Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chun Lv | + 752 word(s) | 752 | 2022-02-08 09:21:49 | | | |
| 2 | Conner Chen | Meta information modification | 752 | 2022-02-09 02:44:53 | | | | |
| 3 | Conner Chen | Meta information modification | 752 | 2022-02-11 07:45:57 | | | | |
| 4 | Conner Chen | Meta information modification | 752 | 2022-02-11 07:46:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lv, C. Cellulose Fiber. Encyclopedia. Available online: https://encyclopedia.pub/entry/19252 (accessed on 08 February 2026).
Lv C. Cellulose Fiber. Encyclopedia. Available at: https://encyclopedia.pub/entry/19252. Accessed February 08, 2026.
Lv, Chun. "Cellulose Fiber" Encyclopedia, https://encyclopedia.pub/entry/19252 (accessed February 08, 2026).
Lv, C. (2022, February 09). Cellulose Fiber. In Encyclopedia. https://encyclopedia.pub/entry/19252
Lv, Chun. "Cellulose Fiber." Encyclopedia. Web. 09 February, 2022.
Copy Citation
Cellulose Fiber (CF) is one of the most abundant natural resources in the world, and it is widely found in agricultural residues, such as rice straw, rice husk, maize straw, bagasse, wood shavings, wood chips, bamboo chips, etc. These agricultural residues are mainly composed of cellulose, hemicellulose, lignin, pectin, wax and some water-soluble materials. Cellulose is the most important component of CF, and its chemical formula is (C6H10O5)n.
cellulose fiber
1. Typical Properties of CFs
Cellulose Fiber (CF) is one of the most abundant natural resources in the world, and it is widely found in agricultural residues, such as rice straw, rice husk, maize straw, bagasse, wood shavings, wood chips, bamboo chips, etc. These agricultural residues are mainly composed of cellulose, hemicellulose, lignin, pectin, wax and some water-soluble materials. Cellulose is the most important component of CF, and its chemical formula is (C6H10O5)n. Cellulose is a macromolecular polysaccharide composed of glucose, which is a straight-chain polymer formed by linking countless D-glucopyranose anhydrides with β(1–4) glycosides, and its structure is regular and unbranched. Cellulose has a large number of hydroxyl groups on the molecular chain, which promote the formation of intramolecular and intermolecular hydrogen bonds [1]. CFs commonly used for geopolymer reinforcement include bast fibers, leaf fibers, stem fibers, etc. [2][3], as shown in Figure 1.
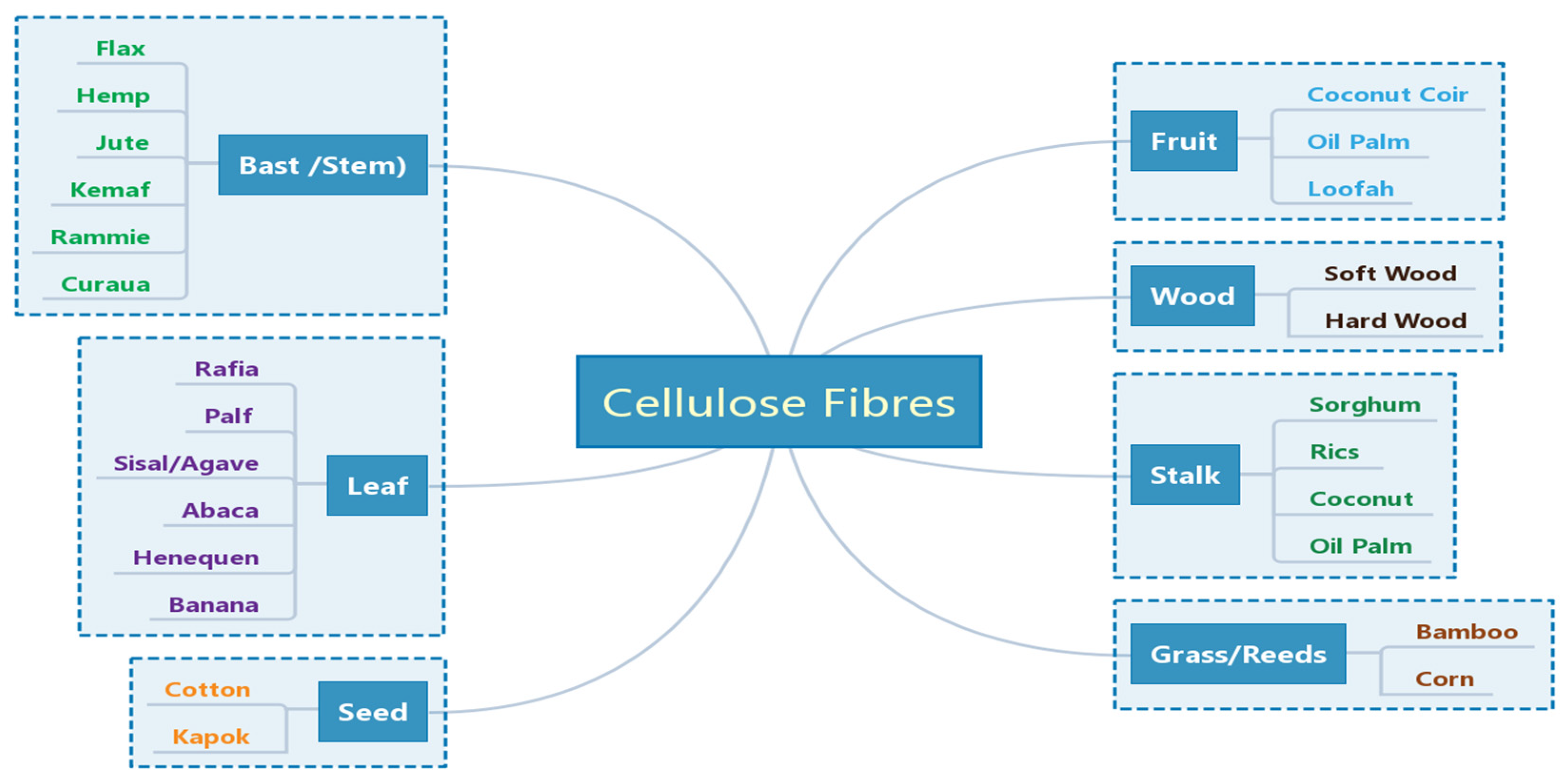
Figure 1. Classification of CFs used for reinforcement the geopolymers. Reprinted with permission from ref. [3]. Copyright 2020 Copyright MDPI.
It can be seen from Table 1 that the density of CFs is roughly similar, with little difference, between 1.1 and 1.6 g·cm−3. The density of bast fiber is basically about 1.5 g·cm−3, its tensile strength is relatively large, and the tensile strength of fruit coconut husk fiber is relatively small.
Table 1. Mechanical properties of typical fibers.
| Fiber Type | Fiber Name | Density/ (g cm−3) |
Tensile Strength/MPa | Specific Strength/(S ρ−1) | Tensile Modulus/GPa | Specific Modulus/(E ρ−1) | Elongation at Break/% | Ref. |
|---|---|---|---|---|---|---|---|---|
| Bast | Flax | 1.5 | 800–1500 | 535–1000 | 27.6–80 | 18.4–53 | 1.2–3.2 | [4] |
| Hemp | 1.48 | 550–900 | 372–608 | 70 | 47.3 | 2–4 | [5] | |
| Jute | 1.46 | 393–800 | 269–548 | 10–30 | 6.85–20.6 | 1.5–1.8 | [6] | |
| Kenaf | 1.45 | 930 | 641 | 53 | 36.55 | 1. 6 | [7] | |
| Ramie | 1.5 | 220–938 | 147–625 | 44–128 | 29.3–85 | 2–3.8 | [8] | |
| Leaf | Abaca | 1.5 | 400 | 267 | 12 | 8 | 3–10 | [9] |
| Sisal | 1.45 | 530–640 | 366–441 | 9.4–22 | 6.5–15.2 | 3–7 | [8] | |
| Banana Leaf | 1.35 | 600 | 444 | 17.85 | 13.2 | 3.36 | [8] | |
| Coconut leaf | 1.15 | 500 | 435 | 2. 5 | 2.17 | 20 | [10] | |
| Seed | cotton | 1.6 | 287–597 | 179–373 | 5.5–12.6 | 3.44–7.9 | 7–8 | [10] |
| Grass | bamboo | 1.1 | 500 | 454 | 35.91 | 32.6 | 1.4 | [10] |
| Fruit | Coconut shell | 1.2 | 175 | 146 | 4–6 | 3.3–5 | 30 | [8] |
| Wood | Soft wood | 1.5 | 1000 | 667 | 40 | 26.67 | 4.4 | [10] |
2. Fiber-Reinforced Geopolymer Composites
Geopolymers can be prepared in two ways: alkali excitation and acid excitation. According to the different active raw materials, alkali excitation methods are mainly divided into alkali-silicate glass body cementing materials and alkali-silicate mineral cementing materials. Alkali-silicate glass body cementing materials mainly use amorphous silicate glass bodies as raw materials, such as slag, fly ash, various metallurgical slags, coal gangue, etc., and the main raw alkali-silicate mineral cementing material is a crystalline mineral, such as clay, feldspar and other tailings.
2.1. The Polymerization Mechanism of Geopolymer
Under the condition of strong alkali, the silicon-oxygen bonds and aluminum-oxygen bonds of active materials such as kaolin are broken to form oligomers of polymer monomers, namely oligomeric silicon-oxygen tetrahedra and aluminum-oxygen tetrahedra. Under the same conditions, oligomeric silicon-oxygen tetrahedrons and aluminum-oxygen tetrahedrons are dehydrated and polymerized to form geopolymers with a three-dimensional network structure in space [11]. It is generally believed that the reaction of geopolymers can be divided into four processes: dissolution, diffusion, polymerization and solidification. Using metakaolin as the active material and (NaOH) or (KOH) as the alkali activator, the reaction mechanism of the resulting geopolymer is shown in Figure 2 [12][13].
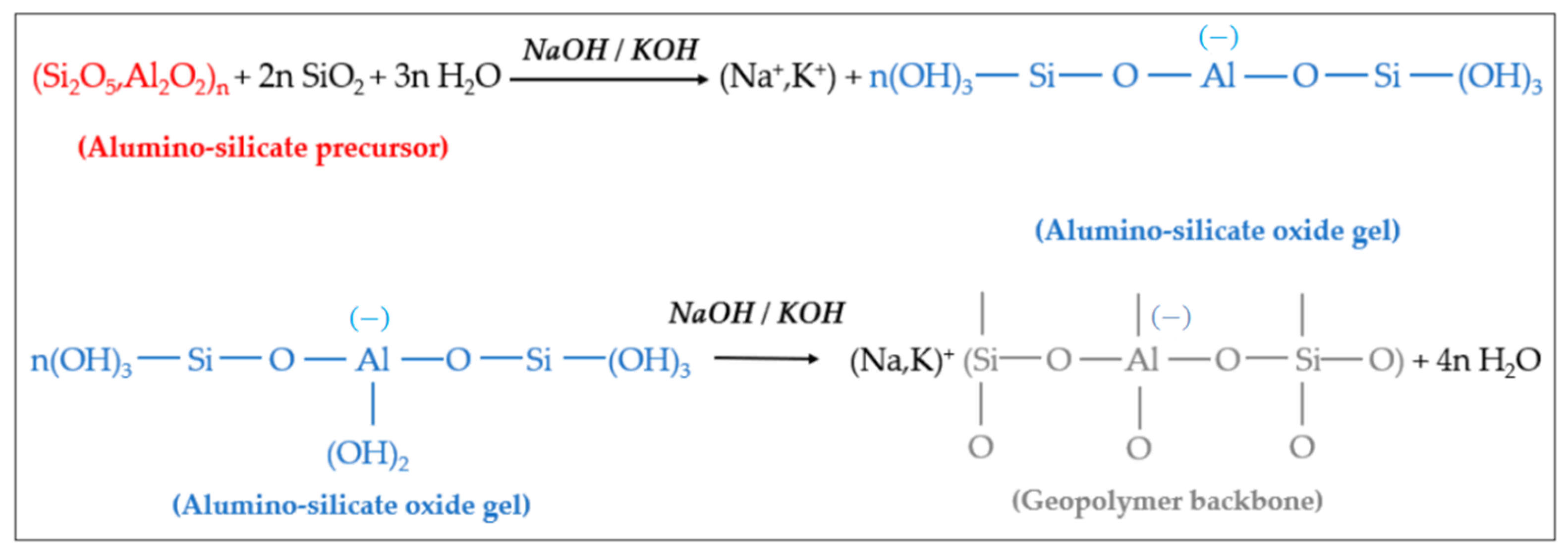
Figure 2. Hydrolysis and polycondensation of aluminum-silicate precursors and formation of geopolymers.
It can be seen from Figure 2 that the aluminosilicate raw materials (precursors) gradually dissolve in (NaOH) or (KOH) alkali activator, producing a large amount of silicon and aluminum monomers. These monomers gradually diffuse in the solution from the surface to the inside, and quickly undergo a polycondensation reaction to form silico−alumina oligomers. The oligomer gel phase solidifies and hardens to form geopolymers.
2.2. Fiber Matrix Interface Bonding Mechanisms
A geopolymer composite is composed of fiber and a matrix with different properties, and the interface between the fiber and matrix is formed. The interface of the composite includes the geometric surface of the matrix and the fiber in contact with each other and the transition area, which is an extremely complex microstructure. Adjusting the bonding state of the fiber and the matrix interface, and optimizing the characteristics of the interface layer between the fiber and the matrix can make the geopolymer composites achieve the best performance. Improving the interfacial adhesion between the fiber reinforcement and the matrix is the most critical factor in the interface control technology of composites. The bonding forms of the fiber and matrix interface generally include interdiffusion, electrostatic adhesion, chemical bonding and mechanical interlocking [13][14]. According to the microscopic morphology of the bonding of fibers and geopolymers, the interface bonding is usually mainly in the form of mechanical interlocking.
References
- Liu, J.; Lv, C. Research progress on durability of cellulose fiber-reinforced cement-based composites. Int. J. Polym. Sci. 2021, 2021, 1014531.
- Yan, L.; Kasal, B.; Huang, L. A review of recent research on the use of cellulosic fibres, their fibre fabric reinforced cementitious, geo-polymer and polymer composites in civil engineering. Compos. Part B Eng. 2016, 92, 94–132.
- Camargo, M.M.; Taye, E.A.; Roether, J.A.; Redda, D.T.; Boccaccini, A.R. A review on natural fiber-reinforced geopolymer and cement-based composites. Materials 2020, 13, 4603.
- Bos, H.L.; Oever, M.; Peters, O. Tensile and compressive properties of flax fibres for natural fibre reinforced composites. J. Mater. Sci. 2002, 37, 1683–1692.
- Pickering, K.L.; Beckermann, G.W.; Alam, S.N.; Foreman, N.J. Optimising industrial hemp fibre for composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 461.
- Summerscales, J.; Dissanayake, N.; Virk, A.S.; Hall, W. A review of bast fibres and their composites. Part1-fibres as reinforcements. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1329–1335.
- Mustafa, A.; Abdollah, M.; Shuhimi, F.F.; Ismail, N.; Amiruddin, H.; Umehara, N. Selection and verification of kenaf fibres as an alternative friction material using weighted decision matrix method. Mater. Des. 2015, 67, 577–582.
- Kumar, R.; Obrai, S.; Sharma, A. Chemical modifications of natural fiber for composite material. Chem. Sin. 2011, 2, 219–228.
- Haque, M.; Rahman, R.; Islam, N.; Huque, M.; Hasan, M. Mechanical properties of polypropylene composites reinforced with chemically treated coir and abaca fiber. J. Reinf. Plast. Compos. 2010, 29, 2253–2261.
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Chopped glass and recycled newspaper as reinforcement fibers in injection molded poly (lactic acid) (pla) composites: A comparative study. Compos. Sci. Technol. 2015, 66, 1813–1824.
- Arioza, E.; Ariozb, O.; Mete Kockar, O. An Experimental Study on the Mechanical and Microstructural Properties of Geopolymers. Procedia Eng. 2012, 42, 100–105.
- Sambucci, M.; Sibai, A.; Valente, M. Recent advances in geopolymer technology. A potential eco-friendly solution in the construction materials industry: A review. J. Compos. Sci. 2021, 5, 109.
- Duxson, P.; Lukey, G.C.; Separovic, F.; van Deventer, J.S.J. Effect of alkali cations on aluminum incorporation in geopolymeric gels. Ind. Eng. Chem. Res. 2005, 44, 832–839.
- Rao, J.; Zhou, Y.; Fan, M. Revealing the interface structure and bonding mechanism of coupling agent treated WPC. Polymers 2018, 10, 266.
More
Information
Subjects:
Materials Science, Textiles
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
4 times
(View History)
Update Date:
11 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




