
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mihnea-Alexandru Gaman | + 1905 word(s) | 1905 | 2022-02-08 05:15:44 | | | |
| 2 | Lindsay Dong | Meta information modification | 1905 | 2022-02-10 04:12:02 | | |
Video Upload Options
Psoriasis is a chronic, immune-mediated inflammatory dermatosis characterized by the appearance of erythematous plaques, covered by white scales, occasionally pruritogenic, and distributed mainly on the extensor areas. Oxidative stress is defined as an imbalance or a transient or chronic increase in the levels of free oxygen/nitrogen radicals, either as a result of the exaggerated elevation in their production or the decrease in their ability to be eliminated by antioxidant systems. Although the pathogenesis of psoriasis remains far from elucidated, there are studies that delineate an involvement of oxidative stress in this skin disorder.
1. Introduction
2. The Involvement of Oxidative Stress in Psoriasis
According to the data in the literature, oxidative stress plays an important role in initiating and perpetuating chronic diseases, e.g., cardiovascular, liver, neurological, metabolic, endocrinological and dermatological disorders [11]. Of the latter, psoriasis is an important representative, affecting a high percentage of the general population. The way in which these parameters reflect the level of oxidative stress in the body is difficult to assess, as so far there are no clear correlations between the oxidative balance and various oxidizing enzymes, antioxidant molecules or oxidation products, as the latter are generated in the body via multiple mechanisms including in physiological conditions [12][13]. The main source of reactive oxygen species remains the mitochondria, namely the mitochondrial inner membrane. Moreover, reactive oxygen species generation also results in the alteration of the main cellular components: lipids, carbohydrates and proteins [12][13]. Thus, at the lipid level, an increased number of reactive oxygen species is responsible for the enzymatic and non-enzymatic peroxidation of polyunsaturated fatty acids and LDL (with the formation of oxidized LDL), affecting the cell membrane and eventually leading to apoptosis [14]. Regarding the action of reactive oxygen species on the protein and carbohydrate components, their presence is associated with the formation of carbonyl-type compounds, advanced glycation end-products and advanced oxidation protein products, with an additional role of stimulating reactive oxygen species-generating processes at the cellular level. In addition to mitochondrial free radicals, a large number of reactive species also result from the action of pro-oxidant enzymes, such as xanthine oxidase, nitric oxide synthase, myeloperoxidase and NADPH oxidase. The body’s defense mechanisms against the increase in ROS levels above the physiological levels consist of enzymatic antioxidant systems (superoxide dismutase, catalase, glutathione peroxidase, etc.) which act as scavengers that capture the already formed free radicals, but also non-enzymatic antioxidant systems (glutathione, antioxidant vitamins) that have the role of interrupting reactive species-generating reactions [14]. Thus, glutathione, one of the main antioxidant systems, has the role of decreasing the reactive oxygen species concentrations via donation of a hydrogen ion with a neutralizing role. Thus, oxidized glutathione is generated [15]. Moreover, the increase in reactive oxygen species also causes changes at the nuclear level, by activating the transcription factors Nrf2 (nuclear factor erythroid related factor 2) and NF-kB (nuclear factor kappa light chain enhancer of activated B cells) which further contribute to the anti-oxidative stress defense mechanisms by inducing the synthesis of antioxidant molecules [14].
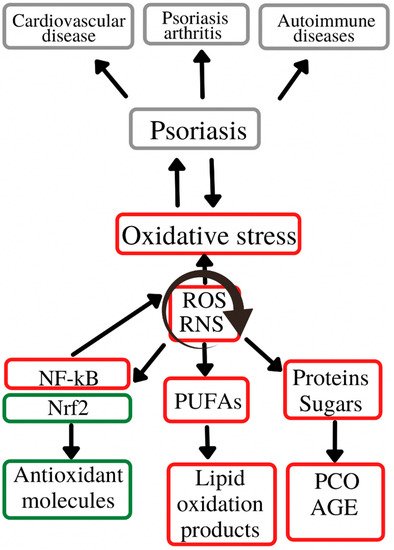
3. Conclusions
References
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475.
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M.; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J. Investig. Dermatol. 2013, 133, 377–385.
- Sarkar, R.; Chugh, S.; Bansal, S. General measures and quality of life issues in psoriasis. Indian Dermatol. Online J. 2016, 7, 481–488.
- Oliveira, M.D.F.S.P.D.; Rocha, B.D.O.; Duarte, G.V. Psoriasis: Classical and emerging comorbidities. An. Bras. Dermatol. 2015, 90, 9–20.
- Kamiya, K.; Kishimoto, M.; Sugai, J.; Komine, M.; Ohtsuki, M. Risk Factors for the Development of Psoriasis. Int. J. Mol. Sci. 2019, 20, 4347.
- Pleńkowska, J.; Gabig-Cimińska, M.; Mozolewski, P. Oxidative Stress as an Important Contributor to the Pathogenesis of Pso-riasis. Int. J. Mol. Sci. 2020, 21, 6206.
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763.
- Poljšak, B.; Dahmane, R. Free Radicals and Extrinsic Skin Aging. Dermatol. Res. Pract. 2012, 2012, 1–4.
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free Radical-Induced Damage to DNA: Mechanisms and Measurement. Free Radic. Biol. Med. 2002, 32, 1102–1115.
- Bickers, D.R.; Athar, M. Oxidative Stress in the Pathogenesis of Skin Disease. J. Investig. Dermatol. 2006, 126, 2565–2575.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; DELLA-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772.
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical Study of Oxidative Stress Markers in the Liver, Kidney and Heart of High Fat Diet Induced Obesity in Rats. Diabetol. Metab. Syndr. 2011, 3, 17–18.
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6175804.
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 1–32.
- Armstrong, J.S.; Steinauer, K.K.; Hornung, B.; Irish, J.; Lecane, P.; Birrell, G.; Peehl, D.M.; Knox, S.J. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002, 9, 252–263.
- Sürücü, H.A.; Aksoy, N.; Ozgöztas, O.; Sezen, H.; Yesilova, Y.; Turan, E. Prolidase Activity in Chronic Plaque Psoriasis Pa-tients. Postepy Dermatol. Alergol. 2015, 32, 82–87.
- Chandrashekar, L.; Kumari, G.R.K.; Rajappa, M.; Revathy, G.; Munisamy, M.; Thappa, D. 25-hydroxy vitamin D and ischaemia-modified albumin levels in psoriasis and their association with disease severity. Br. J. Biomed. Sci. 2015, 72, 56–60.
- Emre, S.; Metin, A.; Demirseren, D.D.; Kilic, S.; Isikoglu, S.; Erel, O. The Relationship between Oxidative Stress, Smoking and the Clinical Severity of Psoriasis: Oxidative Stress and Smoking in Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e370–e375.
- Gabr, S.A.; Al-Ghadir, A.H. Role of cellular oxidative stress and cytochrome c in the pathogenesis of psoriasis. Arch. Dermatol. Res. 2012, 304, 451–457.
- Ferretti, G.; Bacchetti, T.; Campanati, A.; Simonetti, O.; Liberati, G.; Offidani, A. Correlation between Lipoprotein(a) and Lipid Peroxidation in Psoriasis: Role of the Enzyme Paraoxonase-1: Lp(a) and Paraoxonase-1 in Psoriasis. Br. J. Dermatol. 2012, 166, 204–207.
- Kadam, D.P.; Suryakar, A.N.; Ankush, R.D.; Kadam, C.Y.; Deshpande, K.H. Role of Oxidative Stress in Various Stages of Psoriasis. Indian J. Clin. Biochem. 2010, 25, 388–392.
- Kural, B.V.; Örem, A.; Çimşit, G.; Yandı, Y.E.; Calapoǧlu, M. Evaluation of the atherogenic tendency of lipids and lipoprotein content and their relationships with oxidant–antioxidant system in patients with psoriasis. Clin. Chim. Acta 2003, 328, 71–82.
- Baz, K.; Cimen, M.Y.B.; Kokturk, A.; Yazici, A.C.; Eskandari, G.; Ikizoglu, G.; Api, H.; Atik, U. Oxidant/Antioxidant Status in Patients with Psoriasis. Yonsei Med. J. 2003, 44, 987–990.
- Wójcik, P.; Gęgotek, A.; Wroński, A.; Jastrząb, A.; Żebrowska, A.; Skrzydlewska, E. Effect of redox imbalance on protein modifications in lymphocytes of psoriatic patients. J. Biochem. 2019, 167, 323–331.
- Esmaeili, B.; Mansouri, P.; Doustimotlagh, A.H.; Izad, M. Redox imbalance and IL-17 responses in memory CD4+ T cells from patients with psoriasis. Scand. J. Immunol. 2018, 89, e12730.
- Nemati, H.; Khodarahmi, R.; Sadeghi, M.; Ebrahimi, A.; Rezaei, M.; Vaisi-Raygani, A. Antioxidant Status in Patients with Psoriasis: Antioxidant in Patients with Psoriasis. Cell Biochem. Funct. 2014, 32, 268–273.
- Pujari, V.M.; Ireddy, S.; Itagi, I.; Kumar, H.S. The Serum Levels of Malondialdehyde, Vitamin e and Erythrocyte Catalase Activity in Psoriasis Patients. J. Clin. Diagn. Res. 2014, 8, CC14-6.
- Karaman, A.; Aliagaoglu, C.; Pirim, I. Sister chromatid exchange analysis in patients with psoriasis. Exp. Dermatol. 2008, 17, 524–529.
- Yildirim, M.; Inaloz, H.S.; Baysal, V.; Delibas, N. The role of oxidants and antioxidants in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 34–36.
- Oszukowska, M.; Kozłowska, M.; Kaszuba, A. Paraoxonase-1 and other factors related to oxidative stress in psoriasis. Adv. Dermatol. Allergol. 2020, 37, 92–96.
- Husni, M.E.; Tang, W.H.W.; Lucke, M.; Chandrasekharan, U.M.; Ms, D.M.B.; Hazen, S.L. Correlation of High-Density Lipoprotein-Associated Paraoxonase 1 Activity with Systemic Inflammation, Disease Activity, and Cardiovascular Risk Factors in Psoriatic Disease. Arthritis Rheumatol. 2018, 70, 1240–1250.
- He, L.; Qin, S.; Dang, L.; Song, G.; Yao, S.; Yang, N.; Li, Y. Psoriasis decreases the anti-oxidation and anti-inflammation properties of high-density lipoprotein. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 1709–1715.
- Usta, M.; Turan, E.; Aral, H.; Inal, B.B.; Gurel, M.S.; Guvenen, G. Serum paraoxonase-1 activities and oxidative status in patients with plaque-type psoriasis with/without metabolic syndrome. J. Clin. Lab. Anal. 2011, 25, 289–295.
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological Alter-ations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells 2018, 7, 159.
- Kirmit, A.; Kader, S.; Aksoy, M.; Bal, C.; Nural, C.; Aslan, O. Trace elements and oxidative stress status in patients with psoriasis. Adv. Dermatol. Allergol. 2020, 37, 333–339.
- Skutnik-Radziszewska, A.; Maciejczyk, M.; Fejfer, K.; Krahel, J.; Flisiak, I.; Kołodziej, U.; Zalewska, A. Salivary Antioxidants and Oxidative Stress in Psoriatic Patients: Can Salivary Total Oxidant Status and Oxidative Status Index Be a Plaque Psoriasis Biomarker? Oxid. Med. Cell. Longev. 2020, 2020, 9086024.
- Kızılyel, O.; Akdeniz, N.; Metin, M.S.; Elmas, Ö.F. Investigation of Oxidant and Antioxidant Levels in Patients with Psoriasis. Turk. J. Med. Sci. 2019, 49, 1085–1088.
- Ergun, T.; Yazici, V.; Yavuz, D.; Seckin-Gencosmanoglu, D.; Ozen, G.; Salman, A.; Direskeneli, H.; Inanc, N. Advanced Gly-cation End Products, a Potential Link between Psoriasis and Cardiovascular Disease: A Case-Control Study. Indian J. Dermatol. 2019, 64, 201–206.
- Haberka, M.; Bańska-Kisiel, K.; Bergler-Czop, B.; Biedroń, M.; Brzezińska-Wcisło, L.; Okopień, B.; Gąsior, Z. Mild to Moderate Psoriasis Is Associated with Oxidative Stress, Subclinical Atherosclerosis, and Endothelial Dysfunction. Pol. Arch. Intern. Med. 2018, 128, 434–439.
- Papagrigoraki, A.; Giglio, M.; Cosma, C.; Maurelli, M.; Girolomoni, G.; Lapolla, A. Advanced Glycation End Products are Increased in the Skin and Blood of Patients with Severe Psoriasis. Acta Derm. Venereol. 2017, 97, 782–787.
- Dilek, N.; Dilek, A.R.; Taşkın, Y.; Erkinüresin, T.; Yalçın, Ö.; Saral, Y. Contribution of myeloperoxidase and inducible nitric oxide synthase to pathogenesis of psoriasis. Adv. Dermatol. Allergol. 2016, 33, 435–439.
- Damasiewicz-Bodzek, A.; Wielkoszyński, T. Advanced Protein Glycation in Psoriasis: Protein Glycation in Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 172–179.
- Ozdemir, M.; Kiyici, A.; Balevi, A.; Mevlitoğlu, I.; Peru, C. Assessment of Ischaemia-Modified Albumin Level in Patients with Psoriasis: Psoriasis and Ischaemia-Modified Albumin. Clin. Exp. Dermatol. 2012, 37, 610–614.
- Singal, A.; Asha, K.; Sharma, S.B.; Arora, V.K.; Aggarwal, A. Dyslipidaemia & oxidative stress in patients of psoriasis: Emerging cardiovascular risk factors. Indian J. Med. Res. 2017, 146, 708–713.
- Sunitha, S.; Rajappa, M.; Thappa, D.M.; Chandrashekar, L.; Munisamy, M.; Revathy, G. Is the Ratio of Antibodies Against Oxidized LDL to Oxidized LDL an Indicator of Cardiovascular Risk in Psoriasis? Oman Med. J. 2016, 31, 390–393.
- Ikonomidis, I.; Makavos, G.; Papadavid, E.; Varoudi, M.; Andreadou, I.; Gravanis, K.; Theodoropoulos, K.; Pavlidis, G.; Tri-antafyllidi, H.; Parissis, J.; et al. Similarities in Coronary Function and Myocardial De-formation between Psoriasis and Coronary Artery Disease: The Role of Oxidative Stress and Inflammation. Can. J. Cardiol. 2015, 31, 287–295.
- Hernández-Collazo, A.A.; Pérez-Méndez, O.; López-Olmos, V.; Delgado-Rizo, V.; Muñoz-Valle, J.F.; Martínez-López, E.; Villanueva-Quintero, D.G.; Domínguez-Díaz, C.; Fafutis-Morris, M.; Alvarado-Navarro, A. Association between rs662 (A > G) and rs854560 (A > T) polymorphisms in PON1 gene and the susceptibility for psoriasis in mestizo population of Western Mexico. Mol. Biol. Rep. 2021, 48, 183–194.
- Guarneri, F.; Sapienza, D.; Papaianni, V.; Marafioti, I.; Guarneri, C.; Mondello, C.; Roccuzzo, S.; Asmundo, A.; Cannavò, S.P. Association between Genetic Polymorphisms of Glutathione S-Transferase M1/T1 and Psoriasis in a Population from the Area of the Strict of Messina (Southern Italy). Free Radic. Res. 2020, 54, 57–63.
- Solak, B.; Karkucak, M.; Turan, H.; Ocakoğlu, G.; Şebnem Özemri, S.; Uslu, E.; Yakut, T.; Erdem, T. Glutathione S-Transferase M1 and T1 Gene Polymorphisms in Patients with Chronic Plaque-Type Psoriasis: A Case-Control Study. Med. Princ. Pract. 2015, 25, 155–158.
- Chang, Y.-C.; Wu, W.-M.; Huang, Y.-H.; Hung, S.-I.; Tsai, H.-Y.; Hsu, L.-A. The (CCTTT) n pentanucleotide repeat polymorphism in the inducible nitric oxide synthase gene promoter and the risk of psoriasis in Taiwanese. Arch. Dermatol. Res. 2015, 307, 425–432.
- Asefi, M.; Vaisi-Raygani, A.; Khodarahmi, R.; Nemati, H.; Rahimi, Z.; Tavilani, H.; Pourmotabbed, T. Methylentetrahydrofolatereductase (rs1801133) polymorphism and psoriasis: Contribution to oxidative stress, lipid peroxidation and correlation with vascular adhesion protein 1, preliminary report. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1192–1198.
- Asefi, M.; Vaisi-Raygani, A.; Bahrehmand, F.; Kiani, A.; Rahimi, Z.; Nomani, H.; Ebrahimi, A.; Tavilani, H.; Pourmotabbed, T. Paraoxonase 1 (PON1) 55 Polymorphism, Lipid Profiles and Psoriasis: Paraoxonase 55 Polymorphism and Psoriasis. Br. J. Dermatol. 2012, 167, 1279–1286.
- Schnorr, O.; Schuier, M.; Kagemann, G.; Wolf, R.; Walz, M.; Ruzicka, T.; Mayatepek, E.; Laryea, M.; Suschek, C.V.; Kolb-Bachofen, V.; et al. Arginase-1 overexpression induces cationic amino acid transporter-1 in psoriasis. Free Radic. Biol. Med. 2005, 38, 1073–1079.
- Vasku, V.; Kankova, K.; Vasku, A.; Muzik, J.; Hollá, L.I.; Semrádová, V.; Vácha, J. Gene polymorphisms (G82S, 1704G/T, 2184A/G and 2245G/A) of the receptor of advanced glycation end products (RAGE) in plaque psoriasis. Arch. Dermatol. Res. 2002, 294, 127–130.
- Kılıc, S.; Emre, S.; Metin, A.; Isıkoglu, S.; Erel, O. Effect of the systemic use of methotrexate on the oxidative stress and paraoxonase enzyme in psoriasis patients. Arch. Dermatol. Res. 2013, 305, 495–500.
- Wacewicz, M.; Socha, K.; Soroczyńska, J.; Niczyporuk, M.; Aleksiejczuk, P.; Ostrowska, J.; Borawska, M.H. Concentration of Selenium, Zinc, Copper, Cu/Zn Ratio, Total Antioxidant Status and c-Reactive Protein in the Serum of Patients with Psoriasis Treated by Narrow-Band Ultraviolet B Phototherapy: A Case-Control Study. J. Trace Elem. Med. Biol. 2017, 44, 109–114.
- Karadag, A.S.; Uzunçakmak, T.K.; Ozkanli, S.; Oguztuzun, S.; Moran, B.; Akbulak, O.; Ozlu, E.; Zemheri, I.E.; Bilgili, S.G.; Akdeniz, N. An investigation of cytochrome p450 (CYP) and glutathioneS-transferase (GST) isoenzyme protein expression and related interactions with phototherapy in patients with psoriasis vulgaris. Int. J. Dermatol. 2016, 56, 225–231.
- Pektas, S.; Akoglu, G.; Metin, A.; Neselioglu, S.; Erel, O. Evaluation of systemic oxidant/antioxidant status and paraoxonase 1 enzyme activities in psoriatic patients treated by narrow band ultraviolet B phototherapy. Redox Rep. 2013, 18, 200–204.
- Pastore, S.; Mariani, V.; Lulli, D.; Gubinelli, E.; Raskovic, D.; Mariani, S.; Stancato, A.; De Luca, C.; Pecorelli, A.; Valacchi, G.; et al. Glutathione peroxidase activity in the blood cells of psoriatic patients correlates with their responsiveness to Efalizumab. Free Radic. Res. 2011, 45, 585–599.
- Elango, T.; Dayalan, H.; Gnanaraj, P.; Malligarjunan, H.; Subramanian, S. Impact of methotrexate on oxidative stress and apoptosis markers in psoriatic patients. Clin. Exp. Med. 2014, 14, 431–437.
- Barygina, V.; Becatti, M.; Soldi, G.; Prignano, F.; Lotti, T.; Nassi, P.; Wright, D.; Taddei, N.; Fiorillo, C. Altered redox status in the blood of psoriatic patients: Involvement of NADPH oxidase and role of anti-TNF-α therapy. Redox Rep. 2013, 18, 100–106.




