Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Charlotte Gupta | + 2185 word(s) | 2185 | 2022-01-25 04:39:23 | | | |
| 2 | Rita Xu | Meta information modification | 2185 | 2022-02-09 03:10:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gupta, C. Cardiometabolic Risk and Chronobiology. Encyclopedia. Available online: https://encyclopedia.pub/entry/19254 (accessed on 07 February 2026).
Gupta C. Cardiometabolic Risk and Chronobiology. Encyclopedia. Available at: https://encyclopedia.pub/entry/19254. Accessed February 07, 2026.
Gupta, Charlotte. "Cardiometabolic Risk and Chronobiology" Encyclopedia, https://encyclopedia.pub/entry/19254 (accessed February 07, 2026).
Gupta, C. (2022, February 09). Cardiometabolic Risk and Chronobiology. In Encyclopedia. https://encyclopedia.pub/entry/19254
Gupta, Charlotte. "Cardiometabolic Risk and Chronobiology." Encyclopedia. Web. 09 February, 2022.
Copy Citation
Cardiovascular disease (CVD) poses a serious health and economic burden worldwide. Modifiable lifestyle factors are a focus of research into reducing the burden of CVD, with diet as one of the most investigated factors. Specifically, the timing and regularity of food intake is an emerging research area, with approaches such as time-restricted eating (TRE) receiving much attention.
chrono-nutrition
meal timing
eating habits
metabolic health
cardiovascular
sleep timing
1. Introduction: Cardiometabolic Risk and Chronobiology
Cardiovascular disease (CVD) is an umbrella term used to describe medical conditions that affect the heart, blood vessels, and cardiometabolic health [1][2]. CVD is the leading cause of death globally [3][4] and, importantly, is largely preventable [5]. Modifiable lifestyle factors, including sub-optimal diet, physical inactivity, excessive alcohol consumption, and smoking, account for up to 90% of the risk factors associated with CVD [2].
The Heart Foundation Australia [2] emphasises a need to focus on modifiable lifestyle factors as part of future research into reducing the burden of CVD. When investigating how modifiable lifestyle factors influence CVD, research to date has focused on factors such as diet, physical activity, smoking, and alcohol consumption [1][5][6][7]. However, as the rates of CVD continue to grow [2][4], the focus of research has widened to include other lifestyle factors that could also be important contributors to CVD risk, such as sleep [8][9][10]. Attention has also turned to exploring possible interactions between lifestyle factors to identify novel approaches to reducing CVD risk. While sub-optimal diet is a well-established risk factor for CVD [11][12][13], a new focus is emerging on the timing and regularity of food intake [14][15][16]. Given that the timing and regularity of sleep is also a consideration in relation to CVD risk factors [8][17], the next step is to explore the timing of both sleep and food intake, alongside their expanding understanding of biological timing systems.
Identifying the mechanisms underlying CVD is integral to reducing the burden of disease [18][19]. Disruption to human circadian rhythms is one such mechanism that has received scientific attention, with several key reviews demonstrating the relationship between circadian disruption and cardiometabolic health [9][18][20][21]. Circadian rhythms are biological and behavioural rhythms with a period of approximately 24 h [22][23]. While it is well-established that the circadian system, controlled by a central clock located in the suprachiasmatic nucleus in the brain, is influenced by light [24], more recent evidence demonstrates that peripheral clocks throughout the body are influenced by other external behaviours, such as meal timing [25][26]. Furthermore, while sleep timing affects light exposure and therefore the central clock, the timing of sleep has also been linked to the timing of peripheral clocks [18].
Optimal functioning of the circadian system is essential for good health [27]. Circadian disruption occurs when human sleep–wake and eating–fasting rhythms are not appropriately timed; that is, they do not align with human light–dark cycle [18][28]. One of the leading causes of circadian disruption is working non-standard hours including shift work [9]. Shift work disrupts the usual sleep–wake cycle as workers may be on shift during times when the body is primed to be sleeping (i.e., at night), attempting sleep when the body is primed to be awake (i.e., during the day), or woken during a sleep period when working an on-call schedule [29][30]. This leads to inadequate sleep in shift work populations [31][32], contributing to circadian disruption, which is recognised as a major contributor to CVD risk in shift workers [9]. As a result of circadian disruption, natural physiological processes, such as metabolism, digestion, energy expenditure, and blood pressure, are misaligned [9][20][23][24][26][33][34][35][36][37][38][39]. This misalignment is proposed to play a critical role in the development of long-term health problems, such as CVD [1][9].
Eating at a time when the body is not primed to digest food is a challenge to multiple physiological systems, including the circadian system [14]. The typical eating window (the time from first to last time of energy consumption across the day) spans 14 h of the day in healthy, synchronised individuals [40][41]. Spreading eating events across the day (i.e., beyond the 14 h eating window) to include eating late at night is associated with weight gain and increased insulin resistance [42], which are two markers of CVD. Importantly, the typical eating window may be longer in obese populations [43]. In response to these novel findings, time-restricted eating (TRE), whereby the eating window is shortened [44], has been proposed to manage weight and cardiometabolic health. Indeed, several recent studies investigating TRE and cardiometabolic health [14][45][46][47][48][49][50][51] have demonstrated improved cardiometabolic outcomes with TRE, including reductions in systolic and diastolic blood pressure, reductions in fat mass, and improved insulin sensitivity [50][51][52]. While some of these studies measured sleep as an outcome after a TRE intervention [53], they did not consider sleep as a predictor of cardiometabolic health outcomes and therefore missed the opportunity to control for or to consider the impact of chronic inadequate sleep. This is potentially problematic as circadian disruption resulting from inadequate sleep may independently influence the same cardiovascular outcomes influenced by TRE [8][54]. Furthermore, there is a known relationship between food intake and sleep [55][56][57] such that inadequate sleep can lead to altered meal timing [55][58] and increased cravings for certain foods [59][60], and different foods and nutrients can impact sleep quality [61][62]. These interactions highlight the need to better understand the relationships between eating patterns, such as TRE, sleep, circadian misalignment, and cardiometabolic health.
2. Time-Restricted Eating, Circadian Disruption, and Cardiometabolic Health
Eating habits are a well-researched lifestyle factor contributing to CVD [63]. Poor eating habits, such as poor diet quality, have been linked to key cardiometabolic risk factors for CVD [11][12][64][65], including increased blood pressure, body mass index, serum lipids, total cholesterol, and low-density lipoprotein cholesterol [12][66][67]. As such, improving eating habits is a critical part of reducing CVD risk [13][68]. However, it has become apparent that the timing of food intake is also an important consideration [38], primarily due to the burden of altered eating patterns on the circadian system [14].
Eating at times when the body is not primed to digest food (e.g., at night [16]) can compromise metabolism and thus lead to an increase in the likelihood of developing CVD [38][44]. Indeed, observational studies have reported a relationship between irregular eating patterns and increased risk for metabolic syndrome [69][70][71], in addition to a relationship between habitual night eating and arterial stiffness, a preclinical sign of CVD [72]. Altered eating patterns are characteristic of shift work [55][73][74][75], with shift workers reporting that they change the timing of eating to accommodate their shift schedule; for example, eating during the night when working night shifts [55]. Misalignment of eating rhythms with the internal circadian system is thought to contribute to the relationship between shift work and CVD [73]. Research in non-shift workers has shown that erratic eating patterns are associated with increased CVD risk after controlling for dietary composition [76]; thus, highlighting that in addition to ‘what is eaten’, ‘when eating occurs’ also influences CVD risk. Collectively, recent research highlights the need to optimise eating time to reduce circadian disruption and consequently reduce the prevalence of CVD.
As previously discussed, TRE is a unique strategy that has gained popularity as a way to optimise the timing of food intake [14][16][38]. While typical eating windows for most individuals span 14 h of the day, a TRE approach shortens the eating window to between 4 and 10 h [14][77] (Figure 1, example 8 h eating window). It is important to note that the optimal timing of a shortened eating window (i.e., starting the eating window early in the day or later in the day) requires systematic study [44]. In a recent review by Regmi and Heilbronn [78], the authors argued that while the early morning (e.g., Figure 1, middle panel) may be considered an optimal time to start the eating window for maximal metabolic benefits (e.g., improving insulin sensitivity and lipid absorption), this would mean that people would miss eating dinner at a traditional time (6–8 pm), which is a typical family and group eating time [79]. This may therefore present social challenges. In contrast, the same 8 h eating window could start at 12 pm and include the typical dinner time (e.g., Figure 1, right panel). While this arrangement extends the overnight fast to the same degree and may be perceived to be less socially challenging, metabolic benefits may be reduced due to circadian considerations [78].
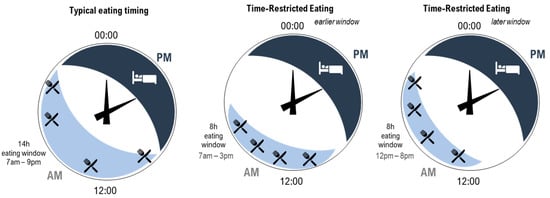
Figure 1. Illustration of three patterns of eating (light blue shading with knife and fork) and sleeping (dark blue shading with bed) across hours on 24 h clocks. Left—typical eating arrangement within a 14 h eating window starting at 7 am. Middle—time-restricted eating within an 8 h eating window starting at 7 am. Right—time-restricted eating within an 8 h eating window starting at 12 pm. All arrangements include the same number of eating occasions (indicated by knife and fork). For the time-restricted eating patterns, the time between the first and last eating occasion is shortened, increasing the length of the overnight fast.
TRE may lead to improved cardiovascular outcomes [16]. For example, a recent systematic review and meta-analysis of eleven studies found significantly lower fasting glucose values for participants on a TRE pattern (with eating windows ranging from 6 h to 12 h) compared to those eating ad libitum [50]. Other studies have similarly demonstrated improvements following TRE in blood pressure, body weight, cholesterol, glucose metabolism, and the gut microbiome [22][50][51][78][80][81]. Furthermore, TRE circumvents some of the challenges of typical dieting approaches, which often require individuals to employ restrictive behaviours, because the quality and quantity of the food eaten in TRE regimes does not change [77][82], as only the window of eating is altered.
In shortening the eating window, TRE reduces the amount of time the body is required to metabolise food and lengthens the daily fast period, arguably allowing for greater metabolic recovery [14][16][22][41][50]. As introduced in Figure 1, a further opportunity for improving cardiometabolic outcomes arises from the ability to consider the timing of the eating window to reduce circadian disruption. In people with typical diurnal rhythm, starting a shortened eating window early in the day avoids circadian misalignment of eating behaviours and related impairment in the way in which food is processed by the body [15][38]. Indeed, evidence suggests that an earlier eating window is associated with more effective cardiometabolic outcomes compared to a later eating window [14][16][41][77]. As previously discussed, circadian disruption plays a significant role in the development of CVD due to misaligned daily rhythms, such as metabolism, digestion, and blood pressure [1]. Since food acts as a signal for peripheral circadian clocks [16][18], eating food at biologically inappropriate times can lead to misalignment between central and peripheral clocks [83]. Therefore, carefully timed TRE may be an effective and relatively straightforward strategy to minimise circadian disruption [48] and ultimately contribute to a reduced burden of CVD [38][77]. However, while day interventions are the focus of much of the existing TRE literature [14][38][45][50][84], consideration of the impacts of practicing TRE at other times, such as during the night, has been less well-documented. Research in this area is of particular interest for night workers who typically eat during the night [55]. Recent laboratory research has demonstrated the beneficial effect for glucose metabolism of maintaining a daytime eating window even while working (simulated) night shifts [85][86][87][88]. To extend this research, additional consideration of TRE for those working night shifts is needed; in particular, this would determine the impact and feasibility of shortening the daytime eating window while supporting the need to sleep during the day (Figure 2).
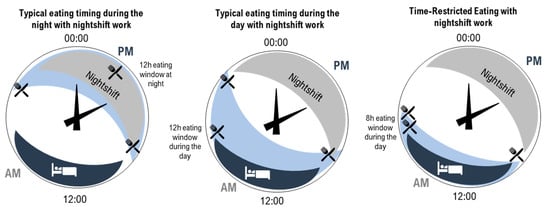
Figure 2. Illustration of three patterns of eating (light blue shading with knife and fork) and sleeping (dark blue shading with bed) on 24 h clocks. Left—typical eating arrangement for a night-shift worker with a 12 h eating window during the night. Middle—example of an eating arrangement for a night-shift worker with a 12 h eating window during the day. Right—time-restricted eating for a night-shift worker with an 8 h eating window during the day (right).
Consideration of sleep in the context of night work is critical. While TRE can be designed to reduce circadian disruption, one of the biggest contributors to circadian disruption is an altered sleep–wake cycle [23][26]. Authors hypothesise that TRE may not have the same benefits for cardiometabolic health if individuals are experiencing circadian disruption due to inadequate sleep.
3. Inadequate Sleep, Circadian Disruption, and Cardiometabolic Health
Inadequate sleep is highly prevalent globally, with adults commonly obtaining less than the optimal 7–9 h of sleep per night [89][90][91][92][93]. For example, up to one-third of Australians do not achieve 7 h of sleep per night [89][90]. This is problematic, as chronic inadequate sleep challenges the circadian system [19] and consequently impacts cardiometabolic health [1][10][19][94]. Several studies have demonstrated the link between short sleep (considered sleep of ≤6 h in duration [95]) and adverse cardiometabolic outcomes including obesity, hypertension, poor glucose regulation, and insulin resistance [19][95][96][97][98][99][100]. Importantly, extended sleep (i.e., sleep >9 h) is also associated with adverse cardiometabolic health effects [101][102], such as increased blood pressure [103][104], and this could be a bi-directional relationship, with long sleep a symptom of CVD [105]. This suggests a U-shape relationship between sleep and cardiometabolic health, with 7–9 h considered an optimum amount for favourable cardiometabolic outcomes [102]. Ensuring adequate sleep duration (i.e., 7–9 h per 24 h) is therefore a key strategy to reduce the risk of CVD.
Inadequate sleep is a common outcome of shift-work schedules [37]. Many shift workers, particularly those engaged in night work, experience some degree of circadian disruption [9][106]. This is thought to be a major contributor to the prevalence of cardiometabolic issues in shift workers, such as elevated post-prandial glucose levels, obesity, and CVD in the long-term [9][19].
References
- Cannon, C.P. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin. Cornerstone 2007, 8, 11–28.
- Heart Foundation Australia. Cardiovascular Disease (CVD) Risk Assessment and Management. 2021. Available online: https://www.heartfoundation.org.au/conditions/cvd-risk-assessment-and-management (accessed on 13 September 2021).
- World Health Organisation. Cardiovascular Disease (CVD). 2015. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 13 September 2021).
- Roever, L.; Tse, G.; Biondi-Zoccai, G. Trends in cardiovascular disease in Australia and in the world. Eur. J. Prev. Cardiol. 2018, 25, 1278–1279.
- Djoussé, L.; Driver, J.A.; Gaziano, J.M. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA 2009, 302, 394–400.
- Phoi, Y.Y.; Bonham, M.P.; Rogers, M.; Dorrian, J.; Coates, A.M. Content Validation of a Chrononutrition Questionnaire for the General and Shift Work Populations: A Delphi Study. Nutrients 2021, 13, 4087.
- Memon, A.R.; Gupta, C.C.; Crowther, M.E.; Ferguson, S.A.; Tuckwell, G.A.; Vincent, G.E. Sleep and physical activity in university students: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 58, 101482.
- Cappuccio, F.P.; Cooper, D.; D'Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 2011, 32, 1484–1492.
- Kervezee, L.; Kosmadopoulos, A.; Boivin, D.B. Metabolic and cardiovascular consequences of shift work: The role of circadian disruption and sleep disturbances. Eur. J. Neurosci. 2020, 51, 396–412.
- Sofer, T.; Goodman, M.O.; Bertisch, S.M.; Redline, S. Longer sleep improves cardiovascular outcomes: Time to make sleep a priority. Eur. Heart J. 2021, 42, 3358–3360.
- Belin, R.J.; Greenland, P.; Allison, M.; Martin, L.; Shikany, J.M.; Larson, J.; Tinker, L.; Howard, B.V.; Lloyd-Jones, D.; Van Horn, L. Diet quality and the risk of cardiovascular disease: The Women’s Health Initiative (WHI). Am. J. Clin. Nutr. 2011, 94, 49–57.
- Kromhout, D. Diet and cardiovascular diseases. J. Nutr. Health Aging 2001, 5, 144–149.
- Siri-Tarino, P.W.; Krauss, R.M. Diet, lipids, and cardiovascular disease. Curr. Opin. Lipidol. 2016, 27, 323–328.
- Chaix, A.; Manoogian, E.N.; Melkani, G.C.; Panda, S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu. Rev. Nutr. 2019, 39, 291–315.
- Manoogian, E.N.; Chaix, A.; Panda, S. When to eat: The importance of eating patterns in health and disease. J. Biol. Rhythm. 2019, 34, 579–581.
- Melkani, G.C.; Panda, S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J. Physiol. 2017, 595, 3691–3700.
- Altman, N.G.; Izci-Balserak, B.; Schopfer, E.; Jackson, N.; Rattanaumpawan, P.; Gehrman, P.R.; Patel, N.P.; Grandner, M.A. Sleep duration versus sleep insufficiency as predictors of cardiometabolic health outcomes. Sleep Med. 2012, 13, 1261–1270.
- Reutrakul, S.; Knutson, K.L. Consequences of circadian disruption on cardiometabolic health. Sleep Med. Clin. 2015, 10, 455–468.
- Knutson, K.L. Sleep duration and cardiometabolic risk: A review of the epidemiologic evidence. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 731–743.
- Fatima, N.; Rana, S. Metabolic implications of circadian disruption. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 513–526.
- Rüger, M.; Scheer, F.A. Effects of circadian disruption on the cardiometabolic system. Rev. Endocr. Metab. Disord. 2009, 10, 245–260.
- Świątkiewicz, I.; Woźniak, A.; Taub, P.R. Time-Restricted Eating and Metabolic Syndrome: Current Status and Future Perspectives. Nutrients 2021, 13, 221.
- Vetter, C. Circadian disruption: What do we actually mean? Eur. J. Neurosci. 2020, 51, 531–550.
- Albrecht, U. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 2012, 74, 246–260.
- Roenneberg, T.; Kantermann, T.; Juda, M.; Vetter, C.; Allebrandt, K.V. Light and the human circadian clock. In Circadian Clocks; Springer: Berlin/Heidelberg, Germany, 2013; pp. 311–331.
- Vetter, C.; Scheer, F.A. Circadian biology: Uncoupling human body clocks by food timing. Curr. Biol. 2017, 27, R656–R658.
- Morris, C.J.; Aeschbach, D.; Scheer, F.A. Circadian system, sleep and endocrinology. Mol. Cell. Endocrinol. 2012, 349, 91–104.
- Almoosawi, S.; Vingeliene, S.; Gachon, F.; Voortman, T.; Palla, L.; Johnston, J.D.; Van Dam, R.M.; Darimont, C.; Karagounis, L.G. Chronotype: Implications for epidemiologic studies on chrono-nutrition and cardiometabolic health. Adv. Nutr. 2019, 10, 30–42.
- Vincent, G.E.; Kovac, K.; Signal, L.; Reynolds, A.C.; Paterson, J.; Sprajcer, M.; Ferguson, S.A. What factors influence the sleep of on-call workers? Behav. Sleep Med. 2021, 19, 255–272.
- Gupta, C.C.; Dominiak, M.; Kovac, K.; Reynolds, A.C.; Ferguson, S.A.; Hilditch, C.J.; Sprajcer, M.; Vincent, G.E. On-call work and sleep: The importance of switching on during a callout and switching off after a call. Ind. Health 2021.
- Åkerstedt, T. Shift work and disturbed sleep/wakefulness. Occup. Med. 2003, 53, 89–94.
- Folkard, S.; Tucker, P. Shift work, safety and productivity. Occup. Med. 2003, 53, 95–101.
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102.
- Archer, S.N.; Oster, H. How sleep and wakefulness influence circadian rhythmicity: Effects of insufficient and mistimed sleep on the animal and human transcriptome. J. Sleep Res. 2015, 24, 476–493.
- Buxton, O.M.; Cain, S.W.; O’Connor, S.P.; Porter, J.H.; Duffy, J.F.; Wang, W.; Czeisler, C.A.; Shea, S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012, 4, 129ra43.
- Chellappa, S.L.; Vujovic, N.; Williams, J.S.; Scheer, F.A. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol. Metab. 2019, 30, 767–779.
- Haus, E.; Smolensky, M. Biological clocks and shift work: Circadian dysregulation and potential long-term effects. Cancer Causes Control 2006, 17, 489–500.
- Manoogian, E.N.; Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 2017, 39, 59–67.
- Shaw, E.; Leung, G.K.; Jong, J.; Coates, A.M.; Davis, R.; Blair, M.; Huggins, C.E.; Dorrian, J.; Banks, S.; Kellow, N.J. The impact of time of day on energy expenditure: Implications for long-term energy balance. Nutrients 2019, 11, 2383.
- Veronda, A.C.; Kline, C.E.; Irish, L.A. The impact of circadian timing on energy balance: An extension of the energy balance model. Health Psychol. Rev. 2021, 1–43.
- Manoogian, E.N.; Chow, L.S.; Taub, P.R.; Laferrère, B.; Panda, S. Time-restricted eating for the prevention and management of metabolic diseases. Endocr. Rev. 2021, bnab027.
- Dashti, H.S.; Gómez-Abellán, P.; Qian, J.; Esteban, A.; Morales, E.; Scheer, F.A.; Garaulet, M. Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. Am. J. Clin. Nutr. 2021, 113, 154–161.
- Cahill, L.E. About Time: Eating Timing Is a Complex Risk Factor for Obesity; Oxford University Press: Oxford, UK, 2021.
- Chawla, S.; Beretoulis, S.; Deere, A.; Radenkovic, D. The Window Matters: A Systematic Review of Time Restricted Eating Strategies in Relation to Cortisol and Melatonin Secretion. Nutrients 2021, 13, 2525.
- Gabel, K.; Cienfuegos, S.; Kalam, F.; Ezpeleta, M.; Varady, K.A. Time-Restricted Eating to Improve Cardiovascular Health. Curr. Atheroscler. Rep. 2021, 23, 22.
- Kesztyüs, D.; Fuchs, M.; Cermak, P.; Kesztyüs, T. Associations of time-restricted eating with health-related quality of life and sleep in adults: A secondary analysis of two pre-post pilot studies. BMC Nutr. 2020, 6, 76.
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: The TREAT randomized clinical trial. JAMA Intern. Med. 2020, 180, 1491–1499.
- Manoogian, E.N.; Zadourian, A.; Lo, H.C.; Gutierrez, N.R.; Shoghi, A.; Rosander, A.; Pazargadi, A.; Wang, X.; Fleischer, J.G.; Golshan, S. Protocol for a randomised controlled trial on the feasibility and effects of 10-hour time-restricted eating on cardiometabolic disease risk among career firefighters doing 24-hour shift work: The Healthy Heroes Study. BMJ Open 2021, 11, e045537.
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290.
- Pellegrini, M.; Cioffi, I.; Evangelista, A.; Ponzo, V.; Goitre, I.; Ciccone, G.; Ghigo, E.; Bo, S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 17–33.
- Wilkinson, M.J.; Manoogian, E.N.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020, 31, 92–104.e5.
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221.e13.
- McStay, M.; Gabel, K.; Cienfuegos, S.; Ezpeleta, M.; Lin, S.; Varady, K.A. Intermittent Fasting and Sleep: A Review of Human Trials. Nutrients 2021, 13, 3489.
- Kecklund, G.; Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ 2016, 355, i5210.
- Gupta, C.C.; Coates, A.M.; Dorrian, J.; Banks, S. The factors influencing the eating behaviour of shiftworkers: What, when, where and why. Ind. Health 2019, 57, 419–453.
- Al Khatib, H.; Harding, S.; Darzi, J.; Pot, G. The effects of partial sleep deprivation on energy balance: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2017, 71, 614–624.
- Chaput, J.-P. Sleep patterns, diet quality and energy balance. Physiol. Behav. 2014, 134, 86–91.
- Crispim, C.A.; Zalcman, I.; Dáttilo, M.; Padilha, H.G.; Edwards, B.; Waterhouse, J.; Tufik, S.; de Mello, M.T. The influence of sleep and sleep loss upon food intake and metabolism. Nutr. Res. Rev. 2007, 20, 195–212.
- Gupta, C.C.; Ferguson, S.A.; Aisbett, B.; Dominiak, M.; Chappel, S.E.; Sprajcer, M.; Fullagar, H.H.; Khalesi, S.; Guy, J.H.; Vincent, G.E. Hot, Tired and Hungry: The Snacking Behaviour and Food Cravings of Firefighters during Multi-Day Simulated Wildfire Suppression. Nutrients 2020, 12, 1160.
- Heath, G.; Roach, G.D.; Dorrian, J.; Ferguson, S.A.; Darwent, D.; Sargent, C. The effect of sleep restriction on snacking behaviour during a week of simulated shiftwork. Accid. Anal. Prev. 2012, 45, 62–67.
- Binks, H.; Vincent, G.E.; Gupta, C.; Irwin, C.; Khalesi, S. Effects of diet on sleep: A narrative review. Nutrients 2020, 12, 936.
- Gupta, C.C.; Irwin, C.; Vincent, G.E.; Khalesi, S. The Relationship Between Diet and Sleep in Older Adults: A Narrative Review. Curr. Nutr. Rep. 2021, 1–13.
- Head, G.A. Cardiovascular and metabolic consequences of obesity. Front. Physiol. 2015, 6, 32.
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361, k2139.
- Funtikova, A.N.; Navarro, E.; Bawaked, R.A.; Fíto, M.; Schröder, H. Impact of diet on cardiometabolic health in children and adolescents. Nutr. J. 2015, 14, 118.
- Hooper, L. Primary prevention of CVD: Diet and weight loss. BMJ Clin. Evid. 2007, 2007, 0219.
- Ferns, G.A. New and emerging risk factors for CVD: Symposium on ‘Diet and CVD’. Proc. Nutr. Soc. 2008, 67, 223–231.
- Makarem, N.; Sears, D.D.; St-Onge, M.P.; Zuraikat, F.M.; Gallo, L.C.; Talavera, G.A.; Castaneda, S.F.; Lai, Y.; Aggarwal, B. Variability in Daily Eating Patterns and Eating Jetlag Are Associated with Worsened Cardiometabolic Risk Profiles in the American Heart Association Go Red for Women Strategically Focused Research Network. J. Am. Heart Assoc. 2021, 10, e022024.
- Pot, G.K.; Almoosawi, S.; Stephen, A.M. Meal irregularity and cardiometabolic consequences: Results from observational and intervention studies. Proc. Nutr. Soc. 2016, 75, 475–486.
- Sierra-Johnson, J.; Undén, A.L.; Linestrand, M.; Rosell, M.; Sjogren, P.; Kolak, M.; De Faire, U.; Fisher, R.M.; Hellénius, M.L. Eating meals irregularly: A novel environmental risk factor for the metabolic syndrome. Obesity 2008, 16, 1302–1307.
- Wennberg, M.; Gustafsson, P.E.; Wennberg, P.; Hammarström, A. Irregular eating of meals in adolescence and the metabolic syndrome in adulthood: Results from a 27-year prospective cohort. Public Health Nutr. 2016, 19, 667–673.
- Zhang, X.; Wu, Y.; Na, M.; Lichtenstein, A.H.; Xing, A.; Chen, S.; Wu, S.; Gao, X. Habitual night eating was positively associated with progress of arterial stiffness in chinese adults. J. Am. Heart Assoc. 2020, 9, e016455.
- Lowden, A.; Moreno, C.; Holmbäck, U.; Lennernäs, M.; Tucker, P. Eating and shift work—effects on habits, metabolism, and performance. Scand. J. Work Environ. Health 2010, 36, 150–162.
- Shaw, E.; Dorrian, J.; Coates, A.M.; Leung, G.K.; Davis, R.; Rosbotham, E.; Warnock, R.; Huggins, C.E.; Bonham, M.P. Temporal pattern of eating in night shift workers. Chronobiol. Int. 2019, 36, 1613–1625.
- Binks, H.; Vincent, G.; Christopher, I.; Heidke, P.; Vandelanotte, C.; Williams, S.; Khalesi, S. Associations between sleep and lifestyle in Australian nursing students: A cross-sectional study. Collegian 2021, 28, 97–105.
- Cahill, L.E.; Chiuve, S.E.; Mekary, R.A.; Jensen, M.K.; Flint, A.J.; Hu, F.B.; Rimm, E.B. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation 2013, 128, 337–343.
- Queiroz, J.d.N.; Macedo, R.C.O.; Tinsley, G.M.; Reischak-Oliveira, A. Time-restricted eating and circadian rhythms: The biological clock is ticking. Crit. Rev. Food Sci. Nutr. 2021, 17, 2863–2875.
- Regmi, P.; Heilbronn, L.K. Time-restricted eating: Benefits, mechanisms, and challenges in translation. Iscience 2020, 23, 101161.
- Dunbar, R. Breaking bread: The functions of social eating. Adapt. Hum. Behav. Physiol. 2017, 3, 198–211.
- Chow, L.S.; Manoogian, E.N.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: A feasibility study. Obesity 2020, 28, 860–869.
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353.
- Waldman, H.S.; Renteria, L.I.; McAllister, M.J. Time-restricted feeding for the prevention of cardiometabolic diseases in high-stress occupations: A mechanistic review. Nutr. Rev. 2020, 78, 459–464.
- Garaulet, M.; Gómez-Abellán, P. Timing of food intake and obesity: A novel association. Physiol. Behav. 2014, 134, 44–50.
- Parr, E.B.; Devlin, B.L.; Lim, K.H.; Moresi, L.N.; Geils, C.; Brennan, L.; Hawley, J.A. Time-Restricted eating as a nutrition strategy for individuals with type 2 diabetes: A feasibility study. Nutrients 2020, 12, 3228.
- Chellappa, S.L.; Qian, J.; Vujovic, N.; Morris, C.J.; Nedeltcheva, A.; Nguyen, H.; Rahman, N.; Heng, S.W.; Kelly, L.; Kerlin-Monteiro, K. Daytime eating prevents internal circadian misalignment and glucose intolerance in night work. Sci. Adv. 2021, 7, eabg9910.
- Gupta, C.C.; Centofanti, S.; Dorrian, J.; Coates, A.; Stepien, J.M.; Kennaway, D.; Wittert, G.; Heilbronn, L.; Catcheside, P.; Noakes, M. Altering meal timing to improve cognitive performance during simulated nightshifts. Chronobiol. Int. 2019, 36, 1691–1713.
- Gupta, C.C.; Centofanti, S.; Dorrian, J.; Coates, A.M.; Stepien, J.M.; Kennaway, D.; Wittert, G.; Heilbronn, L.; Catcheside, P.; Noakes, M. Subjective hunger, gastric upset, and sleepiness in response to altered meal timing during simulated shiftwork. Nutrients 2019, 11, 1352.
- Grant, C.L.; Coates, A.M.; Dorrian, J.; Kennaway, D.J.; Wittert, G.A.; Heilbronn, L.K.; Pajcin, M.; Della Vedova, C.; Gupta, C.C.; Banks, S. Timing of food intake during simulated night shift impacts glucose metabolism: A controlled study. Chronobiol. Int. 2017, 34, 1003–1013.
- Adams, R.J.; Appleton, S.L.; Taylor, A.W.; Gill, T.K.; Lang, C.; McEvoy, R.D.; Antic, N.A. Sleep health of Australian adults in 2016: Results of the 2016 Sleep Health Foundation national survey. Sleep Health 2017, 3, 35–42.
- Metse, A.P.; Bowman, J.A. Prevalence of self-reported suboptimal sleep in Australia and receipt of sleep care: Results from the 2017 National Social Survey. Sleep Health 2020, 6, 100–109.
- Jasani, F.S.; Seixas, A.A.; Madondo, K.; Li, Y.; Jean-Louis, G.; Pagán, J.A. Sleep Duration and Health Care Expenditures in the United States. Med. Care 2020, 58, 770–777.
- Centers for Disease Control and Prevention. Sleep and Sleep Disorders. 2018. Available online: https://www.cdc.gov/sleep/index.html (accessed on 13 September 2021).
- Gupta, C.C.; Duncan, M.J.; Ferguson, S.A.; Rebar, A.; Sprajcer, M.; Khalesi, S.; Booker, L.A.; Binks, H.; Vincent, G.E. The Discrepancy between Knowledge of Sleep Recommendations and the Actual Sleep Behaviour of Australian Adults. Behav. Sleep Med. 2021, 19, 828–839.
- Chapman, J.; Naweed, A.; Wilson, C.; Dorrian, J. Sleep for heart health: Investigating the relationship between work day sleep, days off sleep, and cardiovascular risk in Australian train drivers. Ind. Health 2019, 6.
- Grandner, M.A.; Patel, N.P.; Gehrman, P.R.; Perlis, M.L.; Pack, A.I. Problems associated with short sleep: Bridging the gap between laboratory and epidemiological studies. Sleep Med. Rev. 2010, 14, 239–247.
- Leproult, R.; Deliens, G.; Gilson, M.; Peigneux, P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep 2015, 38, 707–715.
- Van Cauter, E.; Polonsky, K.S.; Scheen, A.J. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr. Rev. 1997, 18, 716–738.
- Dettoni, J.L.; Consolim-Colombo, F.M.; Drager, L.F.; Rubira, M.C.; Cavasin de Souza, S.B.; Irigoyen, M.C.; Mostarda, C.; Borile, S.; Krieger, E.M.; Moreno, H., Jr.; et al. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J. Appl. Physiol. 2012, 113, 232–236.
- Robillard, R.; Lanfranchi, P.A.; Prince, F.; Filipini, D.; Carrier, J. Sleep deprivation increases blood pressure in healthy normotensive elderly and attenuates the blood pressure response to orthostatic challenge. Sleep 2011, 34, 335.
- van Leeuwen, W.M.; Lehto, M.; Karisola, P.; Lindholm, H.; Luukkonen, R.; Sallinen, M.; Härmä, M.; Porkka-Heiskanen, T.; Alenius, H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS ONE 2009, 4, e4589.
- Patel, S.R.; Malhotra, A.; Gottlieb, D.J.; White, D.P.; Hu, F.B. Correlates of long sleep duration. Sleep 2006, 29, 881–889.
- Pizinger, T.M.; Aggarwal, B.; St-Onge, M.-P. Sleep extension in short sleepers: An evaluation of feasibility and effectiveness for weight management and cardiometabolic disease prevention. Front. Endocrinol. 2018, 9, 392.
- Gottlieb, D.J.; Redline, S.; Nieto, F.J.; Baldwin, C.M.; Newman, A.B.; Resnick, H.E.; Punjabi, N.M. Association of usual sleep duration with hypertension: The Sleep Heart Health Study. Sleep 2006, 29, 1009–1014.
- Choi, K.; Lee, J.; Park, H.; Baik, S.; Choi, D.; Kim, S. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int. J. Obes. 2008, 32, 1091–1097.
- Aziz, M.; Ali, S.S.; Das, S.; Younus, A.; Malik, R.; Latif, M.A.; Humayun, C.; Anugula, D.; Abbas, G.; Salami, J. Association of subjective and objective sleep duration as well as sleep quality with non-invasive markers of sub-clinical cardiovascular disease (CVD): A systematic review. J. Atheroscler. Thromb. 2017, 24, 208–226.
- Kuhn, G. Circadian rhythm, shift work, and emergency medicine. Ann. Emerg. Med. 2001, 37, 88–98.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
718
Revisions:
2 times
(View History)
Update Date:
09 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




