
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giselle Amanda Borges E Soares | + 2998 word(s) | 2998 | 2021-12-29 09:26:13 | | | |
| 2 | Conner Chen | Meta information modification | 2998 | 2022-02-10 01:52:45 | | | | |
| 3 | Conner Chen | + 3 word(s) | 3001 | 2022-02-10 02:13:09 | | | | |
| 4 | Conner Chen | Meta information modification | 3001 | 2022-02-10 02:24:35 | | |
Video Upload Options
Essential oils (EOs) are naturally occurring complex mixtures of volatile odor compounds synthesized as secondary metabolites by plants and are extracted through steam distillation, solvent extraction, maceration, cold press extraction, water distillation, and CO2 extraction. Novel methods that are more efficient and provide higher yields include supercritical fluid extraction, microwave-assisted extraction, and ultrasound. Studies conducted on animals and humans have shown that EOs can produce a variety of central nervous system (CNS) targeted pharmacological effects such as anxiolytic effect, neuroprotection, antidepressant effect, anticonvulsant effect, analgesic, and sedative effect, to name a few. As a result, EOs can be used as an adjuvant therapy to prevent and relieve symptoms associated with CNS-based disorders such as insomnia, depression, dementia, Alzheimer’s disease (AD), etc.
1. Role in Pain Management
1.1 Clove Oil
1.2 Lavender Oil
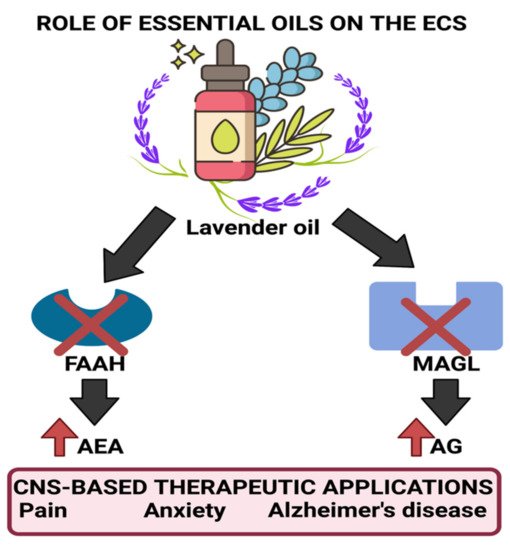
2. Role in Anxiety Relief and Stress Management
2.1 Frankincense oil
2.2 Lavender oil
2.3 Lemongrass oil
3. Role in Depression Management
3.1 Ylang ylang oil
3.2 Cinnamon oil
4. Role in Memory Retention, Neuroprotection, and Alzheimer’s Disease Management
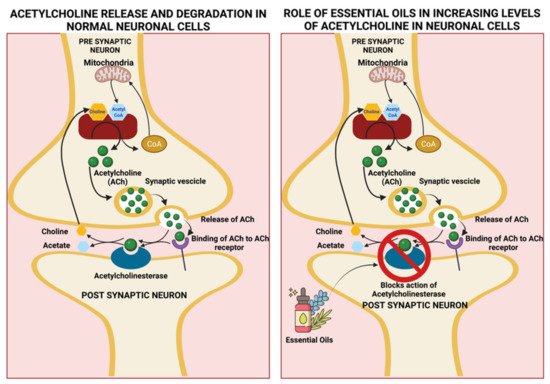
4.1 Eucalyptus oil
4.2 Peppermint oil
4.3 Rosemary oil
4.4 Sage oil
4.5 Sandalwood oil
References
- Lakhan, S.E.; Sheafer, H.; Tepper, D. The Effectiveness of Aromatherapy in Reducing Pain: A Systematic Review and Meta-Analysis. Pain Res. Treat. 2016, 2016, 8158693.
- Yang, S.; Chang, M.C. Chronic Pain: Structural and Functional Changes in Brain Structures and Associated Negative Affective States. Int. J. Mol. Sci. 2019, 20, 3130.
- Sá, R.D.C.D.S.E.; Lima, T.C.; da Nóbrega, F.R.; Brito, A.E.M.D.; de Sousa, D.P. Analgesic-Like Activity of Essential Oil Constituents: An Update. Int. J. Mol. Sci. 2017, 12, 2932.
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A Natural Compound with Versatile Pharmacological Actions. Nat. Prod. Commun. 2010, 5, 1999–2006.
- Chung, G.; Rhee, J.N.; Jung, S.J.; Kim, J.S.; Oh, S.B. Modulation of CaV2.3 calcium channel currents by eugenol. J. Dent. Res. 2008, 87, 137–141.
- Cho, J.S.; Kim, T.H.; Lim, J.M.; Song, J.H. Effects of eugenol on Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2008, 1243, 53–62.
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635.
- Pokajewicz, K.; Bialon, M.; Svydenko, L.; Fedin, R.; Hudz, N. Chemical Composition of the Essential Oil of the New Cultivars of Lavandula angustifolia Mill. Bred in Ukraine. Molecules 2021, 26, 5681.
- Aoshima, H.; Hamamoto, K. Potentiation of GABAA Receptors Expressed in Xenopus Oocytes by Perfume and Phytoncid. Biosci. Biotechnol. Biochem. 1999, 63, 743–748.
- Dal Bo, W.; Luiz, A.P.; Martins, D.F.; Mazzardo-Martins, L.; Santos, A.R. Eugenol reduces acute pain in mice by modulating the glutamatergic and tumor necrosis factor alpha (TNF-alpha) pathways. Fundam. Clin. Pharmacol. 2013, 27, 517–525.
- Dhiman, P.; Malik, N.; Khatkar, A. Lead optimization for promising monoamine oxidase inhibitor from eugenol for the treatment of neurological disorder: Synthesis and in silico based study. BMC Chem. 2019, 13, 38.
- Holanda Pinto, S.A.; Pinto, L.M.; Guedes, M.A.; Cunha, G.M.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Antinoceptive effect of triterpenoid alpha,beta-amyrin in rats on orofacial pain induced by formalin and capsaicin. Phytomedicine 2008, 15, 630–634.
- Venâncio, A.M.; Marchioro, M.; Estavam, C.S.; Melo, M.S.; Santana, M.T.; Onofre, A.S.C.; Guimarães, A.G.; Oliveira, M.G.B.; Alves, P.B.; Pimentel, H.D.C.; et al. Ocimum basilicum leaf essential oil and (-)-linalool reduce orofacial nociception in rodents: A behavioral and electrophysiological approach. Braz. J. Farmacogn. 2011, 21, 1043–1051.
- Andersen, P.; Bliss, T.V.; Skrede, K.K. Unit analysis of hippocampal polulation spikes. Exp. Brain Res. 1971, 13, 208–221.
- Peana, A.T.; De Montis, M.G.; Nieddu, E.; Spano, M.T.; D’Aquila, P.S.; Pippia, P. Profile of spinal and supra-spinal antinociception of (-)-linalool. Eur. J. Pharmacol. 2004, 485, 165–174.
- Peana, A.T.; D’Aquila, P.S.; Chessa, M.L.; Moretti, M.D.; Serra, G.; Pippia, P. (-)-Linalool produces antinociception in two experimental models of pain. Eur. J. Pharmacol. 2003, 460, 37–41.
- Tashiro, S.; Yamaguchi, R.; Ishikawa, S.; Sakurai, T.; Kajiya, K.; Kanmura, Y.; Kuwaki, T.; Kashiwadani, H. Odour-induced analgesia mediated by hypothalamic orexin neurons in mice. Sci. Rep. 2016, 6, 37129.
- Johnson, S.A.; Rodriguez, D.; Allred, K. A Systematic Review of Essential Oils and the Endocannabinoid System: A Connection Worthy of Further Exploration. Evid. Based Complementary Altern. Med. 2020, 2020, 8035301.
- Anxiety and Depression Association of America. Anxiety, Facts and Statistics. Available online: https://adaa.org/understanding-anxiety/facts-statistics (accessed on 1 May 2021).
- MayoClinic. Anxiety Disorders, Diagnosis and Treatment. Available online: https://www.mayoclinic.org/diseases-conditions/anxiety/diagnosis-treatment/drc-20350967 (accessed on 1 May 2021).
- Mertens, M.; Buettner, A.; Kirchhoff, E. The volatile constituents of frankincense—A review. Flavour Fragr. J. 2009, 24, 279–300.
- Okano, S.; Honda, Y.; Kodama, T.; Kimura, M. The Effects of Frankincense Essential Oil on Stress in Rats. J. Oleo Sci. 2019, 68, 1003–1009.
- Scuteri, D.; Hamamura, K.; Sakurada, T.; Watanabe, C.; Sakurada, S.; Morrone, L.A.; Rombola, L.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Efficacy of Essential Oils in Pain: A Systematic Review and Meta-Analysis of Preclinical Evidence. Front. Pharmacol. 2021, 12, 640128.
- Lehrner, J.; Marwinski, G.; Lehr, S.; Johren, P.; Deecke, L. Ambient odors of orange and lavender reduce anxiety and improve mood in a dental office. Physiol Behav. 2005, 86, 92–95.
- Conrad, P.; Adams, C. The effects of clinical aromatherapy for anxiety and depression in the high risk postpartum woman—A pilot study. Complementary Ther. Clin. Pract. 2012, 18, 164–168.
- Okano, K.; Kaczmarzyk, J.R.; Dave, N.; Gabrieli, J.D.E.; Grossman, J.C. Sleep quality, duration, and consistency are associated with better academic performance in college students. NPJ Sci. Learn. 2019, 4, 16.
- Lillehei, A.S.; Halcon, L.L.; Savik, K.; Reis, R. Effect of Inhaled Lavender and Sleep Hygiene on Self-Reported Sleep Issues: A Randomized Controlled Trial. J. Altern. Complementary Med. 2015, 21, 430–438.
- Faydali, S.; Cetinkaya, F. The Effect of Aromatherapy on Sleep Quality of Elderly People Residing in a Nursing Home. Holist. Nurs. Pract. 2018, 32, 8–16.
- Lizarraga-Valderrama, L.R. Effects of essential oils on central nervous system: Focus on mental health. Phytother. Res. 2021, 35, 657–679.
- Ekpenyong, C.E.; Akpan, E.E. Use of Cymbopogon citratus essential oil in food preservation: Recent advances and future perspectives. Crit. Rev. Food Sci. Nutr. 2017, 57, 2541–2559.
- Costa, C.A.; Cury, T.C.; Cassettari, B.O.; Takahira, R.K.; Florio, J.C.; Costa, M. Citrus aurantium L. essential oil exhibits anxiolytic-like activity mediated by 5-HT(1A)-receptors and reduces cholesterol after repeated oral treatment. BMC Complementary Altern. Med. 2013, 13, 42.
- Costa, C.A.; Kohn, D.O.; de Lima, V.M.; Gargano, A.C.; Florio, J.C.; Costa, M. The GABAergic system contributes to the anxiolytic-like effect of essential oil from Cymbopogon citratus (lemongrass). J. Ethnopharmacol. 2011, 137, 828–836.
- Goes, T.C.; Ursulino, F.R.; Almeida-Souza, T.H.; Alves, P.B.; Teixeira-Silva, F. Effect of Lemongrass Aroma on Experimental Anxiety in Humans. J. Altern. Complementary Med. 2015, 21, 766–773.
- NIH. Depression. Available online: https://www.nimh.nih.gov/health/topics/depression/ (accessed on 1 May 2021).
- MayoClinic. Depression (Major Depressive Disorder). Available online: https://www.mayoclinic.org/diseases-conditions/depression/symptoms-causes/syc-20356007 (accessed on 1 May 2021).
- Tan, L.T.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Abdul Kadir, H.; Chan, K.G.; Goh, B.H. Traditional Uses, Phytochemistry, and Bioactivities of Cananga odorata (Ylang-Ylang). Evid. Based Complementary Alternat. Med. 2015, 2015, 896314.
- Zhang, N.; Zhang, L.; Feng, L.; Yao, L. The anxiolytic effect of essential oil of Cananga odorata exposure on mice and determination of its major active constituents. Phytomedicine 2016, 23, 1727–1734.
- Jung, D.J.; Cha, J.Y.; Kim, S.E.; Ko, I.G.; Jee, Y.S. Effects of Ylang-Ylang aroma on blood pressure and heart rate in healthy men. J. Exerc. Rehabil. 2013, 9, 250–255.
- Zhang, N.; Zhang, L.; Feng, L.; Yao, L. Cananga odorata essential oil reverses the anxiety induced by 1-(3-chlorophenyl) piperazine through regulating the MAPK pathway and serotonin system in mice. J. Ethnopharmacol. 2018, 219, 23–30.
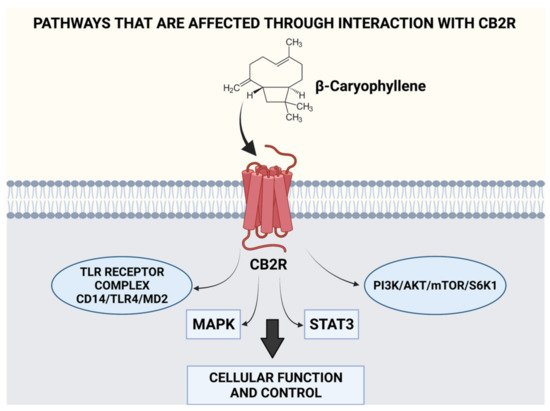
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A systematic review on the neuroprotective perspectives of beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388.
- Galaj, E.; Xi, Z.X. Potential of Cannabinoid Receptor Ligands as Treatment for Substance Use Disorders. CNS Drugs 2019, 33, 1001–1030.
- Johnson, W.G. Late-onset neurodegenerative diseases--the role of protein insolubility. J. Anat. 2000, 196 Pt 4, 609–616.
- Hwang, E.S.; Kim, H.B.; Lee, S.; Kim, M.J.; Kim, K.J.; Han, G.; Han, S.Y.; Lee, E.A.; Yoon, J.H.; Kim, D.O.; et al. Antidepressant-like effects of beta-caryophyllene on restraint plus stress-induced depression. Behav. Brain Res. 2020, 380, 112439.
- Gertsch, J. Anti-inflammatory cannabinoids in diet: Towards a better understanding of CB(2) receptor action? Commun. Integr. Biol 2008, 1, 26–28.
- Paula-Freire, L.I.; Andersen, M.L.; Gama, V.S.; Molska, G.R.; Carlini, E.L. The oral administration of trans-caryophyllene attenuates acute and chronic pain in mice. Phytomedicine 2014, 21, 356–362.
- Katsuyama, S.; Mizoguchi, H.; Kuwahata, H.; Komatsu, T.; Nagaoka, K.; Nakamura, H.; Bagetta, G.; Sakurada, T.; Sakurada, S. Involvement of peripheral cannabinoid and opioid receptors in beta-caryophyllene-induced antinociception. Eur. J. Pain 2013, 17, 664–675.
- Sohrabi, R.; Pazgoohan, N.; Seresht, H.R.; Amin, B. Repeated systemic administration of the cinnamon essential oil possesses anti-anxiety and anti-depressant activities in mice. Iran. J. Basic. Med. Sci. 2017, 20, 708–714.
- Chen, Y.F.; Wang, Y.W.; Huang, W.S.; Lee, M.M.; Wood, W.G.; Leung, Y.M.; Tsai, H.Y. Trans-Cinnamaldehyde, An Essential Oil in Cinnamon Powder, Ameliorates Cerebral Ischemia-Induced Brain Injury via Inhibition of Neuroinflammation Through Attenuation of iNOS, COX-2 Expression and NFkappa-B Signaling Pathway. Neuromolecular. Med. 2016, 18, 322–333.
- Iwasaki, Y.; Tanabe, M.; Kobata, K.; Watanabe, T. TRPA1 agonists--allyl isothiocyanate and cinnamaldehyde--induce adrenaline secretion. Biosci. Biotechnol. Biochem. 2008, 72, 2608–2614.
- Association, A.S. What Is Alzheimer’s Disease? Available online: https://www.alz.org/alzheimers-dementia/what-is-alzheimers (accessed on 1 May 2021).
- National Institute on Ageing. Alzheimer’s Disease Fact Sheet. Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet (accessed on 1 May 2021).
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 7.
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646.
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364.
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419.
- Brett, A.E.; A.Webster, A. Chapter 132—Acetylcholinesterase and its Inhibitors. In Primer on the Autonomic Nervous System (Third Edition), 3rd ed.; Robertson, D., Burnstock, G., Paton, J.F.R., Biaggioni, I., Low, P.A., Eds.; Academic Press: Cambridge, MA, USA, 2012.
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and Anti-Aging Potentials of Essential Oils from Aromatic and Medicinal Plants. Front. Aging Neurosci. 2017, 9, 168.
- Hsouna, A.B.; Touj, N.; Hammami, I.; Dridi, K.; Al-Ayed, A.S.; Hamdi, N. Chemical Composition and in vivo Efficacy of the Essential Oil of Mentha piperita L. in the Suppression of Crown Gall Disease on Tomato Plants. J. Oleo Sci. 2019, 68, 419–426.
- Masomeh, L.; Narges, M.; Hassan, R.A.H. Peppermint and Its Functionality: A Review. Arch. Clin. Microbiol. 2017, 7, 4.
- Umezu, T. Evaluation of the effects of plant-derived essential oils on central nervous system function using discrete shuttle-type conditioned avoidance response in mice. Phytother. Res. 2012, 26, 884–891.
- Kennedy, D.; Okello, E.; Chazot, P.; Howes, M.J.; Ohiomokhare, S.; Jackson, P.; Haskell-Ramsay, C.; Khan, J.; Forster, J.; Wightman, E. Volatile Terpenes and Brain Function: Investigation of the Cognitive and Mood Effects of Mentha × Piperita L. Essential Oil with In Vitro Properties Relevant to Central Nervous System Function. Nutrients 2018, 10, 1029.
- Bhadania, M.; Joshi, H.; Patel, P.; Kulkarni, V.H. Protective effect of menthol on beta-amyloid peptide induced cognitive deficits in mice. Eur. J. Pharmacol. 2012, 681, 50–54.
- Elyemni, M.; Louaste, B.; Nechad, I.; Elkamli, T.; Bouia, A.; Taleb, M.; Chaouch, M.; Eloutassi, N. Extraction of Essential Oils of Rosmarinus officinalis L. by Two Different Methods: Hydrodistillation and Microwave Assisted Hydrodistillation. Sci. World J. 2019, 2019, 3659432.
- Villareal, M.O.; Ikeya, A.; Sasaki, K.; Arfa, A.B.; Neffati, M.; Isoda, H. Anti-stress and neuronal cell differentiation induction effects of Rosmarinus officinalis L. essential oil. BMC Complementary Altern. Med. 2017, 17, 549.
- Habtemariam, S. The Therapeutic Potential of Rosemary (Rosmarinus officinalis) Diterpenes for Alzheimer’s Disease. Evid. Based Complementary Altern. Med. 2016, 2016, 2680409.
- Khedher, M.R.B.; Khedher, S.B.; Chaieb, I.; Tounsi, S.; Hammami, M. Chemical composition and biological activities of Salvia officinalis essential oil from Tunisia. EXCLI J. 2017, 16, 160–173.
- Moss, M.; Rouse, M.; Moss, L. Aromas of Salvia Species Enhance Everyday Prospective Memory Performance in Healthy Young Adults. Adv. Chem. Eng. Sci. 2014, 4, 339–346.
- Lopresti, A.L. Salvia (Sage): A Review of its Potential Cognitive-Enhancing and Protective Effects. Drugs R D 2017, 17, 53–64.
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2003, 28, 53–59.
- Braun, N.A.; Sim, S.; Kohlenberg, B.; Lawrence, B.M. Hawaiian sandalwood: Oil composition of Santalum paniculatum and comparison with other sandal species. Nat. Prod. Commun. 2014, 9, 1365–1368.
- Safwat, Y.N.; Elsayed, M.M. Sandalwood oil neuroprotective effects on middle cerebral artery occlusion model of ischemic brain stroke. Farmacogn. Mag. 2020, 16, 117–122.
- Misra, B.B.; Dey, S. Biological Activities of East Indian Sandalwood Tree, Santalum album. PeerJ 2013, 1, e96v1.
- . Misra, B.B.; Dey, S. Evaluation of in vivo anti-hyperglycemic and antioxidant potentials of alpha-santalol and sandalwood oil. Phytomedicine 2013, 20, 409–416.
- Hoferl, M.; Hutter, C.; Buchbauer, G. A Pilot Study on the Physiological Effects of Three Essential Oils in Humans. Nat. Prod. Commun. 2016, 11, 1561–1564.





