Psoriasis is a chronic, immune-mediated inflammatory dermatosis characterized by the appearance of erythematous plaques, covered by white scales, occasionally pruritogenic, and distributed mainly on the extensor areas. Oxidative stress is defined as an imbalance or a transient or chronic increase in the levels of free oxygen/nitrogen radicals, either as a result of the exaggerated elevation in their production or the decrease in their ability to be eliminated by antioxidant systems. Although the pathogenesis of psoriasis remains far from elucidated, there are studies that delineate an involvement of oxidative stress in this skin disorder.
1. Introduction
Psoriasis is a chronic, immune-mediated inflammatory dermatosis characterized by the appearance of erythematous plaques, covered by white scales, occasionally pruritogenic, and distributed mainly on the extensor areas (elbows, knees, scalp, chest)
[1]. It is one of the most common chronic dermatological diseases, with a worldwide prevalence and incidence that varies significantly depending on geographical area, age and sex. For example, its prevalence varies from 0–2.1% in the pediatric population to 0.91–8.5% in adults, while its incidence is 40.8 cases in 100,000 people in the former and 78.9–230 cases in 100,000 in the latter
[2]. Psoriasis is a debilitating condition, which significantly affects the quality of life and impacts on the life expectancy of individuals who suffer from it. Subjects diagnosed with this dermatological disorder experience feelings of depression, social stigma, and frequently associate other chronic systemic diseases, e.g., cardiovascular disease, obesity, diabetes, psoriasis no longer being considered a condition which strictly involves the integument
[3][4][3,4]. The pathophysiology of the disease is still incompletely elucidated and multiple factors are said to be involved in its initiation and perpetuation, e.g., stressors, genetic factors, environmental factors (air pollutants, cigarette smoke), etc.
[5]. In the last decade, a new theory is trying to explain the pathophysiology of this condition by looking at the role played by oxidative stress and chronic inflammation in the initiation of keratinocyte proliferation and differentiation that underline psoriasis
[6]. Oxidative stress is defined as an imbalance or a transient or chronic increase in the levels of free oxygen/nitrogen radicals, either as a result of the exaggerated elevation in their production or the decrease in their ability to be eliminated by antioxidant systems
[7]. Due to its role as a barrier and its direct exposure to environmental factors, the skin is an important source of free radicals that play, when in low concentrations, an essential role in the defense against microorganisms and in cell differentiation
[8]. When their concentration increases, leading to oxidative stress, free radicals appear to be involved in DNA alteration, cell protein degradation, lipid oxidation, apoptosis, tissue injury, altered response of T-helper cells and secretion of interleukin-17 (IL-17)
[9]. As all these are essential stages in the initiation and perpetuation of psoriasis, the hypothesis that oxidative stress plays a key role in the pathophysiology of this chronic dermatosis has emerged
[10].
2. The Involvement of Oxidative Stress in Psoriasis
According to the data in the literature, oxidative stress plays an important role in initiating and perpetuating chronic diseases, e.g., cardiovascular, liver, neurological, metabolic, endocrinological and dermatological disorders [11][90]. Of the latter, psoriasis is an important representative, affecting a high percentage of the general population. The way in which these parameters reflect the level of oxidative stress in the body is difficult to assess, as so far there are no clear correlations between the oxidative balance and various oxidizing enzymes, antioxidant molecules or oxidation products, as the latter are generated in the body via multiple mechanisms including in physiological conditions [12][13][91,92]. The main source of reactive oxygen species remains the mitochondria, namely the mitochondrial inner membrane. Moreover, reactive oxygen species generation also results in the alteration of the main cellular components: lipids, carbohydrates and proteins [12][13][91,92]. Thus, at the lipid level, an increased number of reactive oxygen species is responsible for the enzymatic and non-enzymatic peroxidation of polyunsaturated fatty acids and LDL (with the formation of oxidized LDL), affecting the cell membrane and eventually leading to apoptosis [14][93]. Regarding the action of reactive oxygen species on the protein and carbohydrate components, their presence is associated with the formation of carbonyl-type compounds, advanced glycation end-products and advanced oxidation protein products, with an additional role of stimulating reactive oxygen species-generating processes at the cellular level. In addition to mitochondrial free radicals, a large number of reactive species also result from the action of pro-oxidant enzymes, such as xanthine oxidase, nitric oxide synthase, myeloperoxidase and NADPH oxidase. The body’s defense mechanisms against the increase in ROS levels above the physiological levels consist of enzymatic antioxidant systems (superoxide dismutase, catalase, glutathione peroxidase, etc.) which act as scavengers that capture the already formed free radicals, but also non-enzymatic antioxidant systems (glutathione, antioxidant vitamins) that have the role of interrupting reactive species-generating reactions [14][93]. Thus, glutathione, one of the main antioxidant systems, has the role of decreasing the reactive oxygen species concentrations via donation of a hydrogen ion with a neutralizing role. Thus, oxidized glutathione is generated [15][94]. Moreover, the increase in reactive oxygen species also causes changes at the nuclear level, by activating the transcription factors Nrf2 (nuclear factor erythroid related factor 2) and NF-kB (nuclear factor kappa light chain enhancer of activated B cells) which further contribute to the anti-oxidative stress defense mechanisms by inducing the synthesis of antioxidant molecules [14][93].
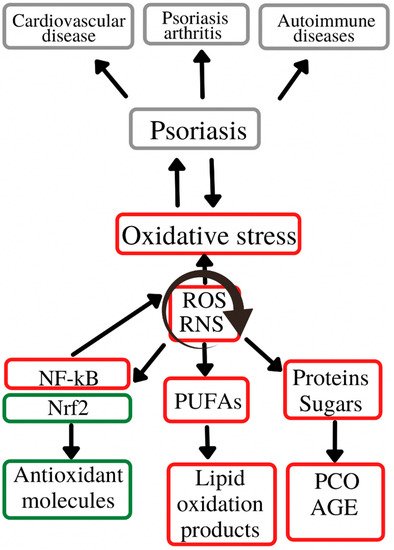
Figure 12 depicts role of oxidative stress in the initiation and evolution of psoriasis and its associated comorbidities.
Figure 12. Oxidative stress in the psoriasis evolution. Oxidative stress plays an important role in the initiation and evolution of psoriasis and its associated-comorbidities/complications (cardiovascular diseases, psoriasis arthritis and autoimmune diseases. These complications are generated by the chronic action of ROS and RNS on the main cell constituents (lipids, proteins and carbohydrates), with a secondary activation of molecular pathways (Nrf2, NF-kB) responsible for inducing synthesis of antioxidant molecules (Nrf2) or pro-oxidant enzymes (NF-kB). ROS—reactive oxygen species; RNS—reactive nitrogen species; Nrf2—nuclear erythroid factor 2-related factor 2; NF-kB—nuclear factor kappa light chain enhancer of activated B cells; PUFAs—polyunsaturated fatty acids; AGE—advanced glycation end-products; PCO—protein carbonyl content.
Thus, following the analysis of the studies, most papers delineated a significant alteration of the redox balance, with a significant decrease in antioxidant enzymes and antioxidant markers and an increase in pro-oxidant molecules in psoriasis. A conundrum of research endeavors which collected venous blood samples from individuals suffering from psoriasis with variable duration of the disease detected low levels of total antioxidant status
[16][17][18][19][20][21][22][23][37,38,46,47,51,54,63,64] and alterations in enzymes involved in decreasing free oxygen radicals concentrations, i.e., catalase
[24][25][26][27][19][21][28][21,22,39,40,47,54,61], superoxide dismutase
[26][19][21][28][22][29][39,47,54,61,63,65], paraoxonase-1
[30][31][26][32][33][20][15,23,39,43,50,51] and glutathione peroxidase
[34][22][25,63]. Several studies with contradictory results were also present, highlighting that in psoriasis a significant elevation in the levels of the aforementioned antioxidant molecules can also occur
[35][34][28][29][14,25,61,65]. We may hypothesize that, in psoriasis, there is a compensatory increase in antioxidant systems in order to counterbalance the elevated levels of oxidative stress. The plasma concentrations of pro-oxidant molecules was notably increased in all studies, reinforcing the theory that oxidative stress is a key player in the pathogenesis of this disease. The main indices assessed were total oxidative status, reactive oxygen species, myeloperoxidase, ischemia modified albumin, advanced glycation end-products and advanced oxidation protein products whose values were higher in subjects with psoriasis versus their healthy counterparts
[35][36][37][38][24][25][39][40][41][16][42][18][43][14,16,19,20,21,22,24,29,33,37,45,46,48]. Moreover, some of these markers were correlated with the duration and severity of the disease, as well as indices of atherosclerosis. Consequently, these data support the contribution of oxidative stress to the development and evolution of the aforementioned illnesses, as well as to the crosstalk of psoriasis and several cardiometabolic comorbidities, i.e., atherosclerosis, hypertension or obesity, with which it is commonly associated
[38][31][44][45][46][20,23,27,32,36]. In addition, several gene polymorphisms in genes encoding molecules with a role in the redox balance, such as glutathione S-transferase M1/glutathione S-transferase T1, paraoxonase-1, methylentetrahydrofolatereductase, glutathione S-transferase and catalase were upregulated or more frequently expressed in psoriasis
[47][48][49][50][51][52][53][54][67,68,69,70,71,72,73,74]. For example, an enzyme with an important role in the psoriasis-associated comorbidities is paraoxonase-1. Paraoxonase-1 plays an antioxidant role, both by protecting LDL against peroxidation and by its direct role in eliminating oxidized forms of fatty acids, thus having both an antioxidant and an antiatherogenic action. Psoriasis is associated with the presence of paraoxonase-1gene polymorphisms that cause a low paraoxonase-1 activity, as well as an increase in oxidative stress
[48][68]. Moreover, certain polymorphisms (paraoxonase-1 55 M > L) appear to be factors that directly impact on one’s risk of developing psoriasis
[52][72].
On one hand, the use of anti-psoriasis therapy displayed promising results in reducing oxidative stress levels. On the other hand, the interpretation of the findings of studies exploring this research topic must be conducted with caution. Only a limited number of papers was published on this subject, involving a small number of cases but a wide range of management options, varying from systemic therapy, i.e., the immunosuppressive agent methotrexate and monoclonal antibodies, to local approaches, e.g., phototherapy. Thus, although biological agents and phototherapy seem to be linked with a decrease in reactive oxygen species and malondialdehyde and an increase in total antioxidant status, these results were not confirmed by all investigated papers. Of these, some highlighted a reduction in total antioxidant status and a paralleled elevation in total oxidative status despite disease amelioration
[55][56][57][58][59][77,81,82,83,88]. Moreover, methotrexate has been proven to display pro-oxidant effects and it is known that this pharmacological agent enhances oxidative stress levels, mainly via post-administration generation of reactive oxygen species and malondialdehyde
[60][76]. However, methotrexate has a dual effect on oxidative stress levels. On one hand, it enhances the generation of reactive oxygen species and, on the other hand, it inhibits the synthesis of nitric oxide. The administration of methotrexate is, thus, associated with a decrease in nitric oxide concentrations
[60][76]. Thus, the antiproliferative and immunosuppressive effects of this pharmacological agent in psoriasis are mainly determined by the aforementioned increase in reactive oxygen species that induces the apoptosis of keratinocytes
[60][55][76,77]. This pro-oxidant effect is also responsible for some of the side effects of this therapy which seem to be alleviated by the administration of antioxidant compounds
[55][77]. Another molecule with antioxidant and protective roles is paraoxonase-1 which in patients with psoriasis treated with methotrexate is present in low concentrations, possibly due to the toxic effect of methotrexate on the liver which is the synthesis site of paraoxonase-1
[55][77].
Other pharmacological agents commonly used in the treatment of psoriasis are TNF-alpha inhibitors, namely infliximab. The use of this drug is associated with a decrease in oxidative stress levels due to its property to block the pro-oxidant role of TNF-alpha. Moreover, as opposed to the use of methotrexate which is linked with an elevation in pro-oxidant levels, infliximab does not influence the pro-oxidant/antioxidant balance or can influence it in the favor of antioxidant molecules
[61][86].
3. Conclusions
Oxidative stress seems to be involvement in the development and evolution of psoriasis and its associated comorbidities, in particular those affecting cardiometabolic health. The utility of the assessment of circulating serum, plasma, urinary and/or skin biomarkers of oxidative stress and of the study of polymorphisms in genes regulating the redox balance remains to be explored in future prospective cohort studies. However, the role that oxidative stress plays in the pathophysiology of psoriasis is indisputable and understanding the mechanisms by which it contributes to the initiation and maintenance of this chronic condition can aid the management of this dermatosis in the near future. Thus, such markers may emerge as essential tools in the early diagnosis of psoriasis, from the subclinical stage, as well as in evaluating the pattern of evolution and the therapeutic response.