Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Textiles
Cellulose Fiber (CF) is one of the most abundant natural resources in the world, and it is widely found in agricultural residues, such as rice straw, rice husk, maize straw, bagasse, wood shavings, wood chips, bamboo chips, etc. These agricultural residues are mainly composed of cellulose, hemicellulose, lignin, pectin, wax and some water-soluble materials. Cellulose is the most important component of CF, and its chemical formula is (C6H10O5)n.
- cellulose fiber
1. Typical Properties of CFs
Cellulose Fiber (CF) is one of the most abundant natural resources in the world, and it is widely found in agricultural residues, such as rice straw, rice husk, maize straw, bagasse, wood shavings, wood chips, bamboo chips, etc. These agricultural residues are mainly composed of cellulose, hemicellulose, lignin, pectin, wax and some water-soluble materials. Cellulose is the most important component of CF, and its chemical formula is (C6H10O5)n. Cellulose is a macromolecular polysaccharide composed of glucose, which is a straight-chain polymer formed by linking countless D-glucopyranose anhydrides with β(1–4) glycosides, and its structure is regular and unbranched. Cellulose has a large number of hydroxyl groups on the molecular chain, which promote the formation of intramolecular and intermolecular hydrogen bonds [31]. CFs commonly used for geopolymer reinforcement include bast fibers, leaf fibers, stem fibers, etc. [35,36], as shown in Figure 1.
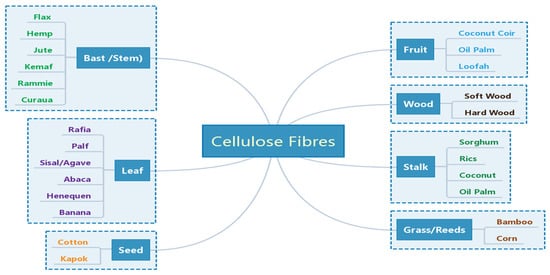
Figure 1. Classification of CFs used for reinforcement the geopolymers. Reprinted with permission from ref. [36]. Copyright 2020 Copyright MDPI.
It can be seen from Table 1 that the density of CFs is roughly similar, with little difference, between 1.1 and 1.6 g·cm−3. The density of bast fiber is basically about 1.5 g·cm−3, its tensile strength is relatively large, and the tensile strength of fruit coconut husk fiber is relatively small.
Table 1. Mechanical properties of typical fibers.
| Fiber Type | Fiber Name | Density/ (g cm−3) |
Tensile Strength/MPa | Specific Strength/(S ρ−1) | Tensile Modulus/GPa | Specific Modulus/(E ρ−1) | Elongation at Break/% | Ref. |
|---|---|---|---|---|---|---|---|---|
| Bast | Flax | 1.5 | 800–1500 | 535–1000 | 27.6–80 | 18.4–53 | 1.2–3.2 | [37] |
| Hemp | 1.48 | 550–900 | 372–608 | 70 | 47.3 | 2–4 | [38] | |
| Jute | 1.46 | 393–800 | 269–548 | 10–30 | 6.85–20.6 | 1.5–1.8 | [39] | |
| Kenaf | 1.45 | 930 | 641 | 53 | 36.55 | 1. 6 | [40] | |
| Ramie | 1.5 | 220–938 | 147–625 | 44–128 | 29.3–85 | 2–3.8 | [41] | |
| Leaf | Abaca | 1.5 | 400 | 267 | 12 | 8 | 3–10 | [42] |
| Sisal | 1.45 | 530–640 | 366–441 | 9.4–22 | 6.5–15.2 | 3–7 | [41] | |
| Banana Leaf | 1.35 | 600 | 444 | 17.85 | 13.2 | 3.36 | [41] | |
| Coconut leaf | 1.15 | 500 | 435 | 2. 5 | 2.17 | 20 | [43] | |
| Seed | cotton | 1.6 | 287–597 | 179–373 | 5.5–12.6 | 3.44–7.9 | 7–8 | [43] |
| Grass | bamboo | 1.1 | 500 | 454 | 35.91 | 32.6 | 1.4 | [43] |
| Fruit | Coconut shell | 1.2 | 175 | 146 | 4–6 | 3.3–5 | 30 | [41] |
| Wood | Soft wood | 1.5 | 1000 | 667 | 40 | 26.67 | 4.4 | [43] |
2. Fiber-Reinforced Geopolymer Composites
Geopolymers can be prepared in two ways: alkali excitation and acid excitation. According to the different active raw materials, alkali excitation methods are mainly divided into alkali-silicate glass body cementing materials and alkali-silicate mineral cementing materials. Alkali-silicate glass body cementing materials mainly use amorphous silicate glass bodies as raw materials, such as slag, fly ash, various metallurgical slags, coal gangue, etc., and the main raw alkali-silicate mineral cementing material is a crystalline mineral, such as clay, feldspar and other tailings.
2.1. The Polymerization Mechanism of Geopolymer
Under the condition of strong alkali, the silicon-oxygen bonds and aluminum-oxygen bonds of active materials such as kaolin are broken to form oligomers of polymer monomers, namely oligomeric silicon-oxygen tetrahedra and aluminum-oxygen tetrahedra. Under the same conditions, oligomeric silicon-oxygen tetrahedrons and aluminum-oxygen tetrahedrons are dehydrated and polymerized to form geopolymers with a three-dimensional network structure in space [44]. It is generally believed that the reaction of geopolymers can be divided into four processes: dissolution, diffusion, polymerization and solidification. Using metakaolin as the active material and (NaOH) or (KOH) as the alkali activator, the reaction mechanism of the resulting geopolymer is shown in Figure 2 [45,46].
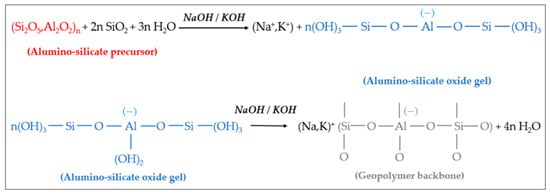
Figure 2. Hydrolysis and polycondensation of aluminum-silicate precursors and formation of geopolymers.
It can be seen from Figure 2 that the aluminosilicate raw materials (precursors) gradually dissolve in (NaOH) or (KOH) alkali activator, producing a large amount of silicon and aluminum monomers. These monomers gradually diffuse in the solution from the surface to the inside, and quickly undergo a polycondensation reaction to form silico−alumina oligomers. The oligomer gel phase solidifies and hardens to form geopolymers.
2.2. Fiber Matrix Interface Bonding Mechanisms
A geopolymer composite is composed of fiber and a matrix with different properties, and the interface between the fiber and matrix is formed. The interface of the composite includes the geometric surface of the matrix and the fiber in contact with each other and the transition area, which is an extremely complex microstructure. Adjusting the bonding state of the fiber and the matrix interface, and optimizing the characteristics of the interface layer between the fiber and the matrix can make the geopolymer composites achieve the best performance. Improving the interfacial adhesion between the fiber reinforcement and the matrix is the most critical factor in the interface control technology of composites. The bonding forms of the fiber and matrix interface generally include interdiffusion, electrostatic adhesion, chemical bonding and mechanical interlocking [46,47]. According to the microscopic morphology of the bonding of fibers and geopolymers, the interface bonding is usually mainly in the form of mechanical interlocking.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27030796
This entry is offline, you can click here to edit this entry!
