Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cardiovascular disease (CVD) poses a serious health and economic burden worldwide. Modifiable lifestyle factors are a focus of research into reducing the burden of CVD, with diet as one of the most investigated factors. Specifically, the timing and regularity of food intake is an emerging research area, with approaches such as time-restricted eating (TRE) receiving much attention.
- chrono-nutrition
- meal timing
- eating habits
- metabolic health
- cardiovascular
- sleep timing
1. Introduction: Cardiometabolic Risk and Chronobiology
Cardiovascular disease (CVD) is an umbrella term used to describe medical conditions that affect the heart, blood vessels, and cardiometabolic health [1,2]. CVD is the leading cause of death globally [3,4] and, importantly, is largely preventable [5]. Modifiable lifestyle factors, including sub-optimal diet, physical inactivity, excessive alcohol consumption, and smoking, account for up to 90% of the risk factors associated with CVD [2].
The Heart Foundation Australia [2] emphasises a need to focus on modifiable lifestyle factors as part of future research into reducing the burden of CVD. When investigating how modifiable lifestyle factors influence CVD, research to date has focused on factors such as diet, physical activity, smoking, and alcohol consumption [1,5,6,7]. However, as the rates of CVD continue to grow [2,4], the focus of research has widened to include other lifestyle factors that could also be important contributors to CVD risk, such as sleep [8,9,10]. Attention has also turned to exploring possible interactions between lifestyle factors to identify novel approaches to reducing CVD risk. While sub-optimal diet is a well-established risk factor for CVD [11,12,13], a new focus is emerging on the timing and regularity of food intake [14,15,16]. Given that the timing and regularity of sleep is also a consideration in relation to CVD risk factors [8,17], the next step is to explore the timing of both sleep and food intake, alongside our expanding understanding of biological timing systems.
Identifying the mechanisms underlying CVD is integral to reducing the burden of disease [18,19]. Disruption to our circadian rhythms is one such mechanism that has received scientific attention, with several key reviews demonstrating the relationship between circadian disruption and cardiometabolic health [9,18,20,21]. Circadian rhythms are biological and behavioural rhythms with a period of approximately 24 h [22,23]. While it is well-established that the circadian system, controlled by a central clock located in the suprachiasmatic nucleus in the brain, is influenced by light [24], more recent evidence demonstrates that peripheral clocks throughout our body are influenced by other external behaviours, such as meal timing [25,26]. Furthermore, while sleep timing affects light exposure and therefore the central clock, the timing of sleep has also been linked to the timing of peripheral clocks [18].
Optimal functioning of the circadian system is essential for good health [27]. Circadian disruption occurs when our sleep–wake and eating–fasting rhythms are not appropriately timed; that is, they do not align with our light–dark cycle [18,28]. One of the leading causes of circadian disruption is working non-standard hours including shift work [9]. Shift work disrupts the usual sleep–wake cycle as workers may be on shift during times when the body is primed to be sleeping (i.e., at night), attempting sleep when the body is primed to be awake (i.e., during the day), or woken during a sleep period when working an on-call schedule [29,30]. This leads to inadequate sleep in shift work populations [31,32], contributing to circadian disruption, which is recognised as a major contributor to CVD risk in shift workers [9]. As a result of circadian disruption, natural physiological processes, such as metabolism, digestion, energy expenditure, and blood pressure, are misaligned [9,20,23,24,26,33,34,35,36,37,38,39]. This misalignment is proposed to play a critical role in the development of long-term health problems, such as CVD [1,9].
Eating at a time when our body is not primed to digest food is a challenge to multiple physiological systems, including the circadian system [14]. The typical eating window (the time from first to last time of energy consumption across the day) spans 14 h of the day in healthy, synchronised individuals [40,41]. Spreading eating events across the day (i.e., beyond the 14 h eating window) to include eating late at night is associated with weight gain and increased insulin resistance [42], which are two markers of CVD. Importantly, the typical eating window may be longer in obese populations [43]. In response to these novel findings, time-restricted eating (TRE), whereby the eating window is shortened [44], has been proposed to manage weight and cardiometabolic health. Indeed, several recent studies investigating TRE and cardiometabolic health [14,45,46,47,48,49,50,51] have demonstrated improved cardiometabolic outcomes with TRE, including reductions in systolic and diastolic blood pressure, reductions in fat mass, and improved insulin sensitivity [50,51,52]. While some of these studies measured sleep as an outcome after a TRE intervention [53], they did not consider sleep as a predictor of cardiometabolic health outcomes and therefore missed the opportunity to control for or to consider the impact of chronic inadequate sleep. This is potentially problematic as circadian disruption resulting from inadequate sleep may independently influence the same cardiovascular outcomes influenced by TRE [8,54]. Furthermore, there is a known relationship between food intake and sleep [55,56,57] such that inadequate sleep can lead to altered meal timing [55,58] and increased cravings for certain foods [59,60], and different foods and nutrients can impact sleep quality [61,62]. These interactions highlight the need to better understand the relationships between eating patterns, such as TRE, sleep, circadian misalignment, and cardiometabolic health.
2. Time-Restricted Eating, Circadian Disruption, and Cardiometabolic Health
Eating habits are a well-researched lifestyle factor contributing to CVD [63]. Poor eating habits, such as poor diet quality, have been linked to key cardiometabolic risk factors for CVD [11,12,64,65], including increased blood pressure, body mass index, serum lipids, total cholesterol, and low-density lipoprotein cholesterol [12,66,67]. As such, improving eating habits is a critical part of reducing CVD risk [13,68]. However, it has become apparent that the timing of food intake is also an important consideration [38], primarily due to the burden of altered eating patterns on the circadian system [14].
Eating at times when our body is not primed to digest food (e.g., at night [16]) can compromise metabolism and thus lead to an increase in the likelihood of developing CVD [38,44]. Indeed, observational studies have reported a relationship between irregular eating patterns and increased risk for metabolic syndrome [69,70,71], in addition to a relationship between habitual night eating and arterial stiffness, a preclinical sign of CVD [72]. Altered eating patterns are characteristic of shift work [55,73,74,75], with shift workers reporting that they change the timing of eating to accommodate their shift schedule; for example, eating during the night when working night shifts [55]. Misalignment of eating rhythms with the internal circadian system is thought to contribute to the relationship between shift work and CVD [73]. Research in non-shift workers has shown that erratic eating patterns are associated with increased CVD risk after controlling for dietary composition [76]; thus, highlighting that in addition to ‘what is eaten’, ‘when eating occurs’ also influences CVD risk. Collectively, recent research highlights the need to optimise eating time to reduce circadian disruption and consequently reduce the prevalence of CVD.
As previously discussed, TRE is a unique strategy that has gained popularity as a way to optimise the timing of food intake [14,16,38]. While typical eating windows for most individuals span 14 h of the day, a TRE approach shortens the eating window to between 4 and 10 h [14,77] (Figure 1, example 8 h eating window). It is important to note that the optimal timing of a shortened eating window (i.e., starting the eating window early in the day or later in the day) requires systematic study [44]. In a recent review by Regmi and Heilbronn [78], the authors argued that while the early morning (e.g., Figure 1, middle panel) may be considered an optimal time to start the eating window for maximal metabolic benefits (e.g., improving insulin sensitivity and lipid absorption), this would mean that people would miss eating dinner at a traditional time (6–8 pm), which is a typical family and group eating time [79]. This may therefore present social challenges. In contrast, the same 8 h eating window could start at 12 pm and include the typical dinner time (e.g., Figure 1, right panel). While this arrangement extends the overnight fast to the same degree and may be perceived to be less socially challenging, metabolic benefits may be reduced due to circadian considerations [78].
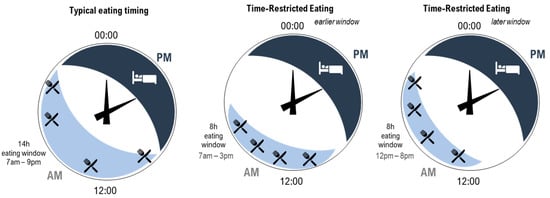
Figure 1. Illustration of three patterns of eating (light blue shading with knife and fork) and sleeping (dark blue shading with bed) across hours on 24 h clocks. Left—typical eating arrangement within a 14 h eating window starting at 7 am. Middle—time-restricted eating within an 8 h eating window starting at 7 am. Right—time-restricted eating within an 8 h eating window starting at 12 pm. All arrangements include the same number of eating occasions (indicated by knife and fork). For the time-restricted eating patterns, the time between the first and last eating occasion is shortened, increasing the length of the overnight fast.
TRE may lead to improved cardiovascular outcomes [16]. For example, a recent systematic review and meta-analysis of eleven studies found significantly lower fasting glucose values for participants on a TRE pattern (with eating windows ranging from 6 h to 12 h) compared to those eating ad libitum [50]. Other studies have similarly demonstrated improvements following TRE in blood pressure, body weight, cholesterol, glucose metabolism, and the gut microbiome [22,50,51,78,80,81]. Furthermore, TRE circumvents some of the challenges of typical dieting approaches, which often require individuals to employ restrictive behaviours, because the quality and quantity of the food eaten in TRE regimes does not change [77,82], as only the window of eating is altered.
In shortening the eating window, TRE reduces the amount of time the body is required to metabolise food and lengthens the daily fast period, arguably allowing for greater metabolic recovery [14,16,22,41,50]. As introduced in Figure 1, a further opportunity for improving cardiometabolic outcomes arises from the ability to consider the timing of the eating window to reduce circadian disruption. In people with typical diurnal rhythm, starting a shortened eating window early in the day avoids circadian misalignment of eating behaviours and related impairment in the way in which food is processed by the body [15,38]. Indeed, evidence suggests that an earlier eating window is associated with more effective cardiometabolic outcomes compared to a later eating window [14,16,41,77]. As previously discussed, circadian disruption plays a significant role in the development of CVD due to misaligned daily rhythms, such as metabolism, digestion, and blood pressure [1]. Since food acts as a signal for peripheral circadian clocks [16,18], eating food at biologically inappropriate times can lead to misalignment between central and peripheral clocks [83]. Therefore, carefully timed TRE may be an effective and relatively straightforward strategy to minimise circadian disruption [48] and ultimately contribute to a reduced burden of CVD [38,77]. However, while day interventions are the focus of much of the existing TRE literature [14,38,45,50,84], consideration of the impacts of practicing TRE at other times, such as during the night, has been less well-documented. Research in this area is of particular interest for night workers who typically eat during the night [55]. Recent laboratory research has demonstrated the beneficial effect for glucose metabolism of maintaining a daytime eating window even while working (simulated) night shifts [85,86,87,88]. To extend this research, additional consideration of TRE for those working night shifts is needed; in particular, this would determine the impact and feasibility of shortening the daytime eating window while supporting the need to sleep during the day (Figure 2).
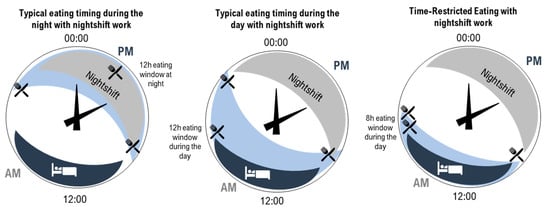
Figure 2. Illustration of three patterns of eating (light blue shading with knife and fork) and sleeping (dark blue shading with bed) on 24 h clocks. Left—typical eating arrangement for a night-shift worker with a 12 h eating window during the night. Middle—example of an eating arrangement for a night-shift worker with a 12 h eating window during the day. Right—time-restricted eating for a night-shift worker with an 8 h eating window during the day (right).
Consideration of sleep in the context of night work is critical. While TRE can be designed to reduce circadian disruption, one of the biggest contributors to circadian disruption is an altered sleep–wake cycle [23,26]. We hypothesise that TRE may not have the same benefits for cardiometabolic health if individuals are experiencing circadian disruption due to inadequate sleep.
3. Inadequate Sleep, Circadian Disruption, and Cardiometabolic Health
Inadequate sleep is highly prevalent globally, with adults commonly obtaining less than the optimal 7–9 h of sleep per night [89,90,91,92,93]. For example, up to one-third of Australians do not achieve 7 h of sleep per night [89,90]. This is problematic, as chronic inadequate sleep challenges the circadian system [19] and consequently impacts cardiometabolic health [1,10,19,94]. Several studies have demonstrated the link between short sleep (considered sleep of ≤6 h in duration [95]) and adverse cardiometabolic outcomes including obesity, hypertension, poor glucose regulation, and insulin resistance [19,95,96,97,98,99,100]. Importantly, extended sleep (i.e., sleep >9 h) is also associated with adverse cardiometabolic health effects [101,102], such as increased blood pressure [103,104], and this could be a bi-directional relationship, with long sleep a symptom of CVD [105]. This suggests a U-shape relationship between sleep and cardiometabolic health, with 7–9 h considered an optimum amount for favourable cardiometabolic outcomes [102]. Ensuring adequate sleep duration (i.e., 7–9 h per 24 h) is therefore a key strategy to reduce the risk of CVD.
Inadequate sleep is a common outcome of shift-work schedules [37]. Many shift workers, particularly those engaged in night work, experience some degree of circadian disruption [9,106]. This is thought to be a major contributor to the prevalence of cardiometabolic issues in shift workers, such as elevated post-prandial glucose levels, obesity, and CVD in the long-term [9,19].
This entry is adapted from the peer-reviewed paper 10.3390/nu14030420
This entry is offline, you can click here to edit this entry!
