
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aloys HITABATUMA | + 6138 word(s) | 6138 | 2022-02-08 04:56:01 | | | |
| 2 | Amina Yu | -134 word(s) | 6004 | 2022-02-09 05:09:44 | | |
Video Upload Options
Metal-organic frameworks (MOFs) are a class of crystalline porous materials systems structured by using metals linked together by organic bridging ligands.
1. metal-organic frameworks (MOFs) -Based Sensors for Food Safety
Over the past decades, there have been many studies and reports on metal-organic frameworks (MOF) synthesis for food safety analysis (Figure 1), encompassing MOF designing, various synthesis methods, and post-synthetic modification, as well as incorporation of biomolecules into MOF [1][2]. Isoreticular expansion, topology-guided design, and modulated synthesis are the most reported methods for MOF synthesis. While, four main classes of post-synthetic modification include covalent post-synthetic modification, post-synthetic metalation modification, dative post-synthetic modification, post-synthetic exchange, and post-synthetic deprotection, and have been reported as tools to overcome different barriers for the application of MOF in food safety analysis [3][4][5]. Besides, incorporation of biomolecules into MOFs has been used as a new strategy to improve MOF efficiency in selectivity, sensitivity, signal amplification, and MOF stability [2]. To accommodate the drawbacks of the lower framework stability of MOFs, carboxylate-based linkers and N-heterocyclic based linkers have been used [6][7].
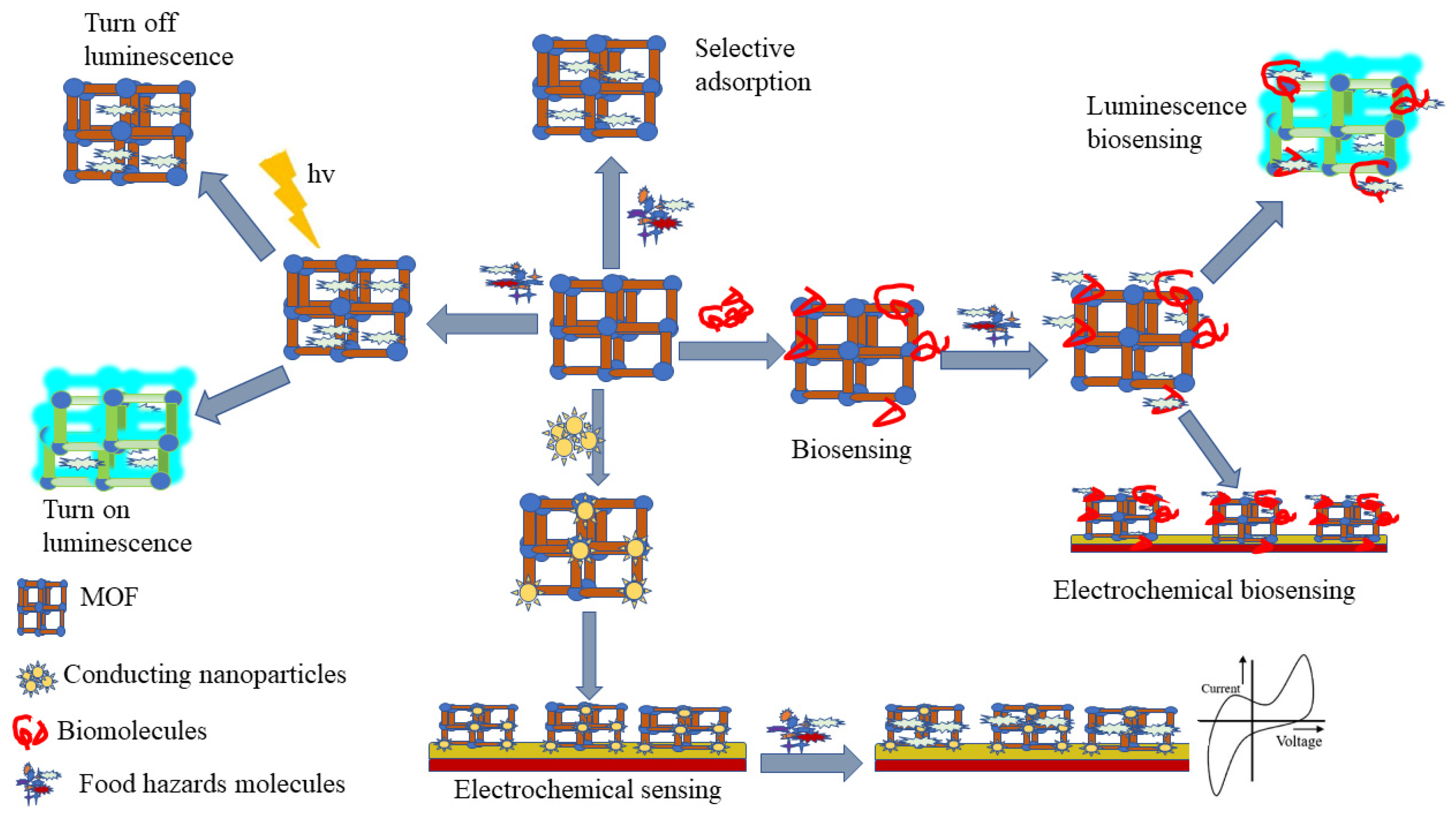
1.1. MOF-Based Electrochemical-Sensing Method
1.2. MOF-Based Chemical Sensing Method
1.3. MOF-Based Biosensing Method
1.4. MOF-Based SERS Sensing Method
2. Use of MOF-Based Sensors for Food Safety Analysis
2.1. Detection of Pathogenic Bacteria
2.2. Detection of Heavy Metals
2.3. Detection of Illegal Food Additives
2.4. Detection of Natural Toxins in Food
2.5. Detection of Drug and Pesticide Residues
2.6. Persistent Organic Pollutants (POPs)
References
- Basaleh, A.; Sheta, S. Manganese metal–organic framework: Chemical stability, photoluminescence studies, and biosensing application. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1726–1737.
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of biomolecules in Metal-Organic Frameworks for advanced applications. Coord. Chem. Rev. 2019, 384, 90–106.
- Wang, P.-L.; Xie, L.-H.; Joseph, E.A.; Li, J.-R.; Su, X.-O.; Zhou, H.-C. Metal–organic frameworks for food safety. Chem. Rev. 2019, 119, 10638–10690.
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent advances in post-synthetic modification of metal–organic frameworks: New types and tandem reactions. Coord. Chem. Rev. 2019, 378, 500–512.
- Xu, W.-Q.; He, S.; Liu, S.-J.; Liu, X.-H.; Qiu, Y.-X.; Liu, W.-T.; Liu, X.-J.; Jiang, L.-C.; Jiang, J.-J. Post-synthetic modification of a metal-organic framework based on 5-aminoisophthalic acid for mercury sorption. Inorg. Chem. Commun. 2019, 108, 107515.
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279.
- Elsabawy, K.M.; Fallatah, A.M. Microwave assisted synthesis and molecular structure visualization of ultrahigh surface area Ni-6,6′-dibromo-indigo coordinated polymeric MOFs stabilized via hydrogen bonding. Inorg. Chem. Commun. 2018, 92, 78–83.
- Kogikoski, S.; Paschoalino, W.J.; Cantelli, L.; Silva, W.; Kubota, L.T. Electrochemical sensing based on DNA nanotechnology. TrAC Trends Anal. Chem. 2019, 118, 597–605.
- Dai, Z.; Liu, H.; Shen, Y.; Su, X.; Xu, Z.; Sun, Y.; Zou, X. Attomolar determination of coumaphos by electrochemical displacement immunoassay coupled with oligonucleotide sensing. Anal. Chem. 2012, 84, 8157–8163.
- Zapp, E.; Brondani, D.; Vieira, I.C.; Scheeren, C.W.; Dupont, J.; Barbosa, A.M.J.; Ferreira, V.S. Biomonitoring of methomyl pesticide by laccase inhibition on sensor containing platinum nanoparticles in ionic liquid phase supported in montmorillonite. Sens. Actuators B Chem. 2011, 155, 331–339.
- Kumar, P.; Kim, K.-H.; Vellingiri, K.; Samaddar, P.; Kumar, P.; Deep, A.; Kumar, N. Hybrid porous thin films: Opportunities and challenges for sensing applications. Biosens. Bioelectron. 2018, 104, 120–137.
- Kumar, P.; Deep, A.; Kim, K.-H. Metal organic frameworks for sensing applications. TrAC Trends Anal. Chem. 2015, 73, 39–53.
- Nasalevich, M.A.; van der Veen, M.; Kapteijn, F.; Gascon, J. Metal–organic frameworks as heterogeneous photocatalysts: Advantages and challenges. CrystEngComm 2014, 16, 4919–4926.
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically conductive porous metal–organic frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579.
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science 2014, 343, 66–69.
- Nesakumar, N.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Electrochemical acetylcholinesterase biosensor based on ZnO nanocuboids modified platinum electrode for the detection of carbosulfan in rice. Biosens. Bioelectron. 2016, 77, 1070–1077.
- Narayan, T.C.; Miyakai, T.; Seki, S.; Dincă, M. High charge mobility in a tetrathiafulvalene-based microporous metal–organic framework. J. Am. Chem. Soc. 2012, 134, 12932–12935.
- Cui, P.; Wang, P.; Zhao, Y.; Sun, W.-Y. Fabrication of desired metal–organic frameworks via postsynthetic exchange and sequential linker installation. Cryst. Growth Des. 2019, 19, 1454–1470.
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352.
- Diamantis, S.A.; Margariti, A.; Pournara, A.D.; Papaefstathiou, G.S.; Manos, M.J.; Lazarides, T. Luminescent metal–organic frameworks as chemical sensors: Common pitfalls and proposed best practices. Inorg. Chem. Front. 2018, 5, 1493–1511.
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367.
- Cheng, W.; Tang, X.; Zhang, Y.; Wu, D.; Yang, W. Applications of metal-organic framework (MOF)-based sensors for food safety: Enhancing mechanisms and recent advances. Trends Food Sci. Technol. 2021, 112, 268–282.
- Liu, B. Metal–organic framework-based devices: Separation and sensors. J. Mater. Chem. 2012, 22, 10094–10101.
- Lei, J.; Qian, R.; Ling, P.; Cui, L.; Ju, H. Design and sensing applications of metal–organic framework composites. TrAC Trends Anal. Chem. 2014, 58, 71–78.
- Chen, L.; Luque, R.; Li, Y. Controllable design of tunable nanostructures inside metal-organic frameworks. Chem. Soc. Rev. 2017, 46, 4614–4630.
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev 2012, 112, 1126–1162.
- Evans, J.D.; Sumby, C.J.; Doonan, C.J. Post-synthetic metalation of metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5933–5951.
- Gao, S.; Zhao, L.; Han, L.; Zhang, Z.; Zhao, H. Synthesis, structure and characterization of two solvatochromic metal–organic frameworks for chemical-sensing applications. CrystEngComm 2018, 20, 2237–2240.
- He, Y.; Li, B.; O’Keeffe, M.; Chen, B. Multifunctional metal-organic frameworks constructed from meta-benzenedicarboxylate units. Chem. Soc. Rev. 2014, 43, 5618–5656.
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356.
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249.
- Khadem, M.; Faridbod, F.; Norouzi, P.; Foroushani, A.R.; Ganjali, M.R.; Shahtaheri, S.J. Biomimetic electrochemical sensor based on molecularly imprinted polymer for dicloran pesticide determination in biological and environmental samples. J. Iran. Chem. Soc. 2016, 13, 2077–2084.
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401.
- Li, B.; Wen, H.-M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal–Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860.
- Nandasiri, M.I.; Jambovane, S.R.; McGrail, B.P.; Schaef, H.T.; Nune, S.K. Adsorption, separation, and catalytic properties of densified metal-organic frameworks. Coord. Chem. Rev. 2016, 311, 38–52.
- Lykourinou, V.; Chen, Y.; Wang, X.-S.; Meng, L.; Hoang, T.; Ming, L.-J.; Musselman, R.L.; Ma, S. Immobilization of MP-11 into a mesoporous metal–organic framework, : A new platform for enzymatic catalysis. J. Am. Chem. Soc. 2011, 133, 10382–10385.
- Wang, C.; Gao, J.; Tan, H. Integrated antibody with catalytic metal–organic framework for colorimetric immunoassay. ACS Appl. Mater. Interfaces 2018, 10, 25113–25120.
- Bhardwaj, S.K.; Bhardwaj, N.; Mohanta, G.C.; Kumar, P.; Sharma, A.L.; Kim, K.-H.; Deep, A. Immunosensing of atrazine with antibody-functionalized Cu-MOF conducting thin films. ACS Appl. Mater. Interfaces 2015, 7, 26124–26130.
- Hintz, H.; Wuttke, S. Postsynthetic modification of an amino-tagged MOF using peptide coupling reagents: A comparative study. Chem. Commun. 2014, 50, 11472–11475.
- Ikezoe, Y.; Fang, J.; Wasik, T.L.; Shi, M.; Uemura, T.; Kitagawa, S.; Matsui, H. Peptide–metal organic framework swimmers that direct the motion toward chemical targets. Nano Lett. 2015, 15, 4019–4023.
- Zhang, H.-T.; Zhang, J.-W.; Huang, G.; Du, Z.-Y.; Jiang, H.-L. An amine-functionalized metal–organic framework as a sensing platform for DNA detection. Chem. Commun. 2014, 50, 12069–12072.
- Patra, S.; Hidalgo Crespo, T.; Permyakova, A.; Sicard, C.; Serre, C.; Chaussé, A.; Steunou, N.; Legrand, L. Design of metal organic framework–enzyme based bioelectrodes as a novel and highly sensitive biosensing platform. J. Mater. Chem. B 2015, 3, 8983–8992.
- Kempahanumakkagari, S.; Kumar, V.; Samaddar, P.; Kumar, P.; Ramakrishnappa, T.; Kim, K.-H. Biomolecule-embedded metal-organic frameworks as an innovative sensing platform. Biotechnol. Adv. 2018, 36, 467–481.
- Bhardwaj, N.; Bhardwaj, S.K.; Mehta, J.; Nayak, M.K.; Deep, A. Bacteriophage conjugated IRMOF-3 as a novel opto-sensor for S. arlettae. New J. Chem. 2016, 40, 8068–8073.
- Kumar, P.; Deep, A.; Paul, A.K.; Bharadwaj, L.M. Bioconjugation of MOF-5 for molecular sensing. J. Porous Mater. 2014, 21, 99–104.
- Kumar, P.; Kumar, P.; Bharadwaj, L.M.; Paul, A.K.; Deep, A. Luminescent nanocrystal metal organic framework based biosensor for molecular recognition. Inorg. Chem. Commun. 2014, 43, 114–117.
- Deep, A.; Bhardwaj, S.K.; Paul, A.K.; Kim, K.-H.; Kumar, P. Surface assembly of nano-metal organic framework on amine functionalized indium tin oxide substrate for impedimetric sensing of parathion. Biosens. Bioelectron. 2015, 65, 226–231.
- Zhang, T.; Zeng, L.; Han, L.; Li, T.; Zheng, C.; Wei, M.; Liu, A. Ultrasensitive electrochemical sensor for p-nitrophenyl organophosphates based on ordered mesoporous carbons at low potential without deoxygenization. Anal. Chim. Acta 2014, 822, 23–29.
- Yuan, J.; Tao, Z.; Yu, Y.; Ma, X.; Xia, Y.; Wang, L.; Wang, Z. A visual detection method for Salmonella Typhimurium based on aptamer recognition and nanogold labeling. Food Control 2014, 37, 188–192.
- Duan, N.; Gong, W.; Wu, S.; Wang, Z. An ssDNA library immobilized SELEX technique for selection of an aptamer against ractopamine. Anal. Chim. Acta 2017, 961, 100–105.
- Suh, S.H.; Dwivedi, H.P.; Choi, S.J.; Jaykus, L.A. Selection and characterization of DNA aptamers specific for Listeria species. Anal. Biochem. 2014, 459, 39–45.
- Liu, X.; Hu, M.; Wang, M.; Song, Y.; Zhou, N.; He, L.; Zhang, Z. Novel nanoarchitecture of Co-MOF-on-TPN-COF hybrid: Ultralowly sensitive bioplatform of electrochemical aptasensor toward ampicillin. Biosens. Bioelectron. 2019, 123, 59–68.
- Wang, S.; Li, Z.; Duan, F.; Hu, B.; He, L.; Wang, M.; Zhou, N.; Jia, Q.; Zhang, Z. Bimetallic cerium/copper organic framework-derived cerium and copper oxides embedded by mesoporous carbon: Label-free aptasensor for ultrasensitive tobramycin detection. Anal. Chim. Acta 2019, 1047, 150–162.
- Chen, M.; Gan, N.; Li, T.; Wang, Y.; Xu, Q.; Chen, Y. An electrochemical aptasensor for multiplex antibiotics detection using Y-shaped DNA-based metal ions encoded probes with NMOF substrate and CSRP target-triggered amplification strategy. Anal. Chim. Acta 2017, 968, 30–39.
- Li, Q.; Gong, S.; Zhang, H.; Huang, F.; Zhang, L.; Li, S. Tailored necklace-like core/shell heterostructure nanowires for high-performance plasmonic SERS detection. Chem. Eng. J. 2019, 371, 26–33.
- Hu, Y.; Liao, J.; Wang, D.; Li, G. Fabrication of gold nanoparticle-embedded metal–organic framework for highly sensitive surface-enhanced raman scattering detection. Anal. Chem. 2014, 86, 3955–3963.
- Liu, J.; White, I.; DeVoe, D.L. Nanoparticle-functionalized porous polymer monolith detection elements for surface-enhanced raman scattering. Anal. Chem. 2011, 83, 2119–2124.
- Zhou, X.; Liu, G.; Zhang, H.; Li, Y.; Cai, W. Porous zeolite imidazole framework-wrapped urchin-like Au-Ag nanocrystals for SERS detection of trace hexachlorocyclohexane pesticides via efficient enrichment. J. Hazard. Mater. 2019, 368, 429–435.
- Qian, K.; Liu, H.; Yang, L.; Liu, J. Functionalized shell-isolated nanoparticle-enhanced Raman spectroscopy for selective detection of trinitrotoluene. Analyst 2012, 137, 4644–4646.
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395.
- Barlow, S.M.; Boobis, A.R.; Bridges, J.; Cockburn, A.; Dekant, W.; Hepburn, P.; Houben, G.F.; König, J.; Nauta, M.J.; Schuermans, J.; et al. The role of hazard- and risk-based approaches in ensuring food safety. Trends Food Sci. Technol. 2015, 46, 176–188.
- Abbaspour, A.; Norouz-Sarvestani, F.; Noori, A.; Soltani, N. Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of staphylococcus aureus. Biosens. Bioelectron. 2015, 68, 149–155.
- Välimaa, A.-L.; Tilsala-Timisjärvi, A.; Virtanen, E. Rapid detection and identification methods for Listeria monocytogenes in the food chain—A review. Food Control 2015, 55, 103–114.
- Huang, Y.; Chen, X.; Duan, N.; Wu, S.; Wang, Z.; Wei, X.; Wang, Y. Selection and characterization of DNA aptamers against Staphylococcus aureus enterotoxin C1. Food Chem. 2015, 166, 623–629.
- Ma, X.; Xu, X.; Xia, Y.; Wang, Z. SERS aptasensor for Salmonella typhimurium detection based on spiny gold nanoparticles. Food Control 2018, 84, 232–237.
- Du, P.Y.; Gu, W.; Liu, X. Multifunctional three-dimensional europium metal-organic framework for luminescence sensing of benzaldehyde and Cu2+ and selective capture of dye molecules. Inorg Chem. 2016, 55, 7826–7828.
- Bhardwaj, N.; Bhardwaj, S.K.; Bhatt, D.; Tuteja, S.K.; Kim, K.-H.; Deep, A. Highly sensitive optical biosensing of Staphylococcus aureus with an antibody/metal–organic framework bioconjugate. Anal. Methods 2019, 11, 917–923.
- Singh, A.; Poshtiban, S.; Evoy, S. Recent advances in bacteriophage based biosensors for food-borne pathogen detection. Sensors 2013, 13, 1763–1786.
- Shabani, A.; Zourob, M.; Allain, B.; Marquette, C.A.; Lawrence, M.F.; Mandeville, R. Bacteriophage-modified microarrays for the direct impedimetric detection of bacteria. Anal. Chem. 2008, 80, 9475–9482.
- Shabani, A.; Marquette, C.A.; Mandeville, R.; Lawrence, M.F. Magnetically-assisted impedimetric detection of bacteria using phage-modified carbon microarrays. Talanta 2013, 116, 1047–1053.
- Raoof, J.-B.; Hosseini, S.R.; Ojani, R.; Mandegarzad, S. MOF-derived Cu/nanoporous carbon composite and its application for electro-catalysis of hydrogen evolution reaction. Energy 2015, 90, 1075–1081.
- Guo, S.; Zhu, Y.; Yan, Y.; Min, Y.; Fan, J.; Xu, Q.; Yun, H. (Metal-organic framework)-polyaniline sandwich structure composites as novel hybrid electrode materials for high-performance supercapacitor. J. Power Source 2016, 316, 176–182.
- Shahrokhian, S.; Ranjbar, S. Aptamer immobilization on amino-functionalized metal-organic frameworks: An ultrasensitive platform for the electrochemical diagnostic of Escherichia coli O157:H7. Analyst 2018, 143, 3191–3201.
- Zhang, Z.; Fu, X.; Li, K.; Liu, R.; Peng, D.; He, L.; Wang, M.; Zhang, H.; Zhou, L. One-step fabrication of electrochemical biosensor based on DNA-modified three-dimensional reduced graphene oxide and chitosan nanocomposite for highly sensitive detection of Hg(II). Sens. Actuators B Chem. 2016, 225, 453–462.
- Liu, Y.; Wang, X.; Wu, H. Reusable DNA-functionalized-graphene for ultrasensitive mercury (II) detection and removal. Biosens. Bioelectron. 2017, 87, 129–135.
- Li, J.; Wang, H.; Guo, Z.; Wang, Y.; Ma, H.; Ren, X.; Du, B.; Wei, Q. A “turn-off” fluorescent biosensor for the detection of mercury (II) based on graphite carbon nitride. Talanta 2017, 162, 46–51.
- Li, Y.; Xia, T.; Zhang, J.; Cui, Y.; Li, B.; Yang, Y.; Qian, G. A manganese-based metal-organic framework electrochemical sensor for highly sensitive cadmium ions detection. J. Solid State Chem. 2019, 275, 38–42.
- Liu, B.-H.; Liu, D.-X.; Yang, K.-Q.; Dong, S.-J.; Li, W.; Wang, Y.-J. A new cluster-based metal-organic framework with triazine backbones for selective luminescent detection of mercury(II) ion. Inorg. Chem. Commun. 2018, 90, 61–64.
- Zhang, Z.; Ji, H.; Song, Y.; Zhang, S.; Wang, M.; Jia, C.; Tian, J.Y.; He, L.; Zhang, X.; Liu, C.S. Fe(III)-based metal-organic framework-derived core-shell nanostructure: Sensitive electrochemical platform for high trace determination of heavy metal ions. Biosens. Bioelectron. 2017, 94, 358–364.
- Chen, L.; Zheng, H.; Zhu, X.; Lin, Z.; Guo, L.; Qiu, B.; Chen, G.; Chen, Z.-N. Metal–organic frameworks-based biosensor for sequence-specific recognition of double-stranded DNA. Analyst 2013, 138, 3490–3493.
- Wang, D.; Ke, Y.; Guo, D.; Guo, H.; Chen, J.; Weng, W. Facile fabrication of cauliflower-like MIL-100(Cr) and its simultaneous determination of Cd2+, Pb2+, Cu2+ and Hg2+ from aqueous solution. Sens. Actuators B Chem. 2015, 216, 504–510.
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472.
- Férey, G.; Serre, C. Large breathing effects in three-dimensional porous hybrid matter: Facts, analyses, rules and consequences. Chem. Soc. Rev. 2009, 38, 1380–1399.
- Wan, Y.; Zou, D.; Cui, Y.; Yang, Y.; Qian, G. A Zn based anionic metal-organic framework for trace Hg2+ ion detection. J. Solid State Chem. 2018, 266, 70–73.
- Petrakis, E.A.; Cagliani, L.R.; Tarantilis, P.A.; Polissiou, M.G.; Consonni, R. Sudan dyes in adulterated saffron (Crocus sativus L.): Identification and quantification by 1H NMR. Food Chem. 2017, 217, 418–424.
- Guo, X.; Wen, F.; Zheng, N.; Luo, Q.; Wang, H.; Wang, H.; Li, S.; Wang, J. Development of an ultrasensitive aptasensor for the detection of aflatoxin B1. Biosens. Bioelectron. 2014, 56, 340–344.
- Rovina, K.; Siddiquee, S. A review of recent advances in melamine detection techniques. J. Food Compos. Anal. 2015, 43, 25–38.
- Rovina, K.; Siddiquee, S. Analytical and advanced methods-based determination of melamine in food products. In Nanobiosensors; Academic Press: Cambridge, MA, USA, 2017; pp. 339–390.
- Hu, Q.; Xu, X.; Fu, Y.; Li, Y. Rapid methods for detecting acrylamide in thermally processed foods: A review. Food Control 2015, 56, 135–146.
- Feng, D.; Wu, Y.; Tan, X.; Chen, Q.; Yan, J.; Liu, M.; Ai, C.; Luo, Y.; Du, F.; Liu, S.; et al. Sensitive detection of melamine by an electrochemiluminescence sensor based on tris(bipyridine)ruthenium(II)-functionalized metal-organic frameworks. Sens. Actuators B Chem. 2018, 265, 378–386.
- Jiang, C.; Lan, L.; Yao, Y.; Zhao, F.; Ping, J. Recent progress in application of nanomaterial-enabled biosensors for ochratoxin A detection. TrAC Trends Anal. Chem. 2018, 102, 236–249.
- Alhamoud, Y.; Yang, D.; Fiati Kenston, S.S.; Liu, G.; Liu, L.; Zhou, H.; Ahmed, F.; Zhao, J. Advances in biosensors for the detection of ochratoxin A: Bio-receptors, nanomaterials, and their applications. Biosens. Bioelectron. 2019, 141, 111418.
- Li, Q.; Xu, P.; Gao, W.; Ma, S.; Zhang, G.; Cao, R.; Cho, J.; Wang, H.-L.; Wu, G. Graphene/graphene-tube nanocomposites templated from cage-containing metal-organic frameworks for oxygen reduction in Li–O2 batteries. Adv. Mater. 2014, 26, 1378–1386.
- Sun, C.-Y.; Liu, S.-X.; Liang, D.-D.; Shao, K.-Z.; Ren, Y.-H.; Su, Z.-M. Highly stable crystalline catalysts based on a microporous metal−organic framework and polyoxometalates. J. Am. Chem. Soc. 2009, 131, 1883–1888.
- Saha, S.; Das, G.; Thote, J.; Banerjee, R. Photocatalytic metal–organic framework from CdS quantum dot incubated luminescent metallohydrogel. J. Am. Chem. Soc. 2014, 136, 14845–14851.
- Cheng, C.; Huang, Y.; Wang, J.; Zheng, B.; Yuan, H.; Xiao, D. Anodic electrogenerated chemiluminescence behavior of graphite-like carbon nitride and its sensing for rutin. Anal. Chem. 2013, 85, 2601–2605.
- Zhang, X.; Wang, H.; Wang, H.; Zhang, Q.; Xie, J.; Tian, Y.; Wang, J.; Xie, Y. Single-layered graphitic-C3N4 quantum dots for two-photon fluorescence imaging of cellular nucleus. Adv. Mater. 2014, 26, 4438–4443.
- Ping, J.; Zhou, Y.; Wu, Y.; Papper, V.; Boujday, S.; Marks, R.S.; Steele, T.W.J. Recent advances in aptasensors based on graphene and graphene-like nanomaterials. Biosens. Bioelectron. 2015, 64, 373–385.
- Hu, S.; Ouyang, W.; Guo, L.; Lin, Z.; Jiang, X.; Qiu, B.; Chen, G. Facile synthesis of Fe3O4/g-C3N4/HKUST-1 composites as a novel biosensor platform for ochratoxin A. Biosens. Bioelectron. 2017, 92, 718–723.
- Zhang, Z.; Huang, Y.; Ding, W.; Li, G. Multilayer interparticle linking hybrid MOF-199 for noninvasive enrichment and analysis of plant hormone ethylene. Anal. Chem. 2014, 86, 3533–3540.
- Hu, Z.; Lustig, W.P.; Zhang, J.; Zheng, C.; Wang, H.; Teat, S.J.; Gong, Q.; Rudd, N.D.; Li, J. Effective detection of mycotoxins by a highly luminescent metal-organic framework. J. Am. Chem. Soc. 2015, 137, 16209–16215.
- Gong, Q.; Hu, Z.; Deibert, B.J.; Emge, T.J.; Teat, S.J.; Banerjee, D.; Mussman, B.; Rudd, N.D.; Li, J. Solution processable MOF yellow phosphor with exceptionally high quantum efficiency. J. Am. Chem. Soc. 2014, 136, 16724–16727.
- Bhandari, G.; Zomer, P.; Atreya, K.; Mol, H.G.J.; Yang, X.; Geissen, V. Pesticide residues in Nepalese vegetables and potential health risks. Environ. Res. 2019, 172, 511–521.
- Alister, C.; Araya, M.; Becerra, K.; Volosky, C.; Saavedra, J.; Kogan, M. Industrial prune processing and its effect on pesticide residue concentrations. Food Chem. 2018, 268, 264–270.
- Bacanli, M.; Basaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466.
- Burns, L.E.; Borts, D.J. Rapid untargeted screening for drug residues in animal tissues with liquid microjunction surface sampling probe mass spectrometry. Anal. Chim. Acta 2019, 1063, 75–81.
- Lozano, A.; Hernando, M.D.; Ucles, S.; Hakme, E.; Fernandez-Alba, A.R. Identification and measurement of veterinary drug residues in beehive products. Food Chem. 2019, 274, 61–70.
- Tong, Z.; Duan, J.; Wu, Y.; Liu, Q.; He, Q.; Shi, Y.; Yu, L.; Cao, H. A survey of multiple pesticide residues in pollen and beebread collected in China. Sci. Total Environ. 2018, 640–641, 1578–1586.
- Parrilla Vázquez, P.; Ferrer, C.; Martínez Bueno, M.J.; Fernández-Alba, A.R. Pesticide residues in spices and herbs: Sample preparation methods and determination by chromatographic techniques. TrAC Trends Anal. Chem. 2019, 115, 13–22.
- Sharma, A.; Istamboulie, G.; Hayat, A.; Catanante, G.; Bhand, S.; Marty, J.L. Disposable and portable aptamer functionalized impedimetric sensor for detection of kanamycin residue in milk sample. Sens. Actuators B Chem. 2017, 245, 507–515.
- Liu, Q.; Ning, D.; Li, W.J.; Du, X.M.; Wang, Q.; Li, Y.; Ruan, W.J. Metal-organic framework-based fluorescent sensing of tetracycline-type antibiotics applicable to environmental and food analysis. Analyst 2019, 144, 1916–1922.
- Zhang, X.; Xu, N.-Y.; Ruan, Q.; Lu, D.-Q.; Yang, Y.-H.; Hu, R. A label-free and sensitive photoluminescence sensing platform based on long persistent luminescence nanoparticles for the determination of antibiotics and 2,4,6-trinitrophenol. RSC Adv. 2018, 8, 5714–5720.
- Vikrant, K.; Tsang, D.C.W.; Raza, N.; Giri, B.S.; Kukkar, D.; Kim, K.H. Potential utility of metal-organic framework-based platform for sensing pesticides. ACS Appl. Mater. Interfaces 2018, 10, 8797–8817.
- Zhou, Y.; Yang, Q.; Zhang, D.; Gan, N.; Li, Q.; Cuan, J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators B Chem. 2018, 262, 137–143.
- Cao, X.; Hong, S.; Jiang, Z.; She, Y.; Wang, S.; Zhang, C.; Li, H.; Jin, F.; Jin, M.; Wang, J. SERS-active metal–organic frameworks with embedded gold nanoparticles. Analyst 2017, 142, 2640–2647.
- Khan, P.M.; Baderna, D.; Lombardo, A.; Roy, K.; Benfenati, E. Chemometric modeling to predict air half-life of persistent organic pollutants (POPs). J. Hazard. Mater. 2020, 382, 121035.
- Aerts, R.; Van Overmeire, I.; Colles, A.; Andjelković, M.; Malarvannan, G.; Poma, G.; Den Hond, E.; Van de Mieroop, E.; Dewolf, M.-C.; Charlet, F.; et al. Determinants of persistent organic pollutant (POP) concentrations in human breast milk of a cross-sectional sample of primiparous mothers in Belgium. Environ. Int. 2019, 131, 104979.
- McComb, J.; Mills, I.G.; Muller, M.; Berntsen, H.F.; Zimmer, K.E.; Ropstad, E.; Verhaegen, S.; Connolly, L. Human blood-based exposure levels of persistent organic pollutant (POP) mixtures antagonise androgen receptor transactivation and translocation. Environ. Int. 2019, 132, 105083.
- Bansal, V.; Kim, K.H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38.
- Wang, L.; Fan, G.; Xu, X.; Chen, D.; Wang, L.; Shi, W.; Cheng, P. Detection of polychlorinated benzenes (persistent organic pollutants) by a luminescent sensor based on a lanthanide metal–organic framework. J. Mater. Chem. A 2017, 5, 5541–5549.




