Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
Metal-organic frameworks (MOFs) have emerged as cutting-edge materials for analytical sensing. MOFs are a class of crystalline porous materials systems structured by using metals linked together by organic bridging ligands.
- foodborne contaminants
- food safety
- food detection
- metal-organic frameworks (MOFs)
- sensing
1. metal-organic frameworks (MOFs) -Based Sensors for Food Safety
Over the past decades, there have been many studies and reports on MOF synthesis for food safety analysis (Figure 3), encompassing MOF designing, various synthesis methods, and post-synthetic modification, as well as incorporation of biomolecules into MOF [23,117]. Isoreticular expansion, topology-guided design, and modulated synthesis are the most reported methods for MOF synthesis. While, four main classes of post-synthetic modification include covalent post-synthetic modification, post-synthetic metalation modification, dative post-synthetic modification, post-synthetic exchange, and post-synthetic deprotection, and have been reported as tools to overcome different barriers for the application of MOF in food safety analysis [28,118,119]. Besides, incorporation of biomolecules into MOFs has been used as a new strategy to improve MOF efficiency in selectivity, sensitivity, signal amplification, and MOF stability [117]. To accommodate the drawbacks of the lower framework stability of MOFs, carboxylate-based linkers and N-heterocyclic based linkers have been used [120,121].
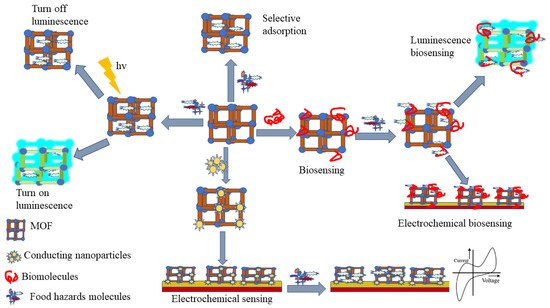
Figure 3. Schematic representation of various MOF-based techniques used for food safety analysis.
1.1. MOF-Based Electrochemical-Sensing Method
The Electrochemical sensing method is one of the major areas in the analytical method, since it easy, reliable, and cheap compared with other analytical methods. Therefore, recent years have seen a great rising of a scientific reports in the development and application of electrochemical-sensing techniques for the successful detection of food safety contaminants [122]. The selectivity and sensitivity of electrochemical methods prove them to be the best candidate for efficient food safety analysis. However, the effectiveness of the electrochemical sensing method is built based on the electrochemical properties of the transducer MOFs or nanomaterials (NMs) (redox reactions of the analyte in electrochemical system). Additionally, the conductivity properties of NMs govern the sensitivity of the electrochemical sensors [123,124]. Therefore, a great effort has concentrated on the improvement of MOFs’ conductivity properties to facilitate the design and synthesis of better and more sensitive MOF-based electrochemical sensors [125,126].
The research on MOF-based methods and electrical conductivity is still in its early stages. As a result of the lack of electrical conduction in their pristine forms, a large number of pre-existing MOF-based sensors are suitable for optical transduction. The organic linkers are redox-inactive and are attached to the hard-metallic cluster via hard oxygen-containing groups. As a result of their insulating properties, pristine MOFs are poor electrical conductors [127,128,129]. To overcome such challenges, various strategies and modifications to increase electrical conductivity have been introduced, such as doping MOFs with specific materials such as nanotubes, nanoparticles (NPs), and selected ionic species [130]. The large specific surface area of the MOFs substrate makes it easier to load nanoparticles, which helps to improve conductivity and amplify electrical signals. For incidence, Talian et al. (2014) reported a realizing tunable electrical conductivity strategy in MOFs, where tetracyanoquinodimethane were used as organic linkers and tetrathiafulvalene were also used as organic linker by Narayan (2102) [129,131]. These changes can be used to improve the conducting properties of MOFs in order to develop potential electrochemical sensor technology based on MOFs [132]. MOF-based sensors can thus be post-modified by modifying their conductive properties and good absorption properties [132].
1.2. MOF-Based Chemical Sensing Method
A chemical sensor method is self-sufficient to provide chemical information of its environment through analytical reaction, whether it is the liquid or the gas phase of the surrounding environment [158]. Luminescent chemical sensing using MOFs has been reported as potential chemical sensors due to their easily induced luminescence, various advantages in structural and components, and their detecting mechanism [23]. Metal ions, organic ligands, and guest species (luminescent guest molecules or nanoparticles) are the most common source of MOFs’ luminescence. Light-emissive organic ligands containing aromatic or conjugated moieties as the linker and lanthanide ions are the most commonly used to fabricate luminescent MOFs [158]. Given that luminescent MOFs (LMOFs) detection capability can be enhanced by host-guest interactions, they have been proposed as excellent candidates for food safety analysis applications. In recent years, prospective applications of LMOFs have been investigated. The sensitivity of MOF-based detection of food contaminants is determined by the sensing method used for signal transduction [159].
Naturally, the sensitivity of LMOFs is linked to MOFs’ high loading capacities and analyte transport facilitation within their structural framework. Furthermore, active analyte incorporation into the MOF framework affects the limits of detection (LODs) for LMOFs [22,126]. Scientists have demonstrated that the fundamental mechanisms involved in the LMOF-based sensing approach are based on variations in the intermolecular distances between the metallic centers and the organic linkers, chemical interactions between the target analyte and the metallic clusters in the MOF framework, and host-guest interactions between the organic ligands and the guest analyte. The luminescent MOF’s working mechanism was mainly based on the occurrence of the fluorescence quenching method. Forster resonance energy transfer (FRET), photoinduced electron transfer (PET), inner filter effect (IFE), and competition of excitation light between MOF and analyte are the most popular quenching mechanisms for fluorescence quenching of MOF-based sensors [27,126,160]. All of these mechanisms are visible through a variety of luminescence-related phenomena such as ligand-localized emissions, ligand-to-metal charge transfer, metal-to-ligand charge transfer, plasma-induced gate oxide damage, sensitization, and metal/excimer/exciplex emissions [158,161].
Furthermore, LMOFs have one intriguing structural component: organic ligands are small, and these ligand molecules can self-quench, resulting in a higher electro-photoluminescence (PL) quantum yield [158]. A subset of LMOFs have a MOF structure that includes a stabilizing organic ligand with a tuned highest occupied molecular orbital and lowest unoccupied molecular orbital energy gap, resulting in a PL quantum yield value closer to one [158,162]. Furthermore, the use of organic linkers in MOFs that can absorb ultraviolet (UV)/visible light can result in fluorescence. Turning off fluorescence is the most common optical intensity quenching method used for signal transduction of LMOFs [162]. This quenching ability is thought to be caused by the overlapping of acceptor and donor electrons. Charges in the redox potential of the in-build moieties, on the other hand, have been recognized to account for quenching in some cases [163]. Furthermore, but not always, the luminescence intensity of the LMOFs increases in turn on fluorescence upon interaction with the guest analyte. This property can be used to quantify the target concentration at the same wavelength as the luminescence intensity increases [163,164].
The interaction MOF-analyte is accompanied by changes in physicochemical properties such as optical and electrical conductivity. Furthermore, LMOF-based optical detection can generate detection signals that can be seen with the naked eye. Overall, the ability to control the charges in the optical characteristics enables high sensitivity detection with low LODs, and this option has been adopted for food safety analysis [164,165]. Nonetheless, LMOF-based food safety analysis warrants future research and development due to identified drawbacks such as variation in the quenching rate and pathways along with medium stabilization and detrimental porosity [165,166,167].
1.3. MOF-Based Biosensing Method
The incorporation of biomolecules into sensing technology has resulted in biosensors being a cost-effective and time-efficient technique for food safety analysis. A biosensor is a self-contained, unified device that contains all of the subsystems required for electronic quantification and data transmission. Interactions of biomolecules that act as biological recognition elements and electrochemical transducers can produce a usable signal [183,184]. MOFs, porous crystalline materials built from the coordination of organic ligands and inorganic metal ions or metal, present ordered and tunable porosity, good crystallinity, and high surface areas, making them excellent for host matrix immobilization of biomolecules [117,185,186,187]. These excellent and unique MOFs’ properties give them outstanding support ability to incorporate biomolecules for modern food safety detection. Some food contaminant molecules can inhibit the activity of specific enzymes use to quantify a targeted analyte. The biosensing approach for food contamination sensing utilizes various kinds of biomolecules and therefore they have been successfully incorporated with MOFs [188,189,190,191,192,193]. Thus, the MOF-based biosensing approach for food safety analysis usually utilizes various biomolecules such as enzymes [194], antibodies (ab) [189,190], peptides [195], bacteriophages [196], and aptamers.
MOFs must be conjugated to biorecognition elements before they can be used in the development of biosensors. These biofunctionalizations can be achieved by using pendant functional groups from linkers’ moiety of MOFs. This procedure, however, provides unnecessary control over the functionalization reaction and may result in a bulk functionalization reaction rather than the intended surface modification. Scientists recently demonstrated that coating the surface of MOFs with silica can improve the condition of their biofunctionalization [211,212,213]. For incidence, the silica coating can play a double role: the improvement of the water stability and dispersibility of the MOFs and the facilitation of their effective surface functionalization. Based on these advantages, the thin assembly of silica-coated water-stable CU3(BTC)2@SiO2 on a conducting substrate was firstly reported [190,211]. Immunosensing has opened up new ways for MOF-based biosensors, in which antibodies serve as identification receptors. They are, of course, organic compounds that regulate peripheral physicochemical properties and govern the grafting procedure in order to improve the sensitivity and selectivity of the biosensing approach [189]. Recently, the development of impedimetric immunosensors technics has been exciting research field due to its ability to prove lab-on-chip devices that are not only easy to be integrated with microfluidic sample chambers, but also easy to calibrate [213,214]. Interestingly, MOFs can be used as nanosized electrode materials in the impedimetric immunosensor fabrication based on their hierarchical chemical assembly and availability of functional groups on them [213].
Aptamer-based sensors are a novel type of biosensor that employs an aptamer as the biological recognition element and possesses a high affinity to the target. Aptamers are oligonucleotides that can specifically bind target molecules based on a combination of hydrogen bonding, electrostatic interaction, van der Waals forces, and their three-dimensional conformation [215]. Many DNA or RNA aptamers with high affinity and specificity have been identified with various targets, including proteins, peptides, amino acids, antibiotics, small chemicals, viruses, whole or parts of cells, and even metal ions. Aptamers are DNA or RNA fragments derived from selection experiments that have a high affinity for a given target [62,216]. The selection of the appropriate aptamer for a given molecule is accomplished in vitro via a systematic evolution of ligands by exponential enrichment (SELEX) process from libraries containing random oligonucleotide sequences. Based on strong interaction, such as π–π stacking, hydrogen bonding, and electrostatic force that can be formed between special functional groups on organic linkers of MOFs and negative charge of nucleic acid, sequences of a series of biosensors have been developed for food safety analysis [204,210]. For incidence, Chen et al. developed an electrochemical biocode based on a nanoscale MOF for the simultaneous detection of multiple antibiotics with a low DL [205].
1.4. MOF-Based SERS Sensing Method
Because of its strong dominance of the interaction and distance targets and nanoparticles, surface-enhanced Raman scattering (SERS) has been widely used in food safety analysis. Because SERS can provide a wealth of structural information, it has been widely used for molecular identification and structural characterization of various compounds, also known as molecular fingerprinting [217]. The widely accepted mechanism for SERS signal enhancement is dominated by electromagnetic field enhancement, which attributes to the localization of surface plasm resonance (LSPR) or hot sport of noble metals and the physical or chemical adsorption of analytes to the surface for metal-analyte charges-transfer production. The adsorptive interaction between suspended colloids and the target in solution is dependent on the slow diffusion of analyte from the bulk solution to the surface of metal nanoparticles (NPs) for the facilitation of molecule-metal interactions [218].
However, various molecules exhibit slow affinity or no affinity for the LSPR areas, limiting the use of SERS techniques. Therefore, much effort has focused on the functionalization of NPs (Au and Ag) with viologen dictations, cyclodextrin, alkanethiolate tri (ethylene glycol), and cysteine, etc., aiming to improve the affinity of the target to the metal surface [219]. The metallic colloids, on the other hand, are easily aggregated, resulting in precipitation into solution and loss of SERS signals [219,220]. Therefore, significant efforts have been made to protect NPs by coating them with organic or inorganic shells such as polymers, transition-metal materials, carbon, and mesoporous silica for mechanical stability and improved signal reproductivity [221]. The majority of these shells are made up of disordered and amorphous structures, and the diffusion of molecules to the metal core is limited. It would be advantageous to develop a SERS detection element with excellent stability and enhanced analyte-metal interactions [222]. Yuling Hu (2014) created a sensitive SERS substrate by embedding AuNPs within MIL-101 using the unique properties of MOFs (high surface areas, tailorable chemistry, and uniform and tunable nanostructured cavities). The SERS substrate that was created was used to detect Rhodamine 6 G and benzidine with detection limits (DL) of 41.75 and 0.54 f mol, respectively. Furthermore, the use of novel SERS in the quantitative analysis of organic pollutant P-phenylenediamine in water and tumor marker alpha-fetoprotein in human serum demonstrated good linearity of 1.0–100.0 ng/mL and 1.0–130.0 ng/mL, respectively [218].
2. Use of MOF-Based Sensors for Food Safety Analysis
2.1. Detection of Pathogenic Bacteria
Food-borne disease is one of the most major public health problems, and failure to detect foodborne pathogens may lead to terrible consequences. Biological hazards cause various infectious diseases [223]. Detection and identification of pathogens is the best way of clinically diagnosing them. Microorganisms are widely distributed in nature and in different ecosystems such as water, soil, air, oceans, food, skin, and the intestinal tracts of humans and animals. While many microorganisms are indispensable in ecosystems, some of them are responsible for diseases [1]. Bacteria that are commonly responsible for outbreaks in different countries include Escherichia coli, Salmonella, Vibrio chorea, Shigella, Listeria monocytogenes, Staphylococcus aureus, Bacillus aureus, Clostridium perfringens, Campylobacter jejuni, and Legionella. All of these pathogens can cause gastrointestinal disease, fever, diarrhea abdominal cramps, vomiting, and nausea and lead to the deleterious consequences on the global economy and human health. Significant improvements in the disinfection in food safety have been achieved such as rigorous, good manufacturing practices and good agricultural practices, but the results of food-borne pathogenic microorganism control are still not optimistic. Therefore, routine monitoring of the quality and safety of food is important for public health [4,58,224].
Based on the diverse structural configuration and exciting optical proprieties, MOFs have attracted huge attention for biosensing applications [225]. LMOFs have a number of distinct advantages over other materials, including crystallinity, nano-to-micro sized structures, stable fluorescence over time and temperature, and readily available functional groups for the conjugation of biorecognition species [225]. For the first time, Neha et al. (2019) reported a non-toxic, biocompatible, and water-stable luminescent biosensor MOF with NH2-MIL-53(Fe) as a fluorescent marker. According to the pre-existing literature, NH2-MIL-53(Fe) was solvothermally prepared [226]. The mixture of FeCl3.6H2O and NH2-BDC in deionized water (same concentration of 5 mmol) were prepared and transferred into sealed containers then treated with autoclave heating at 150 °C over a period of 3 days. The synthesized MOF (NH2-MIL-53) was filtrated, washed twice with water and ethanol, then dried at 70 °C [226]. The conjugate of antibody- NH2-MIL-53 (2 mg mL−1) was prepared in flowing way: NH2-MIL-53 MOF containing amine functional group was mixed with antibody solution (0.1 mg mL−1 into the mixture of 0.1 M PBS, 10 nM EDC, and 5 mM NHS), then incubated at 4 °C overnight for amide linkage formation. The Ab-NH2-MIL-53 conjugate was washed with PBS buffer (three times) to remove any unbound Ab or MOF particles. Complex anti-S. aureus antibody-MOF (Ab-NH2-MIL-53) has been applied to detect different samples, including real samples. The specific binding of complex to bacteria has led to the reduction in fluorescence intensity at the corresponding number of bacteria in solution. Thus, it has given Ab-NH2-MIL-53 biosensors the ability to detect 85 CFU mL−1 as DL with over a wide concentration range 4 × 102–4 × 108 CFU mL−1 of S. aureus [226].
Bacteriophages are a type of bio-recognition element. Bacteriophages are obligate host living parasites that use their tail proteins to recognize the host bacterium with high strain specificity [227]. Therefore, bacteriophages can be used in the development of biosensors with the added benefits of sensor stability in various environmental conditions of pH and/or temperature change, the ability to differentiate viable and dead cells, no sample pre-processing being required, self-signal amplification, and low production cost [227]. Interestingly, bacteriophages can be stable in dried conditions, giving them a distinct advantage over other biomolecules used in biosensor development [228,229]. Neha et al. (2016) designed a bacteriophage-MOF opto-sensor for rapid detection of Staphylococcus arlettae [196] by taking into account the micro-size of the bacteriophages (100–200 nm) [196].
A host-specific bacteriophage to S. arlettae has been conjugated to the surface of metal-organic framework (IRMOF-3) using the covalent attachment. IRMOF-3 was prepared at room temperature condition as reported in the literature by magnetically stirring mixing Zn (NO3)2.6H2O (16 mmol) and 2-amino terephthalic acid (8 mmol) in DMF solution with a total volume of 160 mL. The triethylamine (64 mmol) was slowly added, which led to instant white precipitates formation. Produced IRMOF-3 was collected by filtration and washed three times with DMF solvent then immersed into CH2Cl2 over 72 h, and the product was finally dried under vacuum condition at 70 °C [196]. The highly specific bacteriophage was isolated and purified according to the literature, and the maintained stock solution concentration was 108 PFU mL−1 [196]. Bioconjugation of IRMOF-3 with the S. arlettae-specific bacteriophage process was achieved by adding 2 mg mL−1 of IRMOF-3 into 10 mL Saline Magnesium buffer (pH 7.5) mixed with 2 mL of 25% glutaraldehyde, followed by incubation for 30 min at room temperature; thereafter, 3 mL of bacteriophage solution was added. The function of glutaraldehyde was to catalyze the conjugation reaction of IRMOF-3 with the S. arlettae-specific bacteriophage. Unbounded or loosely bound moieties were separated by washing bacteriophage-IRMOF-3 complex twice with Tris-buffer. The purified probe was stored at 4 °C for further usage after drying in vacuum condition [196]. The detection of S. arlettae was accomplished by observing changes in the photoluminescence intensity of the probe as it interacted with various concentrations of bacterium solution. The proposed bacteriophage-based biosensor had a detection range of 102–1010 CFU mL−1 and a DL of 100 CFU mL−1 [196].
Based on the advantages of electronic (sensitivity, portability, and ease of preparation as key devices), MOFs (high porosity, effective surface area, thermal and chemical stability, and tunable pores sizes), and aptamer (high selectivity, specificity, cheap, and easy to select by SELEX process), Saeed and Saba (2018) reported an electrochemical MOF-based biosensor for detection of E. coli 0157:H7. The synthesis of CU3(BTC)2(HKUST-1) and Cu-MOF/PANI nanocomposites was carried out in accordance with previously published studies, with some modifications [230,231]. The glassy carbon electrode (GCE) was polished with alumina slurry (0.1 M) with a polishing cloth, rinsed with water, and then sonicated in ethanol for 5 min to create the MOF-aptamer biosensor. Figure 4 depicts the synthesis of the complex PANI/MOF/GCE [232]. Aptamer -NH2 groups were covalently linked to PANI/MOF -NH2 groups with GA. In fact, the PANI/MOF surface provided a large number of free amine groups for aptamer immobilization. The developed biosensor was monitored using the cyclic voltammetry (CV) and electro-chemical impedance (EIS) techniques. As a result, using methylene blue (MB) as an electronical indicator, differential pulse voltammetry (DPV) was used to monitor and quantify the interaction between the aptamer and E. coli 0157:H7. The recorded current change (in reduction) of MB was an analytical signal indicator of the relationship with the logarithm of E. coli 0157:H7 concentration in the detection range of 2.1101–2.1107 CFU mL−1 with DL of 2 CFU mL−1 [232].
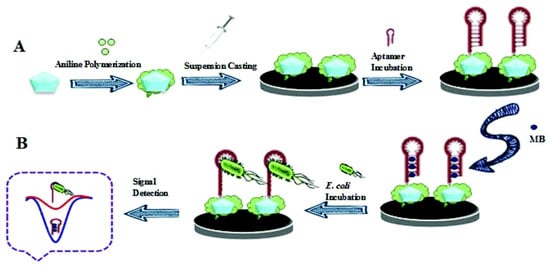
Figure 4. Schematic diagram illustrating (A) aptasensor fabrication and (B) E. coli O157:H7 detection [232]. Copyright permission has been obtained.
2.2. Detection of Heavy Metals
Environmental contamination by heavy metals has been an important issue worldwide. Some of these heavy metals are even not biologically essential, including Pb, Hg, and Cd. Among these heavy metals, Hg is an effective neurotoxin owing to its accumulation in the vital organs and tissues; additionally, its binding to the sulfur-containing proteins and enzymes destroys important cell functions which can lead to disease [233]. Heavy metals can cause toxicity and are a source of severe damage to ecosystems, cause economic losses, and negatively impact the food chain and health due to their lack of biodegradability. There are many ongoing studies on the development of different techniques for the detection of heavy metals at trace levels in the environment, food products, and water, as well as in living organisms [234]. Different studies have been conducted to develop several new methods for heavy metals detection at trace levels. A stripping voltammetric method was developed, and other methods such as mass-spectrograph, plasma-induced spectrum, atomic fluorescence spectrometry, and ultraviolet-visible spectrometry were subsequently developed [235].
While these methods each have advantages, there are also disadvantages, such as complicated procedures for sample pre-treatment, expensive instruments that are operated by professionals, and being time-consuming. In order to overcome these deficiencies, different attempts have been made to establish better sensors for rapid and easy detection of metals including the MOF-based detection method [236,237,238]. Therefore, this study discusses the recently developed MOF-based detection method for sensing heavy metal in water and food. Scientists recently reported that organic linkers on MOFs contain special functional groups that could serve as a source of stacking, hydrogen bonding, and electrostatic interactions with negatively charged molecules. As a result, MOFs can be used as a recognition element in biosensors for small ions or nucleic acid molecules [239]. Furthermore, considerable effort has been expended in obtaining supporting materials with broad properties such as high-water stability, biocompatibility, adsorbent capability, and electrochemical activity for the application of MOF in food safety analysis [238].
Zhang et al. (2017) created a new core-shell nanostructured of Fe-MOF@mFe3O4@mC with an inner cavity and an orderly mesoporous opening structure for incidence. The developed core-shell was attached to porous structure aptamer sequences for heavy metal detection (Pb2+ and As3+). The steps of biosensor fabrication were involved, including the preparation of Fe-MOF@mFe3O4@mC, the immobilization of aptamers, and the detection of Pb2+ and As3+. In the presence of the hallow Fe3O4@mC nanocapsules, the core-shell nanostructured of Fe-MOF@mFe3O4@mC were hydrothermally prepared, with FeCl3 acting as the precursor and 2-amino-terephthalic acid acting as a linker, obtained after calcination of hallow Fe3O4@C nanocapusules, which were synthesized from core-shell SiO2@Fe3O4@C spheres with SiO2 removed. The intensive binding between Fe-MOF and the aptamer sequence could generate a high immobilization force for the aptamer sequences due to supramolecular stacking and hydrogen-bonding interactions. When Fe-MOF is added to a solution containing aptamers, the aptamers tend to approach the surface of the Fe-MOF (Figure 5). As a result, the designed strategy has proven to be a suitable analyzer for traces analyte by detection of heavy metal (Pb2+ and As3+) in river water and blood serum, with a detection range of 0.01 to 10.0 nM and estimated DL of 2.27 and 6.63 PM toward detecting Pb2+ and As3+, respectively [238].
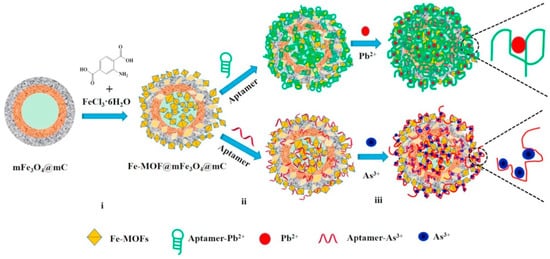
Figure 5. Preparation process for Fe-MOF@mFe3O4@mC nanocomposite and its related aptasensor for detection Pb2+ and As3+ via electrochemical techniques, including (i) the preparation of Fe-MOF@mFe3O4@mC nanocomposite, (ii) the immobilization of the aptamer strands, and (iii) the determination of the heavy metal ions [238]. Copyright permission have been obtained.
Based on various advantages of facile, ecological MOF preparation such as simple instruments, the occurrence of reaction at atmospheric pressure, and convenient reaction process, for the first time, Wang et al. (2015) fabricated a cauliflower-like MIL-100(Cr). After preparation, MIL-100(Cr) was confirmed by FT-IR, XRD, SEM, and XPS to apply in detection of heavy metal ions (Cd2+, Pb2+, Cu2+, and Hg2+) in aqueous solutions at trace amounts [240]. In the concentration range of 0–10 M, a correlation coefficient of Cd2+, Pb2+, Cu2+ and Hg2+ were 0.991, 0.9868, 0.989, 0.997, respectively with DL of 4.4 × 10−8 mol L−1 for Cd2+, 4.8 × 10−8 mol L−1 for Pb2+, 1.1 × 10−8 mol L−1 for Cu2+, and 8.8 10−9 mol L−1 for Hg2+ [240]. Ionic luminescent metal-organic framework (ILMOF) is a new LMOF composed by a charged hybrid material of atoms and organic ligand which contains advantages electrification and intrinsic properties of MOF [241,242,243]. Based on the higher affinity of Hg2+ to the nitrogen atoms, Wan et al. (2018) selected [2, 2′:6′, 2″-Terpyridine]-4, 4′, 4″-tricarboxylic acid (TPTC) to design a MOF with organic ligand which contained multiple nitrogen atoms (N) for Hg2+ detection. The designed Zn-TPTC MOF was performed in the detection of Hg2+ in water with a wide detection range of 10−6–10−4 M, calculated DL was as low as 3.67 nM [243]. Thus, we have a generalized idea of MOF selection and designing using pore size, anionic frameworks, and multiple N sites in the organic ligand.
2.3. Detection of Illegal Food Additives
In recent years, food adulteration has become a public health issue as well as a food safety problem. Sudan dyes have been detected in spice powders, chili sauces, spicy soups, colorful desserts, and even soft drinks [244]. Such illegal synthetic dyes are cheap and easily used as coloring agents to enhance the natural color of products. Adulteration of natural milk with synthetic chemicals is a serious problem for human health [47]. For incidence, melamine (1,3,5-triazine-2,4,6-triamine, C3H6N6) is an industrial chemical compound with high nitrogen content (66% by mass) which used in melamine resins synthesis. Recently, it has been fraudulently added in milk to false a higher level of protein concentration which is evaluated by determination of nitrogen concentration with the Kjeldahl method. The addition of melamine into food products has been a cause of serious diseases and many babies and children were intoxicated [245,246]. Therefore, the detection of illegal additive compounds at trace levels would be advantageous. HPLC coupled with ultraviolet (UV), thin-layer chromatography (TLC), diode array (DAD), and ELISA are still used for detecting toxins and food illegal additives. However, all these methods require complicated and expensive sample pre-treatment, skills of a trained operator, and expensive equipment with low analyte concentration [57,246]. Therefore, the development of a reliable and sensitive detection method which can realize real-time and convenient detection of food adulteration of great importance.
Based on high sensitivity, rapid response, wide linear range, good controllability, low background, and low DL, various scientists have reported on the application of the ECL method as an analytical tool for food safety detection. However, there have been few reports of the application of MOF into ECL systems, because of a lack of redox and luminescence properties in organic ligands of reported MOFs. To overcome this problem, Feng, et al. (2018) designed and synthesized a doped MOF with Tris(2,2′-bipyridyl) dichlororuthenium (II) (Ru (32+) for melamine detection in daily products. The main used building block units were the anionic bio-MOFs-1 [Zn8(ad)4(BPDC)6O.2Me2NH2,8DMF,11H2O] (ad = adeninate; BPDC = biphenyl carboxylate; DMF = dimethylformamide) with columnated zinc-adeninate as a secondary building unity composed of apex-sharing zinc-adeninate octahedral cages, while the Ru(bpy)32+(luminescent cationic) were doped into the MOF and their original electro-chemical and luminescent properties were preserved. The ability of Ru(bpy)32+ to react with amides on melamine (1,3,5-triazine-2,4,6-triamine) has attracted more attention as a potential application in the synthesis of MOF-ECL based method for melamine detection (Figure 6). Under optimum conditions, the ECL intensity was proportional to log (melamine concentration) in the wide detection range of 10−10–10−4 with DL of 3.8 × 10−11 M [247]. The designed method was successfully applied in milk and infant formula powder melamine detection recoveries in the range 98–104% and 95–103%, respectively, obtained from spiked samples [247].
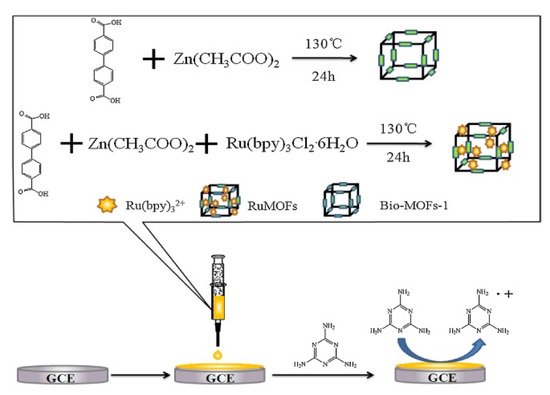
Figure 6. Design process of the ECL sensor for melamine detection in dairy products [247]. Copyright permission has been obtained.
2.4. Detection of Natural Toxins in Food
Food is only one source of nutrients but may also contain potentially harmful natural toxic substances to humans including mycotoxin, a bacterial toxin, animal biotoxin, neurotoxin, and phytotoxin. The toxicological effect of some of these substances can be acute even at a very low dose. Therefore, many classical methods have been developed for toxin detection in food [15,16].
Recently, researchers have been drawn to the combination of MOFs with other superior functional materials such as quantum dots (QDs), polyoxometalates (POMs), polymers, graphene, and carbon nanotubes (CNTs) because this technology may present advantages of their merits while mitigating their shortcomings [248,249,250]. On the other hand, two-dimensional (2D) layered materials like graphitic-phase carbon nitride (g-C3N3) have been widely applied in sensing, drug delivery, and imaging, and they can be regarded as N-substituted graphite in a regular fashion [251,252].
However, the affinity of g-C3N4 for aptamer is low, which may result in aptamer desorption from the material’s surface without the addition of target, lowering the sensor’s stability [253]. To surmount this situation, Hu and his colleagues (2017) referred to Zhang et al.’s (2014) work (the combination of MOF with Carbone nanotube) to combine HKUST-1 with g-C3N4 to form the g-C3N4/HKUST-1 complex, where g-C3N4 were acting as hydrophobic protection of HKUST-1 from water molecules [199,254]. The Fe3O4 was introduced for lowering the background, then the formed Fe3O4-g-C3N4/HKUST-1 composites were to be used in the development of aptasensor for OTA detection in a corn sample as described in Figure 7. The developed composites have a high adsorption capacity for dye-labeled anti-OTA aptamers and can completely quench the dye’s fluorescence via a photoinduced electro transfer (PET) mechanism. In the presence of OTA in solution, it can bind with high affinity to the aptamer, resulting in the leasing of dye-labelled aptamer from quencher (Fe3O4-g-C3N4/HKUST-1) and an increase in fluorescence. The aptasensor’s fluorescence intensity had a linear relationship with the OTA concentration in the range of 5.0–160.0 ng mL−1, with a DL of 2.57 ng mL−1 [199].
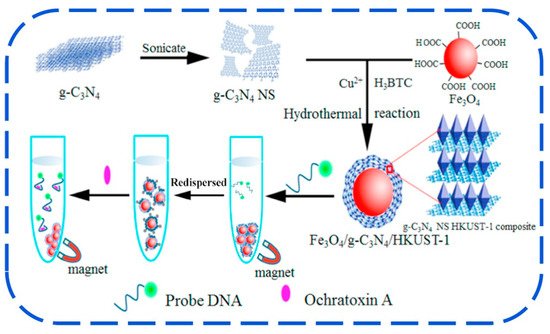
Figure 7. Schematic diagram representing principle of the biosensor based on Fe3O4/g-C3N4/HKUST-1 to detect OTA [199]. Copyright permission has been obtained.
Based on LMOF’s advantages of having an easy-to-functionalize surface and tunable porosity which can promote feasible guest-host interactions, for the first time, LMOF for very fast and sensitive fluorescence-based mycotoxin were developed for OTA detection [255]. Synthesis of Zn(bpdc)2(tppe) (LMOF-21) started from ligand 1,1,2,2-tetrakis(4-(pyridine-4-yl) phenyl) ethane(tppe) synthesis based on a reported process [256] where solid 1,1,2,2-tetraphenylethene (tpe) reacted with liquid bromine to produce 1,1,2,2-tetrakis(4-bromophenyl) ethene (Br4-tpe) with recrystallization purification in dichloromethane/methanol. Br4-tpe and pyridine-4-4bronic acid were reacted in catalysis of palladium (acetate) for the attachment of the pyridine moiety to the tpe moiety. Chloroform and column chromatography were used in the extraction and purification of the product, respectively. Thereafter, a mixture of Zn(NO3)2·6H2O (0.015 g, 0.05 mmol), biphenyl,-4,4′-dicarboxylic acid (H2bpdc, 0.012 g, 0.05 mmol), tppe (0.013 g, 0.02 mmol), N,N-dimethylacetamide (DMA, 8 mL), dimethyl sulfoxide (2 mL), and isopropyl alcohol (2 mL) was added in a 20-mL glass vial. After ultrasonication mixing, the glass vial was sealed and kept at 150 °C for 24 h and then cooled down to room temperature for the filtration process. Optic proprieties evaluation of LMOF-241 proved its ability of blue-green emitting LMOF with an exceptionally high internal quantum yield (92.7%). The developed LMOF was successfully applied in mycotoxin detection via a quenching mechanism with high optical selectivity and the calculated DL was 46 ppb [255].
2.5. Detection of Drug and Pesticide Residues
Pesticides and veterinary drugs are an important tool in agro-business to control insects, weeds and diseases and improve crop and livestock yield by minimizing losses. However, many scientists proved the harmful impact of veterinary drugs and pesticides to the environment as well as to humans via food consumption [257,258]. Utilization of veterinary medicines, especially antibiotics, plays an important role in animal feed production through treatment and disease prevention and growth promotion as well [259]. However, various scientific reports proved that the use of antibiotics in animals can result in antibiotic residues in foodstuffs such as milk, eggs, and meat. These residues may cause side effects such as the transmission of antibiotic-resistant bacteria to humans, immunopathological effects, allergy, mutagenicity, nephropathy, hepatotoxicity, reproductive disorders, bone marrow toxicity, and carcinogenicity through human conception [259,260,261]. On other the side, the routine utilization of pesticides in modern agriculture has increased agricultural crop yield. However, it has proved that pesticides can be serious sources of food safety hazards [262,263]. Therefore, the detection of pesticides and drug residue at trace amounts in food is necessary.
During the last decade, different studies have been carried out to develop different analytical techniques for pesticides and drugs residue detection, including capillary electrophoresis, surface plasmon resonance, HPLC, microbiological methods, immunoassays, and electrochemical immunosensors [60]. Usually, these methods are very expensive, time-consuming, and require expensive equipment and highly-trained technicians. Recently, with featuring tunable intriguing structures, permanent porosity, and structural flexibility, MOFs have been used for pesticide and drugs residue detection in food and the environment [264,265,266]. Therefore, different authors have reported and reviewed the application of MOFs in the detection of pesticide and drug residue detection in food and the environment. For incidence, Vikrant et al. (2018) highlighted recent advancements in MOF-based sensing techniques for pesticides with emphasis on the description of sensing principles of MOFs along with areas of practical applications in pesticide detection [266]. Therefore, this subtitle of the application of MOFs in the detection of pesticide and veterinary drug residues focused on the recently developed MOFs-based analytical techniques for drugs residue detection in food.
LMOFs have tunable intriguing structures, permanent porosity, and intense fluorescence, which has sparked a lot of interest recently for their potential use in fluorometric chemosensors. As a result, Zhou and her coworkers (2018) reasoned that tetracycline (TC) detection and absorption could be accomplished through electron/energy transfer and specific host-guest interactions between TC and MOF by carefully selecting the component metal ions and organic ligands. As a result, a highly stable luminescent zirconium-based MOF (PCN-128Y) for the detection and removal of TC in water was created. PCN-128Y was constructed by tetraphenylethylene (TPE)-based ligand H4ETTC (which can serve as fluorophore and its mission can be quenched by TC) and Zr6 clusters (with coordination sites terminal OH/H2O which can facilitation of TC absorption). The synthesis of PCN-128YZrCl4 started from mixing ultrasonically of H4ETTC (60 mg, 0.072 mmol) and trifluoroacetic acid (0.08 mL) in Pyrex tube contained 8 mL DMF, then was heated at 120 °C for 48 h. The harvested white, solid product was transferred into a mixture of DMF and HCl, then stirred at 100 °C in an oil bath for 12 h. The centrifugation separation was performed and the product washed by with DMF and acetone five times. The yellow product of PCN-128 was obtained after centrifugation and drying at 70 °C for 6 h under vacuum conditions. The application of PCN-128 in TC sensing was successful with significant luminescence quenching (0.1 mM quenched 90% of PCN-128 luminescence) in 1 min [267].
However, the high selectivity was not well achieved where tested antibiotics presented 5–40% fluorescent quenching capacity except TC. Therefore, a nanoscale luminescent MOF (ln-sbdc) was synthesized from In3+ (metal ion) and ligand of trans-4,4-stilbenedicarboxylate (sbdc2−) for recognition of TCs over a series of other kinds of antibiotics in food and the environment [264]. The synthesis of ln-sbdc MOF was performed at room temperature by mixing InCl3 with H2sddc in the DMF-H2O solvent. Synthesized MOFs which were successfully applied in the detection of tetracycline series antibiotics included tetracycline, chlortetracycline, and oxytetracycline with DLs of 0.28–0.30 μM. The selectivity test showed that the other eight tested kinds of antibiotics did not cause an equable change in its emission [264].
The application of MOFs in SERS technology has provided a new route for pesticide detection by embedding NPs with MOFs. Cao (2017) successfully embedded AUNPs into MOFs (MOF-199, Uio-66, and Uio067) for SERS enhancement. The synthesized AuNPs-MOF-199, AuNPs-Uio-66, and AuNPs-Uio-67 composites exhibited excellent SERS activity. The application of developed approaches to the detection of acetamiprid was successfully achieved with DL of 0.02 μM, 0.009 μM, and 0.02 μM [268].
2.6. Persistent Organic Pollutants (POPs)
Persistent organic pollutants (POPs) are various classes of toxic organic compounds that can persist in the environment and have the potential to bio-accumulate in biological organisms, resulting in a variety of health effects in both animals and humans. As a result, POPs have been classified as important environmental and food contaminants due to their resistance to degradation, ability to travel long distances by air, water, and sedimentation to new environmental media located far away from the original released source [269]. These POPs have a long half-life spread in the environment for a long period of time, which may accumulate and increase significantly in the food chain as well as in the living organism and have an adverse effect on human beings and the environment in general [116,270]. Therefore, it is greatly important to establish simple, rapid, low-cost and sensitive analytical methods for trace detection of POPs in food and the environment. Recently, the conventional method has been developed and applied to POPs detection [271]. However, these methods can provide reliable analytical results but generally require complicated sample preparation processes and skilled personnel. Therefore, is urgent to develop new methods that are highly efficient and easy to perform for the detection POPs.
Based on remarkable luminescence properties of lanthanide MOFs (Ln-MOFs) and their applications as luminescent sensors, a new Ln-MOF 1 was synthesized for detection of polychlorinated benzenes including 1,2,4-trichlorobenzene (1,2,4-TCB), 1,2,3,4-tetracholobenzene (1,2,3,4-TCB), 1,2,3,5-tetracholorobenzene (1,2,4,5-tcb), pentacholorobenzene (PeCB), and hexachlorobenzene (HCB). The synthesis of [(Eu2(L)3(DMF)2].DMF.MeOH}n (Ln-MOF 1, H2L = 5-(4H-1,2,4-triazol-4-yl)benzene-1,3-dicarboxylic acid, MeOH = methanol, DMF = N, N-dimethylformamide) was performed through a coordination symmetry approach [272]. The systematical luminescence studies showed that Ln-MOF 1 have a quenching ability on detecting polychlorinated benzenes series, and the increasing of the chlorine atoms number on benzene corresponded to decreasing luminescent intensity [272].
This entry is adapted from the peer-reviewed paper 10.3390/foods11030382
This entry is offline, you can click here to edit this entry!
