
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | HAN QI ZHAO | + 3496 word(s) | 3496 | 2022-01-24 08:45:27 | | | |
| 2 | Vivi Li | Meta information modification | 3496 | 2022-02-10 02:24:12 | | | | |
| 3 | Vivi Li | Meta information modification | 3496 | 2022-02-10 09:09:46 | | |
Video Upload Options
Cold-stored platelets are making a comeback. They were abandoned in the late 1960s in favor of room-temperature stored platelets due to the need for longer post-transfusion platelet recoverability and survivability in patients with chronic thrombocytopenia. However, the current needs for platelet transfusions are rapidly changing. Today, more platelets are given to patients who are actively bleeding, such as ones receiving cardiac surgeries. It has been established that cold-stored platelets are more hemostatically effective, have reduced bacterial growth, and have longer potential shelf lives. These compelling characteristics led to the recent interest in bringing back cold-stored platelets to the blood systems. However, before reinstating cold-stored platelets in the clinics again, a thorough investigation of in vitro storage characteristics and in vivo transfusion effects is required.
1. Introduction
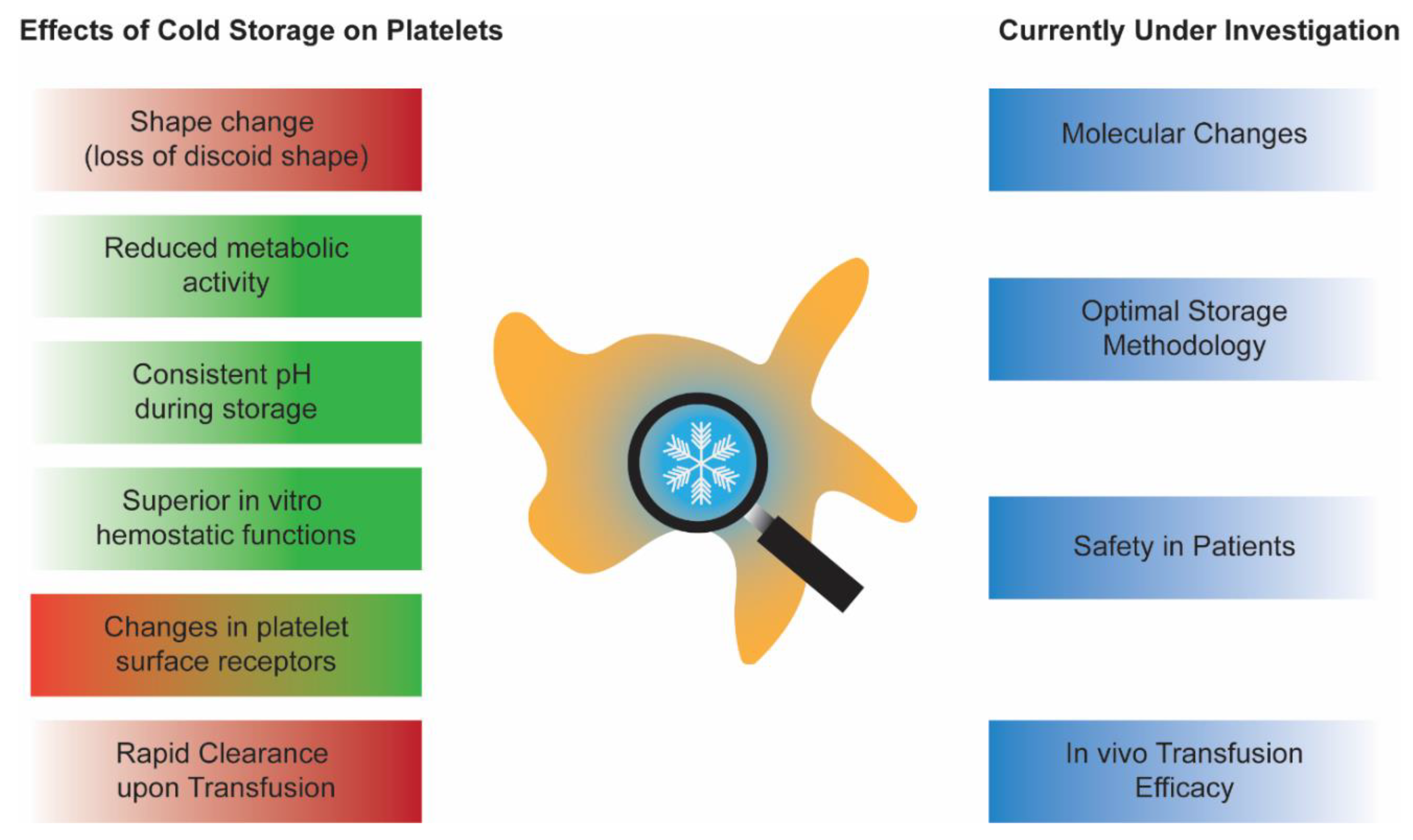
2. CPs In Vitro Characterization: An Update and a Debate
3. “Omics” and Cold-Stored Platelets
4. Reduction of CPs Storage Lesion, a Work in Progress
Results from the recent omics study showed that oxidative stress played a significant role in platelet storage lesion both in RPs and CPs. Hence, to improve CPs’ storage quality, both synthetic and naturally occurring antioxidants have been used as supplements. N-acetylcysteine (NAC) has been used as a supplement to minimize the mitochondrial uncoupling and proton leaks caused by cold-storage [32]. NAC supplement also showed the slight effect of reducing ROS in CPs. Both RPs and CPs with NAC supplement had better correction in a mice tail bleeding experiment than CPs without NAC [32]. However, these experiments had relatively few biological replicates. In addition, the use of platelet additive solution (PAS) could have influenced the results as well. In another study, whole blood-derived platelet rich plasma (PRP) supplemented with NAC and stored in the cold seemed to have reduced platelet activation measured by P-selectin expression compared to RPs [33]. NAC supplement did not result in differences in the phosphatidylserine expression of CPs or RPs. However, the platelets used in this experiment were stored in gas-permeable tubes instead of standardized storage containers, making it difficult to draw solid conclusions [33]. In a follow-up study, the effect of NAC on CPs post-transfusion recovery has been tested using the Prkdcscid mouse model [34]. There was a slight improvement of platelet recovery 2 h post transfusion in the CPs with the NAC group. However, this improved recovery effect was diminished after 24 h post transfusion [34]. It is important to note that in this mouse study, CPs without NAC supplement were not tested due to aggregate formation in the storage bag. The absence of this control makes data interpretation more challenging, as the result could be due to either the storage temperatures or the addition of NAC. Another antioxidant, resveratrol, and a mitochondrial targeting protein, cytochrome c, have been used to reduce oxidative stress in CPs as well [35]. The preliminary results from these supplements showed decreased ROS in resveratrol-treated CPs [35]. However, further investigation of supplemented CPs function and transfusion efficacy are needed.
5. Safer CPs: Pathogen Inactivation and Platelet Additive Solutions
| PAS | Author | Ref | Main Usage |
|---|---|---|---|
| T-PAS | Nair et al. | [21] | Investigation of the clot structure of CPs. |
| T-PAS | Reddoch-Cardenas et al. | [56] | Compared CPs stored in PAS versus RPs and CPs in plasma. |
| T-PAS and Intersol | Getz et al. | [10] | Reduce aggregate formation in CPs using PAS. |
| SSP+ | Six et al. | [47] | Pathogen inactivation (Intercept). |
| SSP+ | Johnson et al. | [49] | Pathogen inactivation (Theraflex UV-platelet System). |
| SSP+ | Marini et al. | [54] | Plasma replacement and test for the percentage of residual plasma required. |
| SSP+ | Wood et al. | [60] | Delayed cold storage. |
| PAS-E | Hegde et al. | [32] | NAC supplementation in CPs |
| PAS-E | Johnson et al. | [57] | Prolonged platelet cold storage. |
| Intersol | Agey et al. | [48] | Pathogen inactivation (Intercept). |
| Isoplate | Reddoch-Cardenas et al. | [58] | Preserving mitochondrial function in CPs. |
| Isoplate and Intersol | Reddoch-Cardenas et al. | [55] | Compare the CPs storage characteristics in two FDA approved PAS. |
| Isoplate and Intersol | Stolla et al. | [59] | PAS in CPs in vivo recoveries using autologous radiolabeled platelet transfusion. |
| PAS-IIIM | Braathen et al. | [61] | Delayed cold storage. |
References
- Murphy, S.; Gardner, F.H. Effect of storage temperature on maintenance of platelet viability—Deleterious effect of refrigerated storage. N. Engl. J. Med. 1969, 280, 1094–1098.
- Josefsson, E.C.; Gebhard, H.H.; Stossel, T.P.; Hartwig, J.H.; Hoffmeister, K.M. The macrophage alphaMbeta2 integrin alphaM lectin domain mediates the phagocytosis of chilled platelets. J. Biol. Chem. 2005, 280, 18025–18032.
- Badlou, B.A.; Spierenburg, G.; Ulrichts, H.; Deckmyn, H.; Smid, W.M.; Akkerman, J.-W.N. Role of glycoprotein Ib? In phagocytosis of platelets by macrophages. Transfusion 2006, 46, 2090–2099.
- Rumjantseva, V.; Grewal, P.K.; Wandall, H.H.; Josefsson, E.C.; Sørensen, A.L.; Larson, G.; Marth, J.D.; Hartwig, J.H.; Hoffmeister, K.M. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat. Med. 2009, 15, 1273–1280.
- Wandall, H.H.; Hoffmeister, K.M.; Sørensen, A.L.; Rumjantseva, V.; Clausen, H.; Hartwig, J.H.; Slichter, S.J. Galactosylation does not prevent the rapid clearance of long-term, 4 °C-stored platelets. Blood 2008, 111, 3249–3256.
- Zucker, M.B.; Borrelli, J. Reversible alterations in platelet morphology produced by anticoagulants and by cold. Blood 1954, 9, 602–608.
- White, J.G.; Krivit, W. An ultrastructural basis for the shape changes induced in platelets by chilling. Blood 1967, 30, 625–635.
- Reddoch, K.M.; Pidcoke, H.F.; Montgomery, R.K.; Fedyk, C.G.; Aden, J.K.; Ramasubramanian, A.K.; Cap, A.P. Hemostatic function of apheresis platelets stored at 4 °C and 22 °C. Shock 2014, 41, 54–61.
- Oliver, A.E.; Tablin, F.; Walker, N.J.; Crowe, J.H. The internal calcium concentration of human platelets increases during chilling. Biochim. Biophys. Acta (BBA)-Biomembr. 1999, 1416, 349–360.
- Getz, T.M.; Montgomery, R.K.; Bynum, J.A.; Aden, J.K.; Pidcoke, H.F.; Cap, A.P. Storage of platelets at 4 °C in platelet additive solutions prevents aggregate formation and preserves platelet functional responses. Transfusion 2016, 56, 1320–1328.
- Bynum, J.A.; Meledeo, M.; Getz, T.M.; Rodriguez, A.C.; Aden, J.K.; Cap, A.P.; Pidcoke, H.F. Bioenergetic profiling of platelet mitochondria during storage: 4 °C storage extends platelet mitochondrial function and viability. Transfusion 2016, 56, S76–S84.
- Ketter, P.M.; Kamucheka, R.; Arulanandam, B.; Akers, K.; Cap, A.P. Platelet enhancement of bacterial growth during room temperature storage: Mitigation through refrigeration. Transfusion 2019, 59, 1479–1489.
- Becker, G.A.; Tuccelli, M.; Kunicki, T.; Chalos, M.K.; Aster, R.H. Studies of platelet concentrates stored at 22 C and 4 C. Transfusion 1973, 13, 61–68.
- Zhao, H.W.; Serrano, K.; Stefanoni, D.; D’Alessandro, A.; Devine, D.V. In Vitro Characterization and Metabolomic Analysis of Cold-Stored Platelets. J. Proteome Res. 2021, 20, 2251–2265.
- Sharma, V.; Shah, M.; Pullela, R.; Cohen, A.J. Platelet Utilization in a Tertiary Care Hospital: An Opportunity for Reduced Transfusion? Blood 2010, 116, 2575.
- Pandey, S.; Belanger, G.A.; Rajbhandary, S.; Cohn, C.S.; Benjamin, R.J.; Bracey, A.W.; Katz, L.M.; Menitove, J.E.; Mintz, P.D.; Gammon, R.R. A survey of US hospitals on platelet inventory management, transfusion practice, and platelet availability. Transfusion 2021, 61, 2611–2620.
- Reddoch-Cardenas, K.; Bynum, J.; Meledeo, M.; Nair, P.; Wu, X.; Darlington, D.; Ramasubramanian, A.; Cap, A. Cold-stored platelets: A product with function optimized for hemorrhage control. Transfus. Apher. Sci. 2018, 58, 16–22.
- Berzuini, A.; Spreafico, M.; Prati, D. One size doesn’t fit all: Should we reconsider the introduction of cold-stored platelets in blood bank inventories? F1000Research 2017, 6, 95.
- Getz, T.M. Physiology of cold-stored platelets. Transfus. Apher. Sci. 2019, 58, 12–15.
- Nair, P.M.; Pandya, S.G.; Dallo, S.F.; Reddoch, K.M.; Montgomery, R.K.; Pidcoke, H.F.; Cap, A.P.; Ramasubramanian, A.K. Platelets stored at 4 °C contribute to superior clot properties compared to current standard-of-care through fibrin-crosslinking. Br. J. Haematol. 2017, 178, 119–129.
- Nair, P.M.; Meledeo, M.A.; Wells, A.R.; Wu, X.; Bynum, J.A.; Leung, K.P.; Liu, B.; Cheeniyil, A.; Ramasubramanian, A.K.; Weisel, J.W.; et al. Cold-stored platelets have better preserved contractile function in comparison with room temperature-stored platelets over 21 days. Transfusion 2021, 61, S68–S79.
- Melchinger, H.; Jain, K.; Tyagi, T.; Hwa, J. Role of Platelet Mitochondria: Life in a Nucleus-Free Zone. Front. Cardiovasc. Med. 2019, 6, 153.
- Koessler, J.; Klingler, P.; Niklaus, M.; Weber, K.; Koessler, A.; Boeck, M.; Kobsar, A. The Impact of Cold Storage on Adenosine Diphosphate-Mediated Platelet Responsiveness. TH Open 2020, 4, e163–e172.
- Miles, J.; Bailey, S.L.; Obenaus, A.M.; Mollica, M.Y.; Usaneerungrueng, C.; Byrne, D.A.; Fang, L.; Flynn, J.R.; Corson, J.; Osborne, B.; et al. Storage temperature determines platelet GPVI levels and function in mice and humans. Blood Adv. 2021, 5, 3839–3849.
- Scorer, T.G.; FitzGibbon, L.; Aungraheeta, R.; Sharma, U.; Peltier, G.C.; McIntosh, C.S.; Reddoch-Cardenas, K.M.; Meyer, A.; Cap, A.P.; Mumford, A.D. TEG PlateletMapping assay results may be misleading in the presence of cold stored platelets. Transfusion 2020, 60, S119–S123.
- Gutmann, C.; Joshi, A.; Mayr, M. Platelet “-omics” in health and cardiovascular disease. Atherosclerosis 2020, 307, 87–96.
- D’Alessandro, A.; Thomas, K.A.; Stefanoni, D.; Gamboni, F.; Shea, S.M.; Reisz, J.A.; Spinella, P.C. Metabolic phenotypes of standard and cold-stored platelets. Transfusion 2019, 60, S96–S106.
- Wang, S.; Jiang, T.; Fan, Y.; Zhao, S. A proteomic approach reveals the variation in human platelet protein composition after storage at different temperatures. Platelets 2018, 30, 403–412.
- Nagy, M.; Mastenbroek, T.G.; Mattheij, N.J.A.; De Witt, S.; Clemetson, K.J.; Kirschner, J.; Schulz, A.S.; Vraetz, T.; Speckmann, C.; Braun, A.; et al. Variable impairment of platelet functions in patients with severe, genetically linked immune deficiencies. Haematologica 2017, 103, 540–549.
- Hickey, M.J.; Hagen, F.S.; Yagi, M.; Roth, G.J. Human platelet glycoprotein V: Characterization of the polypeptide and the related Ib-V-IX receptor system of adhesive, leucine-rich glycoproteins. Proc. Natl. Acad. Sci. USA 1993, 90, 8327–8331.
- Mukai, N.; Nakayama, Y.; Ishi, S.; Murakami, T.; Ogawa, S.; Kageyama, K.; Murakami, S.; Sasada, Y.; Yoshioka, J.; Nakajima, Y. Cold storage conditions modify microRNA expressions for platelet transfusion. PLoS ONE 2019, 14, e0218797.
- Hegde, S.; Wellendorf, A.M.; Zheng, Y.; Cancelas, J.A. Antioxidant prevents clearance of hemostatically competent platelets after long-term cold storage. Transfusion 2020, 61, 557–567.
- Handigund, M.; Bae, T.W.; Lee, J.; Cho, Y.G. Evaluation of in vitro storage characteristics of cold stored platelet concentrates with N acetylcysteine (NAC). Transfus. Apher. Sci. 2016, 54, 127–138.
- Handigund, M.; Kim, J.T.; Bae, T.W.; Lee, J.; Cho, Y.G. N-acetylcysteine reduce the stress induced by cold storage of platelets: A potential way to extend shelf life of platelets. Transfus. Apher. Sci. 2020, 60, 103039.
- Ekaney, M.L.; Grable, M.A.; Powers, W.F.; McKillop, I.H.; Evans, S.L. Cytochrome c and resveratrol preserve platelet function during cold storage. J. Trauma Acute Care Surg. 2017, 83, 271–277.
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2015, 16, 51–67.
- Fredman, G.; Van Dyke, T.E.; Serhan, C.N. Resolvin E1 Regulates Adenosine Diphosphate Activation of Human Platelets. Arter. Thromb. Vasc. Biol. 2010, 30, 2005–2013.
- Reddoch-Cardenas, K.M.; Sharma, U.; Salgado, C.L.; Cantu, C.; Darlington, D.N.; Pidcoke, H.F.; Bynum, J.A.; Cap, A.P. Use of Specialized Pro-Resolving Mediators to Alleviate Cold Platelet Storage Lesion. Transfusion 2020, 60, S112–S118.
- Baghdadi, V.; Yari, F.; Nikougoftar, M.; Rafiee, M.H. Platelets Apoptosis and Clearance in The Presence of Sodium Octanoate during Storage of Platelet Concentrate at 4 °C. Cell J. 2020, 22, 212–217.
- Xiang, B.; Zhang, G.; Zhang, Y.; Wu, C.; Joshi, S.; Morris, A.J.; Ware, J.; Smyth, S.S.; Whiteheart, S.W.; Li, Z. Calcium Ion Chelation Preserves Platelet Function During Cold Storage. Arter. Thromb. Vasc. Biol. 2020, 41, 234–249.
- Lanteri, M.C.; Kleinman, S.H.; Glynn, S.A.; Musso, D.; Hoots, W.K.; Custer, B.S.; Sabino, E.C.; Busch, M.P. Zika virus: A new threat to the safety of the blood supply with worldwide impact and implications. Transfusion 2016, 56, 1907–1914.
- Harrington, T.; Kuehnert, M.J.; Kamel, H.; Lanciotti, R.S.; Hand, S.; Currier, M.; Chamberland, M.E.; Petersen, L.R.; Marfin, A.A. West Nile virus infection transmitted by blood transfusion. Transfusion 2003, 43, 1018–1022.
- Seltsam, A. Pathogen Inactivation of Cellular Blood Products—An Additional Safety Layer in Transfusion Medicine. Front. Med. 2017, 4, 219.
- Listing of Countries in Which Pathogen Inactivation Technology Systems and Products Are in Use. 2015 March. Available online: https://www.aabb.org/docs/default-source/default-document-library/regulatory/eid/prt-systems-in-use-country-listing.pdf (accessed on 24 December 2021).
- Ribault, S.; Faucon, A.; Grave, L.; Nannini, P.; Faure, I.B. Detection of Bacteria in Red Blood Cell Concentrates by the Scansystem Method. J. Clin. Microbiol. 2005, 43, 2251–2255.
- Guinet, F.; Carniel, E.; Leclercq, A. Transfusion-Transmitted Yersinia enterocolitica Sepsis. Clin. Infect. Dis. 2011, 53, 583–591.
- Six, K.R.; Devloo, R.; Compernolle, V.; Feys, H.B. Impact of cold storage on platelets treated with Intercept pathogen inactivation. Transfusion 2019, 59, 2662–2671.
- Agey, A.; Reddoch-Cardenas, K.; McIntosh, C.; Sharma, U.; Cantu, C.; Cap, A.; Bynum, J. Effects of Intercept pathogen reduction treatment on extended cold storage of apheresis platelets. Transfusion 2020, 61, 167–177.
- Johnson, L.; Cameron, M.; Waters, L.; Padula, M.P.; Marks, D.C. The impact of refrigerated storage of UVC pathogen inactivated platelet concentrates on in vitro platelet quality parameters. Vox Sang. 2018, 114, 47–56.
- Bayry, J.; Kazatchkine, M.D.; Kaveri, S.V. Shortage of human intravenous immunoglobulin—reasons and possible solutions. Nat. Clin. Pr. Neurol. 2007, 3, 120–121.
- N’Kaoua, E.; Attarian, S.; Delmont, E.; Campana-Salort, E.; Verschueren, A.; Grapperon, A.-M.; Mestivier, E.; Roche, M. Immunoglobulin shortage: Practice modifications and clinical outcomes in a reference centre. Rev. Neurol. 2021, S0035-3787(21)00768-2.
- Mathur, A.; Swamy, N.; Thapa, S.; Chakraborthy, S.; Jagannathan, L. Adding to platelet safety and life: Platelet additive solutions. Asian J. Transfus. Sci. 2018, 12, 136–140.
- Van der Meer, P.F.; de Korte, D. Platelet Additive Solutions: A Review of the Latest Developments and Their Clinical Implications. Transfus. Med. Hemotherapy 2018, 45, 98–102.
- Marini, I.; Aurich, K.; Jouni, R.; Nowak-Harnau, S.; Hartwich, O.; Greinacher, A.; Thiele, T.; Bakchoul, T. Cold storage of platelets in additive solution: The impact of residual plasma in apheresis platelet concentrates. Haematologica 2019, 104, 207–214.
- Reddoch-Cardenas, K.M.; Montgomery, R.K.; Lafleur, C.B.; Peltier, G.C.; Bynum, J.A.; Cap, A.P. Cold storage of platelets in platelet additive solution: An in vitro comparison of two Food and Drug Administration–approved collection and storage systems. Transfusion 2018, 58, 1682–1688.
- Reddoch-Cardenas, K.M.; Sharma, U.; Salgado, C.L.; Montgomery, R.K.; Cantu, C.; Cingoz, N.; Bryant, R.; Darlington, D.N.; Pidcoke, H.F.; Kamucheka, R.M.; et al. An in vitro pilot study of apheresis platelets collected on Trima Accel system and stored in T-PAS+ solution at refrigeration temperature (1–6 °C). Transfusion 2019, 59, 1789–1798.
- Johnson, L.; Vekariya, S.; Wood, B.; Tan, S.; Roan, C.; Marks, D.C. Refrigeration of apheresis platelets in platelet additive solution (PAS-E) supports in vitro platelet quality to maximize the shelf-life. Transfusion 2021, 61, S58–S67.
- Reddoch-Cardenas, K.M.; Peltier, G.C.; Chance, T.C.; Nair, P.M.; Meledeo, M.A.; Ramasubramanian, A.K.; Cap, A.P.; Bynum, J.A. Cold storage of platelets in platelet additive solution maintains mitochondrial integrity by limiting initiation of apoptosis-mediated pathways. Transfusion 2020, 61, 178–190.
- Stolla, M.; Fitzpatrick, L.; Gettinger, I.; Bailey, S.L.; Pellham, E.; Christoffel, T.; Slichter, S.J. In vivo viability of extended 4 °C-stored autologous apheresis platelets. Transfusion 2018, 58, 2407–2413.
- Wood, B.; Johnson, L.; Hyland, R.A.; Marks, D.C. Maximising platelet availability by delaying cold storage. Vox Sang. 2018, 113, 403–411.
- Braathen, H.; Sivertsen, J.; Lunde, T.H.F.; Kristoffersen, E.K.; Assmus, J.; Hervig, T.A.; Strandenes, G.; Apelseth, T.O. In vitro quality and platelet function of cold and delayed cold storage of apheresis platelet concentrates in platelet additive solution for 21 days. Transfusion 2019, 59, 2652–2661.




