimulate inflammation in contact with the human body [8][9][10]. Synthetic polymers include: polycaprolactone (PCL), poly (vinyl alcohol) (PVA), polylactide (PLA) and poly (lactic-co-glycolic acid) (PLGA) [11][12]; natural polymers include: Chitosan (polysaccharide), cellulose (polysaccharide), gelatin (protein), silk proteins (proteins) [13], etc. In biomedical applications, natural polymers are often perceived as relatively safe, biocompatible and degradable polymers [14]. Cellulose is a linear polysaccharide, the main component of the cell wall, which is currently the most used in biological applications from bacteria [15]. Taokaew et al. composite nanofibers based on bacterial cellulose with Garcinia mangostana peel extract exhibited potent bacterial inhibition against Gram-positive bacteria while treating the membranes with MCF-7 breast cancer cells for 48 h, only 5% of the cancer cells remained viable [16]. The protein class of silk fibroin, unlike other natural polymers, has outstanding elasticity and strength, which is of interest in tissue engineering applications [17][18].
The advantages of synthetic polymers over natural polymers are their stability, superior mechanical properties and degradability. PCL is an aliphatic semicrystalline polymer with hexanoic acid formed by esterolysis [19], which can be excreted through the digestive system, thus making it biocompatible, biodegradable, non-toxic and popular in medical applications, such as drug delivery, wound plug dressings and stents. The use of PCL scaffolds for tissue engineering has been reported in detail by Janmohammadi et al. [20]. Zavan and co-works prepared a bilayer fibrous scaffold with the outer layer of PCL exhibiting the best in vitro response to fibroblasts and weak adhesion to cellular tissues due to the hydrophobicity of PCL to cellular tissues, while the inner layer used gelatin-modified PCL to significantly promote gene expression in endothelial cells [21].
Nanoscale polymers have a wide range of uses in various fields, especially in medical applications, where the electrospinning process (Figure 1) is one of the most convenient ways to prepare nanofiber membranes (1–100 nm) directly and continuously [22][23][24][25][26]. Laboratory electrospinning setup generally has three components: a high voltage electrostatic generator (1–30 kv), a spinning head and a collector (opposite charge target) for the process of producing various continuous nanofiber assemblies with controlled morphology and dimensions from polymer solutions or melts under a high voltage electric field [27][28][29]. The development of the electrospinning (ES) process was a somewhat difficult journey. In the 16th century, William Gibert discovered a conical droplet formed when charged amber approached water droplets. Micro-droplets are also ejected from the conical droplets. The beginning of electrospinning technology is recognized to be in 1934, when Anton Formalas invented a device to prepare polymer fibers by electrostatic force action, presenting for the first time how a polymer solution could form the jet between electrodes. Subsequent years of intermittent patent publication have not attracted much attention from researchers. In 1990, Reneker’s research team at the Acolon University, USA, went deeper into the electrospinning (ES) process and applications, with ES of various organic polymer solutions into fibers, ES process is rapidly becoming a research hotspot, entering a new era of vigorous development [30][31].
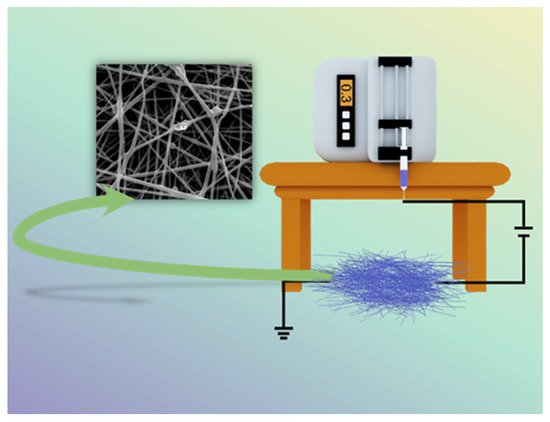
Figure 1. Schematic diagram of electrospinning setup.
To prepare the desired nanofibers, the electrospinning process usually requires setting parameters, the main influencing factors being the system (nature of the polymer, solution viscosity, conductivity and surface tension) [32], process parameters (voltage, receiving distance and flow rate) [33] and environmental factors (temperature and humidity) [34].
Synthetic polymers have modifiable mechanical properties, but the hydrophobic nature contributes to an absence of cell adhesion and the onset of inflammation, which is usually modified by natural polymers. One problem that most natural polymers suffer from is the deficiency of certain mechanical properties [35]. To combine the advantages of various polymers and overcome their limitations, the desired properties have been achieved using blends of these polymers instead of using single polymers by optimizing the ratio between the components of the blend. During drug release, the hydrophobic properties of the polymer seriously affect the release performance of the loaded drug. Huo and co-workers used PCL blended with gelatin to effectively improve the hydrophobic properties of the composite fiber. Drug release demonstrated that the increased PCL content made the composite fiber more hydrophobic, which belonged to the slow release of artemisinin (ART) and enhanced the therapeutic effect [36]. Chen et al. improved the defect of gelatin’s poor hemostasis ability for large wounds by co-mixing sodium alginate, which can form a gel on the wound surface, and achieved hemostasis of rat wounds in only 1.53 min [37].
Nanofiber membranes are prepared by the ES process of polymers, and the application scenario is constrained to fiber scaffolds [38][39][40]. Researchers are not satisfied with this single role, so various functional nanofiber membranes have been prepared by ES in combination with multifunctional materials, among which NNHs show great potential to combine the advantages of nanoparticles with the properties of polymers. Additionally, the is a wide range of application scenarios [38][39][40][41][42][43][44][45]. Of interest is that as with decades of research, the number of electrospun nanoparticle articles published occupies half the number of electrostatic spinning (Figure 2). It shows the pivotal position of NNHs.
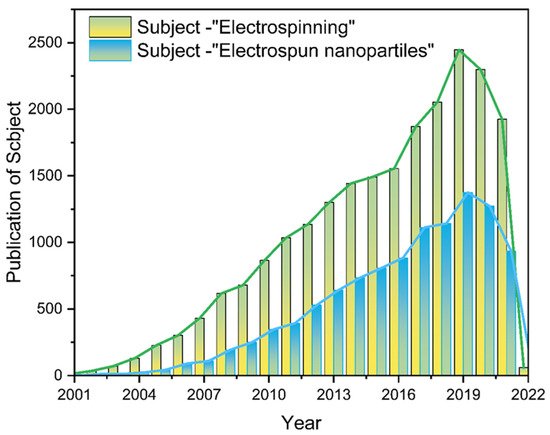
Figure 2. The literature search statistics of “electrostatic spinning” and “electrospun nanoparticles” on the “Web of Science” platform, respectively.
Multiple nanoparticles have been successfully prepared into nanofibers, which mainly include: metal [46], metal oxide [47] and polymer nanoparticles (NPs) [48][49]. The presence of NPs in polymer solution is uniformly dispersed and will be randomly distributed on the surface or inside the nanofibers (NFs). Therefore, the co-blending of NPs with polymer solutions is the most common form. During the process of electrospinning the mixture into fibers, the homogeneity of NPs in the polymer solution and the interfacial interaction between nanoparticles and polymer solution significantly affect the performance of NNHs. To attenuate the effects of the above factors, the NPs can be mixed by physical mixing methods (stirring and sonication), by dissolving the NPs and polymer in different solvents separately or adding a certain amount of surface activator. The electrospun fiber precursor working solution can effectively reduce the phenomenon of nanoparticle agglomeration and even blockage of the spinning heads after mixing uniformly [50][51][52]. Secondly, other processes combined with ES technology can also effectively load NPs into NFs. For instance, the modification of NFs by plasma technology. This methodology promotes the interaction between NPs and easily loads NPs on the nanofiber surface [53]. The electrospray [54] or magnetron sputtering [55] technology allows the uniform spraying of NPs on the fiber surface. The single-fluid ES process requires mixed solutions with a certain viscosity and dispersion. Otherwise, a non-spinnable or agglomerative phenomenon will occur. Multi-fluid dynamics have been studied for many years. The design of the spinneret is especially important. The morphological structure of the nanofibers is similar to the spinneret structure [56]. Multi-fluid dynamics ES processes often require only one fluid available for spinning to form nanofibers [57]. Zheng et al. used TiO2 suspension as the sheath layer and PEO as the core layer, which perfectly avoided the enrichment of TiO2NPs using a modified coaxial electrospinning process, and the uniform TiO2NPs on the nanofiber surface enhanced the absorption of UV light [58]. The NPs are loaded in nanofibers based on the above single-step process, and if the NPs are not well dispersed in the working solution, many self-assembly strategies are available to grow NPs in the fibers by initiation effects in the precursor solution, including: in situ synthesis [59], hydrothermal-assistance [60] and calcination [61].
NNHs prepared by combining the characteristics of biocompatible polymers and multifunctional nanoparticles are already practical in medical applications [62][63]. Researchers is to investigate the preparation of nanofiber and nanoparticle hybrids in-depth by combining different processes with ES; concentrate on the most recent breakthroughs of NNHs in the medical area (Figure 3); and provide clear concepts for research workers in material preparation and application.
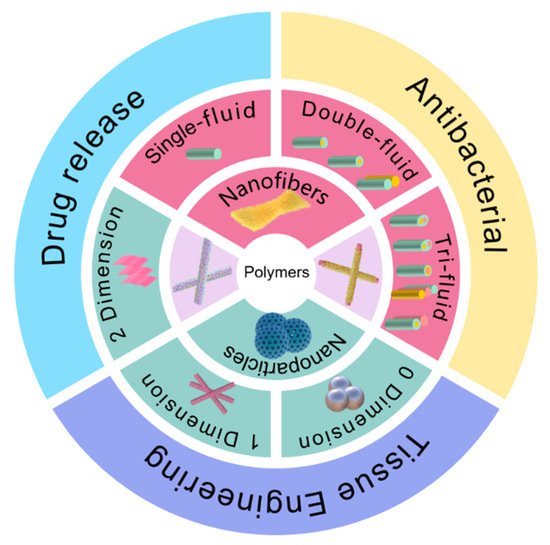
Figure 3. Electrospun fiber and NPs structures and their hybrids in medical direction.
2. The Methods for Creating Polymer-Based Nanofiber-Nanoparticle Hybrids (NNHs)
2.1. Overlapping of Electrospinning and Other Techniques
ES is a top-down molding process for the direct and continuous preparation of nanofibers. NNHs in the preparation of NPs in the spinning precursor solution, due to a certain stability, will not form a homogeneous solution in organic solvents. In addition to the large-scale manufacturing of NNHs, other technologies are often used in further combination with ES.
AgNPs in PCL nanofibers suffer from inhomogeneous dispersion and irregular ion release. To address this problem, Valerini and co-workers employed the magnetron sputtering technique to sputter Ag targets onto the surface of PCL nanofibers at a low power of 500 w. SEM images of PCL NFs after Ag magnetron sputtering show higher contrast. Composite NFs with 22 nm size AgNPs were deposited on the surface. Although the Ag content was only 0.1 wt.%, the PCL-Ag nanofibers showed a faster and stronger antibacterial effect by depositing all AgNPs on the nanofiber surface by magnetron sputtering technique [64]. Immersion of nanofibers (NFs) in NPs suspension loaded with NPs is the simplest approach; however, significant rejection occurs due to the different hydrophilic properties of NPs and NFs, leading to ineffective results. Liu et al. introduced oxygen-containing polar functional groups to the surface of PLLA membranes by a facile plasma technique. Negatively charged pPLLA membranes, impregnated in AgNPs solution, were well aggregated with NPs on the fiber surface driven by electrostatic interactions [65]. Electrospray, a sister technology to electrospun fibers, is commonly employed to generate NPs [66]. Fahimirad and co-workers sprayed chitosan NPs loaded with curcumin onto the surface of PCL/chitosan/curcumin nanofibers by electrospray, which showed no significant change in nanofiber diameter. While effectively promoting the swelling ability and degradation rate of nanofibers, the composite fibers healed 98.5% of the wounds infected by MRSA through wound closure experiments [67].
2.2. Encapsulating Nanoparticles in the Working Fluids
The presence of NPs necessarily affects the spinnability and composite fiber morphology during the preparation of NNHs. NPs appear agglomerated in the working solution, and needle blocking may occur during ES, or the NPs will not appear uniformly on the fibers. These issues can lead to reduced mechanical properties and loss of functional sites in composite fiber membranes. There are three ways to deal with them to avoid a similar situation: (1) disperse NPs uniformly in the working solution by physical methods such as stirring; (2) separately dissolve NPs and polymers in different solvents; (3) modify them using surfactants to promote uniform dispersion. With different natures of nanoparticles and polymer solutions in the mixing process, there is a large interfacial force, and the mixture can be added to a quantitative amount of surfactant, promoting the orderly arrangement of nanoparticles no longer agglomeration. Karagoz and co-workers took this approach by adding a quantitative amount of Triton x-100 (surfactant) to a mixture of ZnO nanorods and DMF, sonicated for 20 min, then added the polymer under rapid stirring until complete dissolution. Fiber diameters were uniformly distributed, and no beads were observed [68].
2.3. Formation of NNHs in the Single-Step Process
With a precursor working solution using suitable solvent and proper dispersion, the NPs are uniformly dispersed in the working solution, which requires only a single-step process to form NNHs by adjusting the ES process parameters (Figure 4A). Lopresti et al. incorporated silica (AS) or clay (CLO) NPs into 10% PLA solution and gathered them by a cylindrical grounded drum at 15 KV. The phenomenon of particle aggregation or clustering was evident with the increase in NPs content of the composite fibers (Figure 4E). Moreover, the composites with a certain number of added NPs exhibited brittleness, but bone cells diffused faster on the composites with higher particle content [69]. Abdelaziz and co-workers encapsulated silver nanoparticles (AgNPs) and hydroxyapatite (HANPs) in PCL/CA solution. Interestingly, the tensile stress of the composite fibers loaded with 10% HANPs was up to 3.39 MPa, well above that of pure PCL/CA nanofibers, but then the tensile stress of the composite fibers with 20% HANPs dropped sharply to 2.22 MPa, perhaps because the mechanical property change of NNHs with the addition of nanoparticles is a parabolic. This interesting phenomenon could contribute to the reference of optimizing the relationship between NNHs proportioning and mechanical properties [70].
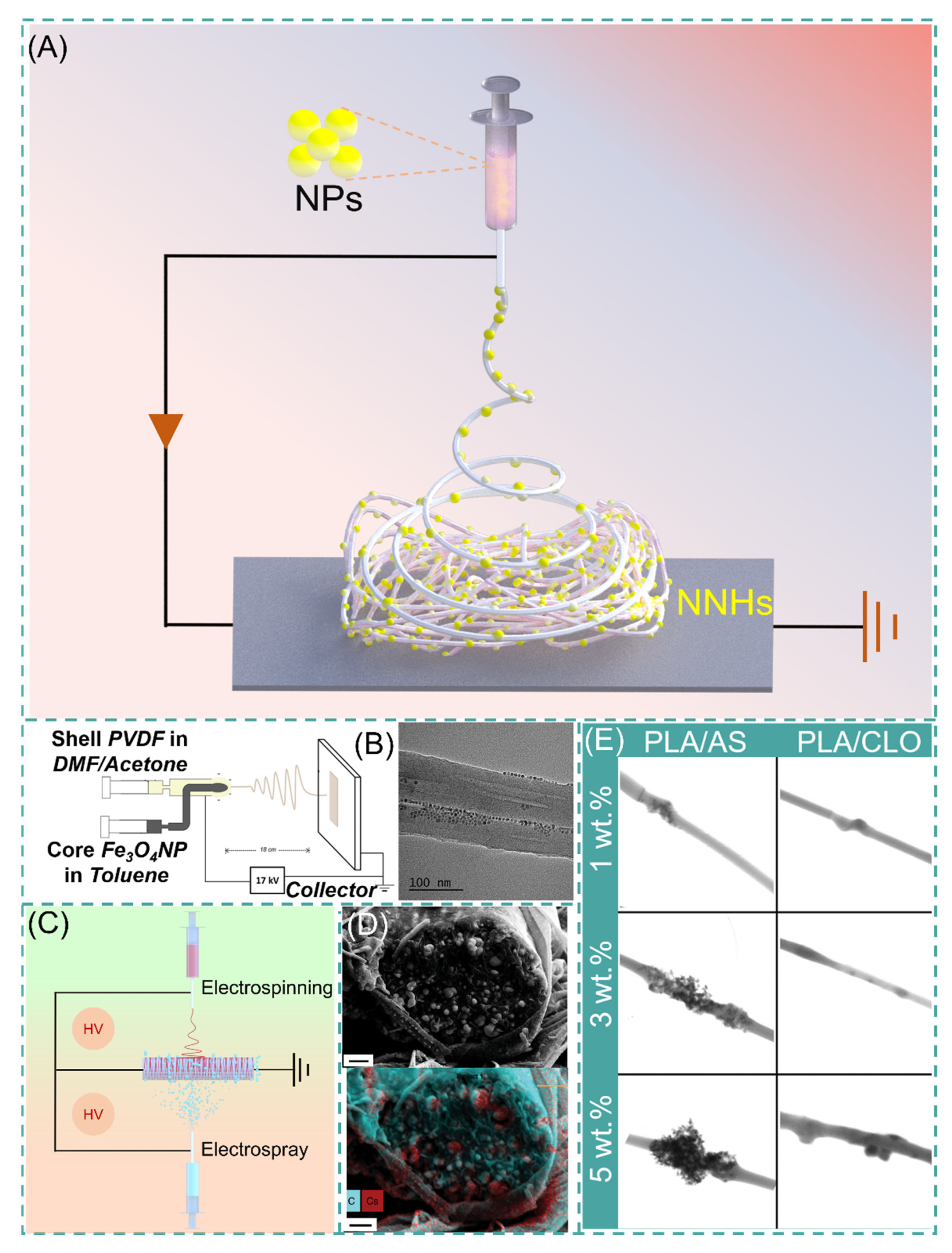
Figure 4. (A) Simple preparation of NNHs based on the single-step process. (B) Schematic diagram of coaxial preparation of NNHs and Transmission Electron Microscopy (TEM), reprinted with permission from Ref. [71]. Copyright 2021 John Wiley and Sons. (C) Schematic diagram of simultaneous electrospinning and electrospraying. (D) Scanning Electron Microscopy (SEM) images of CDP-PVP-PANI fiber cross-sections and Energy dispersive X-Ray spectroscopy map. Reprinted from Ref. [72]. (E) Scanning Transmission Electron Microscopy (STEM) images of PVA/AS or PVA/CLO fibers with different particle concentrations, Reprinted from Ref. [69].
The polymer solution and NPs are divided into different syringes to eliminate the agglomeration of NPs inside the fibers, which are encapsulated in the fibers by coaxial electrospinning or electrospray. Navarro Oliva and co-workers prepared core-shell-structured NNHs by the coaxial electrostatic spinning technique using an Fe3O4 solution as the core layer and a PVDF polymer solution as the shell layer. As shown in Figure 4B, TEM images showed that the NPs were uniformly dispersed inside the fibers without agglomeration [71]. Combining both electrospinning and electrospraying to prepare NNHs is also a convenient method. These two processes are carried out simultaneously, and NPs can be uniformly distributed in the fiber layer (Figure 4C).
The electrospinning process (ESP) has evolved from single-fluid to multi-fluid as research has progressed [73]. Yu’s research team successfully prepared nanofibers with obvious core-shell structures and smoother fiber surfaces by modified tri-axial ESP and achieved intelligent three-stage controlled drug release [74][75]. Pursuing a simple and convenient preparation method, the main preparation method of NNHs is currently the uniaxial ES process. Integrating the preparation of NNHs with more sophisticated ES techniques is a challenge. The incorporation of NPs somewhat reduces the capability of NFs. There are two obvious drawbacks: (1) The addition the NPs reduces the interactions between polymer chains and decreases the mechanical properties of NNHs. (2) NPs are wrapped in fibers and cannot be functionalized. However, Radacsi et al. avoid the defect that NPs are encapsulated by nanofibers and cannot realize the specific surface area enhancement by using cesium dihydrogen phosphate (CDP) for spontaneous nucleation along the solute growth in porous nanofibers; the moist water in the air acts as a solute transport fast channel. Thus, NPs are not only grown inside the fibers but also are uniformly dispersed on the fiber surface. This strategy perfectly avoids the defect that NPs are encapsulated by nanofibers and cannot realize the specific surface area enhancement (Figure 4D) [72].
2.4. The Nanoparticles from Nanofibers through Molecular Self-Assembly
NPs are formed spontaneously by self-assembly strategies [76][77], combining this scheme with ESP, based on post-processing to form NPs spontaneously in fibers, such as in situ synthesis, hydrothermal-assistance and calcination.
2.4.1. In Situ Synthesis
The smooth surface of electrospun fibers and certain stability of the polymer, NPs cannot be perfectly and uniformly attached to the fiber surface by electrostatic adsorption or functional group action. The fiber impregnated with a ligand solution instigates bonding using a reducing agent or other reduction in the composite material to form NPs [78][79]. Lv et al. cross-linked potato starch as a polymer after forming starch fiber mats immersed in AgNO3 solution, and Ag+ was reduced to AgNPs by heating at 60 °C protected from light. The average particle size of AgNPs increased with the concentration of AgNO3, but the particle size decreased significantly and agglomerated together when the concentration reached 100 mg/mL [80]. The technology is also applied to MOF-based nanofibers. Li and co-workers synthesized PW12@UiO-66 crystals in situ on PAA-PVA nanofibers. The surface of the fibers was completely covered by crystals at 12 min of growth, and the MOF crystals were enriched on the surface of NNH but with the disadvantage of random arrangement [81]. Lee and co-workers adopted a hydrodistillation-induced phase separation method to form dense cavities on the surface of PLA nanofibers, followed by uniform growth of ZIF-8 crystals on the porous fiber surface (Figure 5A [82]). Using COF materials with similar properties to MOF, Ma et al. adopted soaking PAN membranes in COF material precursor solutions, where Schiff base condensation occurred at room temperature to form COF spherical particles, and PAN@COF composite fibers showed excellent thermodynamic stability at 300 °C (Figure 5B) [78]. The NNHs prepared by this method, with dense particle aggregation, provide a sufficient number of sites of action that may provide a good solution for the adsorption of tissue fluids or other substances. Similar results from in situ synthesis can be found in Figure 5C [83] and Figure 5D [84].
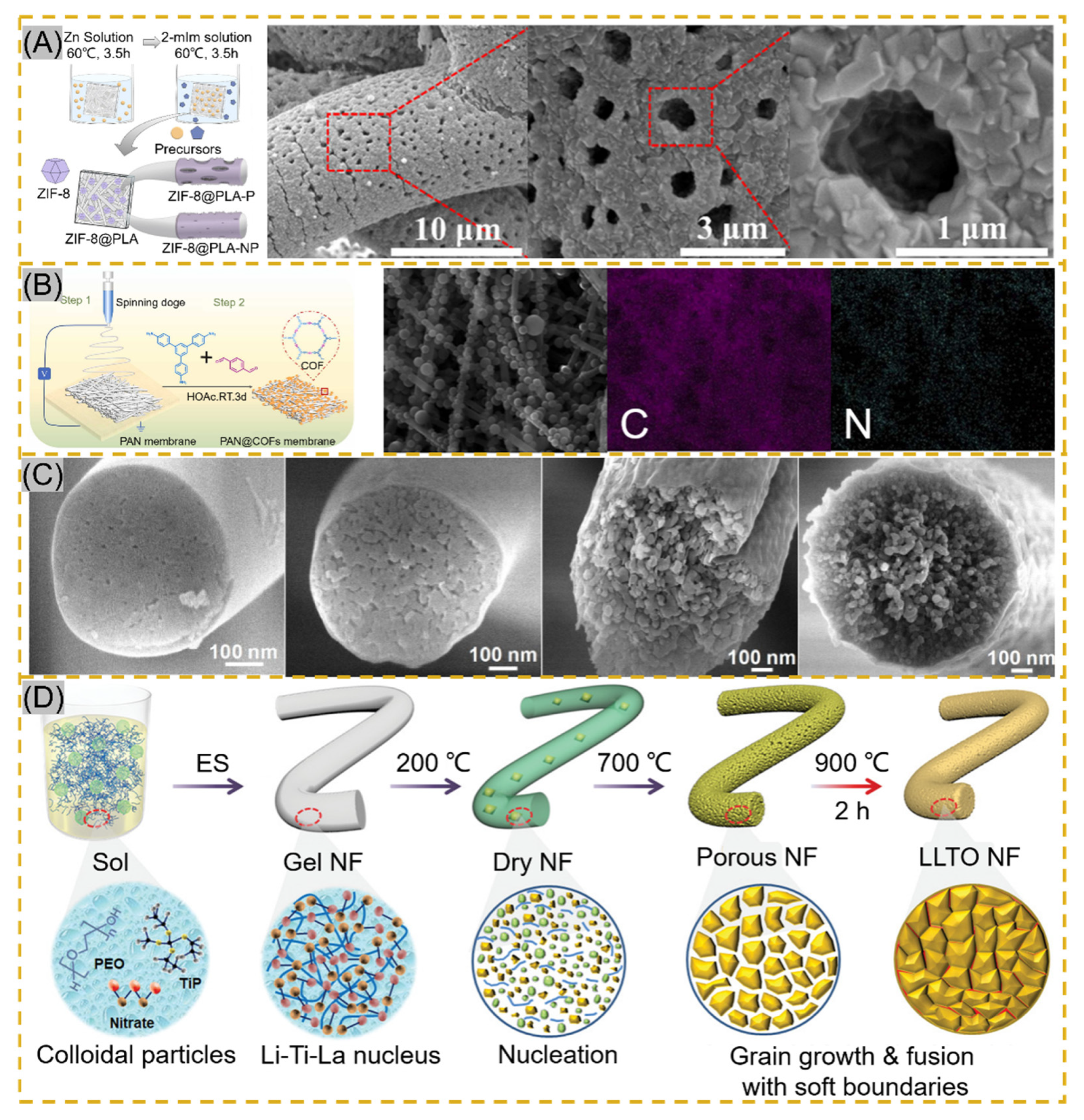
Figure 5. (A) Schematic of in situ ZIF-8 growth on PLA fibers and Scanning Electron Microscopy (STEM) images of ZIF@PLA-P, reprinted with permission from Ref. [82]. Copyright 2021 ACS Publications. (B) Schematic of PAN@COF synthesis and EDS mapping images reprinted with permission from Ref. [78]. Copyright 2022 Elsevier. (C) Transmission Electron Microscopy images of MBNM films prepared based on different contents of PAA, reprinted with permission from Ref. [83]. Copyright 2021 Elsevier. (D) Schematic of LLTO NF process prepared by electrospinning followed by calcination, reprinted with permission from Ref. [84]. Copyright 2021 John Wiley and Sons.
2.4.2. Calcination
Calcination is another effective method to prepare NNHs. Precursor fibers are prepared by the sol–gel method in combination with ESP, and calcination forms nanoparticles in the fibers [85]. Xie fabricated PVP composite fibers, which were maintained at 800 °C for 30 min, and iron-cobalt (FeCo) alloy nanoparticles (30–50 nm) were uniformly distributed in CNFs (150–300 nm), which achieved electrocatalytic degradation of antibiotics in wastewater [86]. Ding’s research team proposed the use of ball-milling precursor sols to form homogeneous nuclear to precisely control crystal nucleation and growth for the purpose of grain refinement to avoid problems, such as the appearance of impurity phases and crack expansion during the preparation of flexible chalcogenide LLTO nanofibers (Figure 5D). Meanwhile, the soft grain boundary is constructed by adopting 200–900 °C stage calcination, which shows the excellent mechanical properties of flexible electronic fiber films based on perovskite ceramic oxide [79]. Nanofibers obtained by unusual calcination are brittle and inflexible. To reduce such a situation, Shan et al. formed porous structures using the template method with sacrificial PAA heated at 700 °C for 2 h to modulate the PAA concentration driving phase separation during calcination stage pyrolysis (Figure 5C) [78]. Similarly, Li and colleagues inherited the MOF structure in ZIF/PAN-Ni-15 composite nanofibers at the sacrifice of ZIFNPs by calcining at 700 °C for 2 h, followed by annealing to form a rosary morphology in the fibers [61].
2.4.3. Hydrothermal-Assistance
Hydrothermal-assistance is a common method to prepare a variety of inorganic oxide crystals, which is based on the conditions of low temperature and isobaric pressure. The uniform distribution of substances avoids the appearance of impurity phases by means of an aqueous medium, obtaining nanoparticles (such as spheres, cubes, flowers, etc.) with diverse morphologies [87][88][89]. The combination of hydrothermal and ESP for the preparation of multilayer heterostructures provides superior performance at a low cost and is green, easy to operate and well received by researchers. Küçük et al. immersed TiO2 fibers (100–200 nm) in an alkaline Ba (OH)2·8H2O solution by a simple hydrothermal reaction, which unexpectedly transformed the composite fibers into tetragonal crystals, demonstrating that metal oxide nanofibers can also be precursors for the preparation of BaTiO3 crystals [90]. Mukhiya and colleagues grew MOF materials on PAN-prepared carbon nanomats (350 nm). First growth of ZIF-67 crystals was attributed to the hydrothermal reaction of cobalt carbonate hydroxide with 2-methylimidazole. The synthesis of ligands by deprotonated 2-methylimidazole with Co2+ was responsible for the crystals’ second growth. The composite fiber membrane has high specific capacity and excellent service life, with strong advantages in energy storage applications [91]. In terms of the same application, Poudel et al. prepared PAN/PMMA nanofiber membranes using a coaxial ESP and sacrificed the internal PMMA after carbonization to obtain 3D hollow carbon nanofibers (3DHPCNF). By adopting two hydrothermal methods, Fe2O3 was first synthesized on the fiber surface, and then this was used as a precursor to hydrothermally generate ZMALDH@Fe2O3/3DHPCNF with ternary metal salts in an autoclave. The LDH lamellar structure was thinned by changing the content of Zn, and the transformation of nanosheets to nanowires was found at high Zn2+ content, which is a novel top-down way to obtain ternary LDH-electrospun hollow carbon nanofibers, providing a new idea for subsequent design [92].
References
- Koh, H.K.; Geller, A.C.; VanderWeele, T.J. Deaths From COVID-19. J. Am. Med. Assoc. 2021, 325, 133–134.
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615.
- Fauci, A.S.; Merad, M.; Swaminathan, S.; Hur, S.; Topol, E.; Fitzgerald, K.; Reis e Sousa, C.; Corbett, K.S.; Bauer, S. From MRNA Sensing to Vaccines. Immunity 2021, 54, 2676–2680.
- Extance, A. MRNA Vaccines: Hope beneath the Hype. Br. Med. J. 2021, 375, n2744.
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the MRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785.
- Ali, K.; Berman, G.; Zhou, H.; Deng, W.; Faughnan, V.; Coronado-Voges, M.; Ding, B.; Dooley, J.; Girard, B.; Hillebrand, W.; et al. Evaluation of MRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 2241–2251.
- Becker, M.L.; Burdick, J.A. Introduction: Polymeric Biomaterials. Chem. Rev. 2021, 121, 10789–10791.
- Safian, M.T.; Umar, K.; Parveen, T.; Yaqoob, A.A.; Ibrahim, M.N.M. Chapter Eight-Biomedical applications of smart polymer composites. In Smart Polymer Nanocomposites: Biomedical and Environmental Applications; Elsevier Inc.: Cambridge, MA, USA, 2021; pp. 183–204.
- Chakraborty, P.; Oved, H.; Bychenko, D.; Yao, Y.; Tang, Y.; Zilberzwige-Tal, S.; Wei, G.; Dvir, T.; Gazit, E. Nanoengineered Peptide-Based Antimicrobial Conductive Supramolecular Biomaterial for Cardiac Tissue Engineering. Adv. Mater. 2021, 33, 2008715.
- Kang, S.; Hou, S.; Chen, X.; Yu, D.G.; Wang, L.; Li, X.; Williams, G.R.R. Energy-saving electrospinning with a concentric Teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421.
- Yaqoob, A.A.; Safian, M.T.; Rashid, M.; Parveen, T.; Umar, K.; Ibrahim, M.N.M. Chapter One-Introduction of Smart Polymer Nanocomposites. In Smart Polymer Nanocomposites: Biomedical and Environmental Applications; Elsevier Inc.: Cambridge, MA, USA, 2021; pp. 1–25.
- Jung, K.; Corrigan, N.; Wong, E.H.H.; Boyer, C. Bioactive Synthetic Polymers. Adv. Mater. 2021, 34, 2105063.
- Sionkowska, A. Collagen Blended with Natural Polymers: Recent Advances and Trends. Prog. Polym. Sci. 2021, 122, 101452.
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive Potential of Natural Biomaterials: Identification, Retention and Assessment of Biological Properties. Signal Transduc. Target. Ther. 2021, 6, 1–28.
- Rosén, T.; Hsiao, B.S.; Söderberg, L.D. Elucidating the Opportunities and Challenges for Nanocellulose Spinning. Adv. Mater. 2021, 33, 2001238.
- Taokaew, S.T.; Chiaoprakobkij, N.; Siripong, P.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Multifunctional Cellulosic Nanofiber Film with Enhanced Antimicrobial and Anticancer Properties by Incorporation of Ethanolic Extract of Garcinia Mangostana Peel. Mater. Sci. Eng. C 2021, 120, 111783.
- Chen, Z.; Zhang, Q.; Li, H.; Wei, Q.; Zhao, X.; Chen, F. Elastin-like Polypeptide Modified Silk Fibroin Porous Scaffold Promotes Osteochondral Repair. Bioact. Mater. 2021, 6, 589–601.
- Bakhshandeh, B.; Nateghi, S.S.; Gazani, M.M.; Dehghani, Z.; Mohammadzadeh, F. A Review on Advances in the Applications of Spider Silk in Biomedical Issues. Int. J. Biol. Macromol. 2021, 192, 258–271.
- Dziadek, M.; Dziadek, K.; Checinska, K.; Zagrajczuk, B.; Golda-Cepa, M.; Brzychczy-Wloch, M.; Menaszek, E.; Kopec, A.; Cholewa-Kowalska, K. PCL and PCL/Bioactive Glass Biomaterials as Carriers for Biologically Active Polyphenolic Compounds: Comprehensive Physicochemical and Biological Evaluation. Bioact. Mater. 2021, 6, 1811–1826.
- Janmohammadi, M.; Nourbakhsh, M.S. Electrospun Polycaprolactone Scaffolds for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 527–539.
- Zavan, B.; Gardin, C.; Guarino, V.; Rocca, T.; Cruz Maya, I.; Zanotti, F.; Ferroni, L.; Brunello, G.; Chachques, J.-C.; Ambrosio, L.; et al. Electrospun PCL-Based Vascular Grafts: In Vitro Tests. Nanomaterials 2021, 11, 751.
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun Structural Hybrids of Acyclovir-Polyacrylonitrile at Acyclovir for Modifying Drug Release. Polymers 2021, 13, 4286.
- Zhang, X.; Guo, S.; Qin, Y.; Li, C. Functional Electrospun Nanocomposites for Efficient Oxygen Reduction Reaction. Chem. Res. Chin. Univ. 2021, 37, 379–393.
- Ning, T.; Zhou, Y.; Xu, H.; Guo, S.; Wang, K.; Yu, D.-G. Orodispersible Membranes from a Modified Coaxial Electrospinning for Fast Dissolution of Diclofenac Sodium. Membranes 2021, 11, 802.
- Xu, H.; Xu, X.; Li, S.; Song, W.-L.; Yu, D.-G.; Annie Bligh, S.W. The Effect of Drug Heterogeneous Distributions within Core-Sheath Nanostructures on Its Sustained Release Profiles. Biomolecules 2021, 11, 1330.
- Wang, Y.; Xu, H.; Wu, M.; Yu, D.-G. Nanofibers-Based Food Packaging. ES Food Agrofor. 2022, 2, 1–23.
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415.
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.-G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770.
- Kang, S.; Zhao, K.; Yu, D.G.; Zheng, X.; Huang, C. Advances in Biosensing and Environmental Monitoring Based on Electrospun Nanofibers. Adv. Fiber Mater. 2022, 9.
- Zhao, K.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Electrospun Functional Nanofiber Membrane for Antibiotic Removal in Water: Review. Polymers 2021, 13, 226.
- Shepa, I.; Mudra, E.; Dusza, J. Electrospinning through the Prism of Time. Mater. Today Chem. 2021, 21, 100543.
- Madruga, L.Y.C.; Kipper, M.J. Expanding the Repertoire of Electrospinning: New and Emerging Biopolymers, Techniques, and Applications. Adv. Heal. Mater. 2021, 2101979.
- Zhang, X.; Xie, L.; Wang, X.; Shao, Z.; Kong, B. Electrospinning Super-Assembly of Ultrathin Fibers from Single- to Multi-Taylor Cone Sites. Appl. Mater. Today 2021, 23, 101272.
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive Polymer Ultrafine Fibers via Electrospinning: Preparation, Physical Properties and Applications. Prog. Mater. Sci. 2021, 115, 100704.
- Wang, Y.; Chen, G.; Zhang, H.; Zhao, C.; Sun, L.; Zhao, Y. Emerging Functional Biomaterials as Medical Patches. ACS Nano 2021, 15, 5977–6007.
- Huo, P.; Han, X.; Zhang, W.; Zhang, J.; Kumar, P.; Liu, B. Electrospun Nanofibers of Polycaprolactone/Collagen as a Sustained-Release Drug Delivery System for Artemisinin. Pharmaceutics 2021, 13, 1228.
- Chen, K.; Pan, H.; Yan, Z.; Li, Y.; Ji, D.; Yun, K.; Su, Y.; Liu, D.; Pan, W. A Novel Alginate/Gelatin Sponge Combined with Curcumin-Loaded Electrospun Fibers for Postoperative Rapid Hemostasis and Prevention of Tumor Recurrence. Int. J. Biol. Macromol. 2021, 182, 1339–1350.
- Zhang, X.; Li, L.; Ouyang, J.; Zhang, L.; Xue, J.; Zhang, H.; Tao, W. Electroactive Electrospun Nanofibers for Tissue Engineering. Nano Today 2021, 39, 101196.
- Zhu, M.; Tan, J.; Liu, L.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. Construction of Biomimetic Artificial Intervertebral Disc Scaffold via 3D Printing and Electrospinning. Mater. Sci. Eng. C 2021, 128, 112310.
- Rickel, A.P.; Deng, X.; Engebretson, D.; Hong, Z. Electrospun Nanofiber Scaffold for Vascular Tissue Engineering. Mater. Sci. Eng. C 2021, 129, 112373.
- Wang, Z.; Li, J.; Shao, C.; Lin, X.; Yang, Y.; Chen, N.; Wang, Y.; Qu, L. Moisture Power in Natural Polymeric Silk Fibroin Flexible Membrane Triggers Efficient Antibacterial Activity of Silver Nanoparticles. Nano Energy 2021, 90, 106529.
- Ho Na, J.; Chan Kang, Y.; Park, S.-K. Electrospun MOF-Based ZnSe Nanocrystals Confined in N-Doped Mesoporous Carbon Fibers as Anode Materials for Potassium Ion Batteries with Long-Term Cycling Stability. Chem. Eng. J. 2021, 425, 131651.
- Zhao, K.; Lu, Z.-H.; Zhao, P.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Modified Tri–Axial Electrospun Functional Core–Shell Nanofibrous Membranes for Natural Photodegradation of Antibiotics. Chem. Eng. J. 2021, 425, 131455.
- Huang, G.-Y.; Chang, W.-J.; Lu, T.-W.; Tsai, I.-L.; Wu, S.-J.; Ho, M.-H.; Mi, F.-L. Electrospun CuS Nanoparticles/Chitosan Nanofiber Composites for Visible and near-Infrared Light-Driven Catalytic Degradation of Antibiotic Pollutants. Chem. Eng. J. 2022, 431, 134059.
- Cui, Z.; Shen, S.; Yu, J.; Si, J.; Cai, D.; Wang, Q. Electrospun Carbon Nanofibers Functionalized with NiCo2S4 Nanoparticles as Lightweight, Flexible and Binder-Free Cathode for Aqueous Ni-Zn Batteries. Chem. Eng. J. 2021, 426, 130068.
- Sharma, D.; Satapathy, B.K. Polymer Substrate-Based Transition Metal Modified Electrospun Nanofibrous Materials: Current Trends in Functional Applications and Challenges. Polym. Rev. 2021, 61, 1–46.
- Jena, S.K.; Sadasivam, R.; Packirisamy, G.; Saravanan, P. Nanoremediation: Sunlight Mediated Dye Degradation Using Electrospun PAN/CuO–ZnO Nanofibrous Composites. Environ. Pollut. 2021, 280, 116964.
- Ding, C.; Breunig, M.; Timm, J.; Marschall, R.; Senker, J.; Agarwal, S. Flexible, Mechanically Stable, Porous Self-Standing Microfiber Network Membranes of Covalent Organic Frameworks: Preparation Method and Characterization. Adv. Funct. Mater. 2021, 31, 2106507.
- Neibolts, N.; Platnieks, O.; Gaidukovs, S.; Barkane, A.; Thakur, V.K.; Filipova, I.; Mihai, G.; Zelca, Z.; Yamaguchi, K.; Enachescu, M. Needle-Free Electrospinning of Nanofibrillated Cellulose and Graphene Nanoplatelets Based Sustainable Poly (Butylene Succinate) Nanofibers. Mater. Today Chem. 2020, 17, 100301.
- Ren, S.; Zhou, Y.; Zheng, K.; Xu, X.; Yang, J.; Wang, X.; Miao, L.; Wei, H.; Xu, Y. Cerium Oxide Nanoparticles Loaded Nanofibrous Membranes Promote Bone Regeneration for Periodontal Tissue Engineering. Bioact. Mater. 2022, 7, 242–253.
- Rasekh, A.; Raisi, A. Electrospun Nanofibrous Polyether-Block-Amide Membrane Containing Silica Nanoparticles for Water Desalination by Vacuum Membrane Distillation. Sep. Purif. Technol. 2021, 275, 119149.
- Jiang, X.; Zhang, S.; Zou, B.; Li, G.; Yang, S.; Zhao, Y.; Lian, J.; Li, H.; Ji, H. Electrospun Nanofiber Membrane as an Effective Polysulfides Adsorption-Catalysis Interlayer for Li-S Batteries. Chem. Eng. J. 2022, 430, 131911.
- Fang, H.; Wang, C.; Li, D.; Zhou, S.; Du, Y.; Zhang, H.; Hang, C.; Tian, Y.; Suga, T. Fabrication of Composite Nanowires for High-Efficient Room-Temperature Removal of Formaldehyde. J. Mater. Sci. Technol. 2021, 91, 5–16.
- Park, K.; Kang, S.; Park, J.; Hwang, J. Fabrication of Silver Nanowire Coated Fibrous Air Filter Medium via a Two-Step Process of Electrospinning and Electrospray for Anti-Bioaerosol Treatment. J. Hazard. Mater. 2021, 411, 125043.
- Enculescu, M.; Costas, A.; Evanghelidis, A.; Enculescu, I. Fabrication of ZnO and TiO2 Nanotubes via Flexible Electro-Spun Nanofibers for Photocatalytic Applications. Nanomaterials 2021, 11, 1305.
- Wang, K.; Wang, P.; Wang, M.; Yu, D.-G.; Wan, F.; Bligh, S.W.A. Comparative Study of Electrospun Crystal-Based and Composite-Based Drug Nano Depots. Mater. Sci. Eng. C 2020, 113, 110988.
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun Tri-Layer Nanodepots for Sustained Release of Acyclovir. J. Alloy. Compd. 2020, 846, 156471.
- Zheng, G.; Peng, H.; Jiang, J.; Kang, G.; Liu, J.; Zheng, J.; Liu, Y. Surface Functionalization of PEO Nanofibers Using a TiO2 Suspension as Sheath Fluid in a Modified Coaxial Electrospinning Process. Chem. Res. Chin. Univ. 2021, 37, 571–577.
- Yang, C.-H.; Hsiao, Y.-C.; Lin, L.-Y. Novel in Situ Synthesis of Freestanding Carbonized ZIF67/Polymer Nanofiber Electrodes for Supercapacitors via Electrospinning and Pyrolysis Techniques. ACS Appl. Mater. Interfaces 2021, 13, 41637–41648.
- Pant, B.; Prasad Ojha, G.; Acharya, J.; Park, M. Ag3PO4-TiO2-Carbon Nanofiber Composite: An Efficient Visible-Light Photocatalyst Obtained from Eelectrospinning and Hydrothermal Methods. Sep. Purif. Technol. 2021, 276, 119400.
- Li, R.; Ke, H.; Shi, C.; Long, Z.; Dai, Z.; Qiao, H.; Wang, K. Mesoporous RGO/NiCo2O4@carbon Composite Nanofibers Derived from Metal-Organic Framework Compounds for Lithium Storage. Chem. Eng. J. 2021, 415, 128874.
- Alturki, A.M. Rationally Design of Electrospun Polysaccharides Polymeric Nanofiber Webs by Various Tools for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2021, 184, 648–665.
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun Polymer Biomaterials. Prog. Polym. Sci. 2019, 90, 1–34.
- Valerini, D.; Tammaro, L.; Vitali, R.; Guillot, G.; Rinaldi, A. Sputter-Deposited Ag Nanoparticles on Electrospun PCL Scaffolds: Morphology, Wettability and Antibacterial Activity. Coatings 2021, 11, 345.
- Liu, Z.; Jia, L.; Yan, Z.; Bai, L. Plasma-Treated Electrospun Nanofibers as a Template for the Electrostatic Assembly of Silver Nanoparticles. N. J. Chem. 2018, 42, 11185–11191.
- Rostamabadi, H.; Falsafi, S.R.; Rostamabadi, M.M.; Assadpour, E.; Jafari, S.M. Electrospraying as a Novel Process for the Synthesis of Particles/Nanoparticles Loaded with Poorly Water-Soluble Bioactive Molecules. Adv. Colloid Interface Sci. 2021, 290, 102384.
- Fahimirad, S.; Abtahi, H.; Satei, P.; Ghaznavi-Rad, E.; Moslehi, M.; Ganji, A. Wound Healing Performance of PCL/Chitosan Based Electrospun Nanofiber Electrosprayed with Curcumin Loaded Chitosan Nanoparticles. Carbohydr. Polym. 2021, 259, 117640.
- Karagoz, S.; Kiremitler, N.B.; Sarp, G.; Pekdemir, S.; Salem, S.; Goksu, A.G.; Onses, M.S.; Sozdutmaz, I.; Sahmetlioglu, E.; Ozkara, E.S.; et al. Antibacterial, Antiviral, and Self-Cleaning Mats with Sensing Capabilities Based on Electrospun Nanofibers Decorated with ZnO Nanorods and Ag Nanoparticles for Protective Clothing Applications. ACS Appl. Mater. Interfaces 2021, 13, 5678–5690.
- Lopresti, F.; Pavia, F.C.; Ceraulo, M.; Capuana, E.; Brucato, V.; Ghersi, G.; Botta, L.; La Carrubba, V. Physical and Biological Properties of Electrospun Poly(d,l-Lactide)/Nanoclay and Poly(d,l-Lactide)/Nanosilica Nanofibrous Scaffold for Bone Tissue Engineering. J. Biomed. Mater. Res. Part A 2021, 109, 2120–2136.
- Abdelaziz, D.; Hefnawy, A.; Al-Wakeel, E.; El-Fallal, A.; El-Sherbiny, I.M. New Biodegradable Nanoparticles-in-Nanofibers Based Membranes for Guided Periodontal Tissue and Bone Regeneration with Enhanced Antibacterial Activity. J. Adv. Res. 2021, 28, 51–62.
- Navarro Oliva, F.S.; Picart, L.; Leon-Valdivieso, C.Y.; Benalla, A.; Lenglet, L.; Ospina, A.; Jestin, J.; Bedoui, F. Coaxial Electrospinning Process toward Optimal Nanoparticle Dispersion in Polymeric Matrix. Polym. Compos. 2021, 42, 1565–1573.
- Radacsi, N.; Campos, F.D.; Chisholm, C.R.I.; Giapis, K.P. Spontaneous Formation of Nanoparticles on Electrospun Nanofibres. Nat. Commun. 2018, 9, 4740.
- Yu, D.G.; Lv, H. Preface-striding into Nano Drug Delivery. Curr. Drug Deliv. 2022, 19, 1–3.
- Ding, Y.; Dou, C.; Chang, S.; Xie, Z.; Yu, D.-G.; Liu, Y.; Shao, J. Core–Shell Eudragit S100 Nanofibers Prepared via Triaxial Electrospinning to Provide a Colon-Targeted Extended Drug Release. Polymers 2020, 12, 2034.
- Chang, S.; Wang, M.; Zhang, F.; Liu, Y.; Liu, X.; Yu, D.-G.; Shen, H. Sheath-Separate-Core Nanocomposites Fabricated Using a Trifluid Electrospinning. Mater. Des. 2020, 192, 108782.
- Chen, J.; Pan, S.; Zhou, J.; Lin, Z.; Qu, Y.; Glab, A.; Han, Y.; Richardson, J.J.; Caruso, F. Assembly of Bioactive Nanoparticles via Metal–Phenolic Complexation. Adv. Mater. 2021, 33, 2108624.
- Zhang, Z.; Yue, Y.-X.; Xu, L.; Wang, Y.; Geng, W.-C.; Li, J.-J.; Kong, X.; Zhao, X.; Zheng, Y.; Zhao, Y.; et al. Macrocyclic-Amphiphile-Based Self-Assembled Nanoparticles for Ratiometric Delivery of Therapeutic Combinations to Tumors. Adv. Mater. 2021, 33, 2007719.
- Ma, J.; Yu, Z.; Liu, S.; Chen, Y.; Lv, Y.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; Liu, M.; et al. Efficient Extraction of Trace Organochlorine Pesticides from Environmental Samples by a Polyacrylonitrile Electrospun Nanofiber Membrane Modified with Covalent Organic Framework. J. Hazard. Mater. 2022, 424, 127455.
- Meng, Q.; Huang, Y.; Ye, J.; Xia, G.; Wang, G.; Dong, L.; Yang, Z.; Yu, X. Electrospun Carbon Nanofibers with In-Situ Encapsulated Ni Nanoparticles as Catalyst for Enhanced Hydrogen Storage of MgH2. J. Alloys Compd. 2021, 851, 156874.
- Lv, H.; Cui, S.; Yang, Q.; Song, X.; Wang, D.; Hu, J.; Zhou, Y.; Liu, Y. AgNPs-Incorporated Nanofiber Mats: Relationship between AgNPs Size/Content, Silver Release, Cytotoxicity, and Antibacterial Activity. Mater. Sci. Eng. C 2021, 118, 111331.
- Li, T.; Zhang, Z.; Liu, L.; Gao, M.; Han, Z. A Stable Metal-Organic Framework Nanofibrous Membrane as Photocatalyst for Simultaneous Removal of Methyl Orange and Formaldehyde from Aqueous Solution. Colloids Surf. A 2021, 617, 126359.
- Lee, J.; Jung, S.; Park, H.; Kim, J. Bifunctional ZIF-8 Grown Webs for Advanced Filtration of Particulate and Gaseous Matters: Effect of Charging Process on the Electrostatic Capture of Nanoparticles and Sulfur Dioxide. ACS Appl. Mater. Interfaces 2021, 13, 50401–50410.
- Shan, H.; Si, Y.; Yu, J.; Ding, B. Facile Access to Highly Flexible and Mesoporous Structured Silica Fibrous Membranes for Tetracyclines Removal. Chem. Eng. J. 2021, 417, 129211.
- Li, X.; Zhang, Y.; Zhang, L.; Xia, S.; Zhao, Y.; Yan, J.; Yu, J.; Ding, B. Synthesizing Superior Flexible Oxide Perovskite Ceramic Nanofibers by Precisely Controlling Crystal Nucleation and Growth. Small 2021, 11, 2106500.
- Lee, S.S.; Bai, H.; Chua, S.C.; Lee, K.W.; Sun, D.D. Electrospun Bi3+/TiO2 Nanofibers for Concurrent Photocatalytic H2 and Clean Water Production from Glycerol under Solar Irradiation: A Systematic Study. J. Cleaner Prod. 2021, 298, 126671.
- Xie, W.; Shi, Y.; Wang, Y.; Zheng, Y.; Liu, H.; Hu, Q.; Wei, S.; Gu, H.; Guo, Z. Electrospun Iron/Cobalt Alloy Nanoparticles on Carbon Nanofibers towards Exhaustive Electrocatalytic Degradation of Tetracycline in Wastewater. Chem. Eng. J. 2021, 405, 126585.
- Chang, Y.; Dong, C.; Zhou, D.; Li, A.; Dong, W.; Cao, X.-Z.; Wang, G. Fabrication and Elastic Properties of TiO2 Nanohelix Arrays through a Pressure-Induced Hydrothermal Method. ACS Nano 2021, 15, 14174–14184.
- Jung, K.-W.; Lee, S.Y.; Choi, J.-W.; Hwang, M.-J.; Shim, W.G. Synthesis of Mg–Al Layered Double Hydroxides-Functionalized Hydrochar Composite via an in Situ One-Pot Hydrothermal Method for Arsenate and Phosphate Removal: Structural Characterization and Adsorption Performance. Chem. Eng. J. 2021, 420, 129775.
- Xu, W.; Li, X.; Peng, C.; Yang, G.; Cao, Y.; Wang, H.; Peng, F.; Yu, H. One-Pot Synthesis of Ru/Nb2O5@Nb2C Ternary Photocatalysts for Water Splitting by Harnessing Hydrothermal Redox Reactions. Appl. Catal. B 2022, 303, 120910.
- Küçük, Ö.; Teber, S.; Cihan Kaya, İ.; Akyıldız, H.; Kalem, V. Photocatalytic Activity and Dielectric Properties of Hydrothermally Derived Tetragonal BaTiO3 Nanoparticles Using TiO2 Nanofibers. J. Alloys Compd. 2018, 765, 82–91.
- Mukhiya, T.; Tiwari, A.P.; Chhetri, K.; Kim, T.; Dahal, B.; Muthurasu, A.; Kim, H.Y. A Metal–Organic Framework Derived Cobalt Oxide/Nitrogen-Doped Carbon Nanotube Nanotentacles on Electrospun Carbon Nanofiber for Electrochemical Energy Storage. Chem. Eng. J. 2021, 420, 129679.
- Poudel, M.B.; Kim, H.J. Confinement of Zn-Mg-Al-Layered Double Hydroxide and α-Fe2O3 Nanorods on Hollow Porous Carbon Nanofibers: A Free-Standing Electrode for Solid-State Symmetric Supercapacitors. Chem. Eng. J. 2022, 429, 132345.
More
Information
Subjects:
Materials Science, Biomaterials
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
814
Revisions:
2 times
(View History)
Update Date:
11 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




