Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Luisa Di Gioia | + 3157 word(s) | 3157 | 2022-01-05 03:54:58 | | | |
| 2 | Amina Yu | -14 word(s) | 3143 | 2022-02-07 07:35:53 | | | | |
| 3 | Amina Yu | + 1 word(s) | 3144 | 2022-02-08 02:09:13 | | | | |
| 4 | Amina Yu | Meta information modification | 3144 | 2022-02-09 02:52:17 | | | | |
| 5 | Amina Yu | -16 word(s) | 3128 | 2022-02-09 03:02:17 | | | | |
| 6 | Amina Yu | Meta information modification | 3128 | 2022-02-10 10:00:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Di Gioia, M.L. Catalytic Synthesis of Glycerol Carbonate. Encyclopedia. Available online: https://encyclopedia.pub/entry/19065 (accessed on 08 February 2026).
Di Gioia ML. Catalytic Synthesis of Glycerol Carbonate. Encyclopedia. Available at: https://encyclopedia.pub/entry/19065. Accessed February 08, 2026.
Di Gioia, Maria Luisa. "Catalytic Synthesis of Glycerol Carbonate" Encyclopedia, https://encyclopedia.pub/entry/19065 (accessed February 08, 2026).
Di Gioia, M.L. (2022, February 01). Catalytic Synthesis of Glycerol Carbonate. In Encyclopedia. https://encyclopedia.pub/entry/19065
Di Gioia, Maria Luisa. "Catalytic Synthesis of Glycerol Carbonate." Encyclopedia. Web. 01 February, 2022.
Copy Citation
Glycerol carbonate (GC) belongs to the family of organic carbonates that are regarded as very typical “green chemistry” products for their unique advantages in many fields, such as high boiling point solvents, pharmaceutical intermediates, and material intermediates.
glycerol
green chemistry
by-product
glycerol carbonate
catalysis
1. Routes for Glycerol Carbonate (GC) Synthesis
There are four main synthetic routes (Figure 1) reported at this stage to convert glycerol into racemic glycerol carbonate, namely (1) reaction of glycerol with phosgene, (2) carbonylation of glycerol by urea, (3) transesterification of glycerol with dimethyl carbonate or ethylene carbonate, and (4) reaction of glycerol with carbon dioxide or carbon monoxide [1][2].
Figure 1. Main synthetic routes to glycerol carbonate.
2. Phosgene Route
As early as 1948, the method to produce glycerol carbonate was developed by Strain [3] by using glycerol and phosgene (Figure 1).
The catalysts used in this route are some common alkali metal salts and alkaline-earth metal salts, such as NaOH, Na2CO3, Ca(OH)2. Subsequent literature reported that pyridine, which is basic due to the lone electron pair on the nitrogen atom accepting protons, can also catalyze the reaction of glycerol and phosgene to produce GC [2].
Although the phosgene route has good reaction performance, it not only needs to be carried out at a very low temperature (−70 °C), but also the raw materials are highly toxic chemical products, which are not suitable for transportation and pollute the environment. At the same time, the by-product HCl will corrode equipment, and the complicated process of preparing glycerol carbonate leads to a long operation cycle, which is contrary to the concept of contemporary green chemical industry and belongs to the elimination process [4][5][6].
3. Carbonylation by Urea
Synthesis of GC from glycerol and urea in the presence of a suitable catalyst is an attractive method of production, also known as the urea method. The advantages of this reaction system are as follows: the raw materials glycerol and urea are non-toxic, cheap, and easy to obtain, no solvent is added in the reaction, the reaction conditions are mild, and the glycerol conversion rate is high [7].
The by-product of the reaction is only NH3, which can be recovered and coupled with CO2 to generate urea, realizing the indirect synthesis of GC from CO2 and glycerol. It can be argued that urea represents an activated form of CO2. The industrial process utilizes the Bosch–Meiser urea route, developed in 1922 [8]. This makes urea a green and good alternative source for carbonylation reactions. [9].
Nevertheless, in order to promote the reaction balance to shift to the right, it is necessary to continuously remove the generated ammonia gas, which will pollute the environment, under reduced pressure (40–50 mbar), thus increasing investment costs.
The reaction mechanism of the urea route has been studied extensively over the past years. Rubio-Marcos et al. [10] proposed that the hydroxyl group of glycerol attacks the carbonyl carbon atom in urea under the action of the catalyst. After the removal of ammonia gas, a ring-closure reaction will occur to produce glycerol carbonate. Nevertheless, the water loss at this stage can result in the formation of 4-(hydroxymethyl)oxazolidin-2-one as side product; furthermore, GC, after its formation, could subsequently react with urea to yield (2-oxo-1,3-dioxolan-4-yl)methyl carbamate, as shown in Scheme 1.
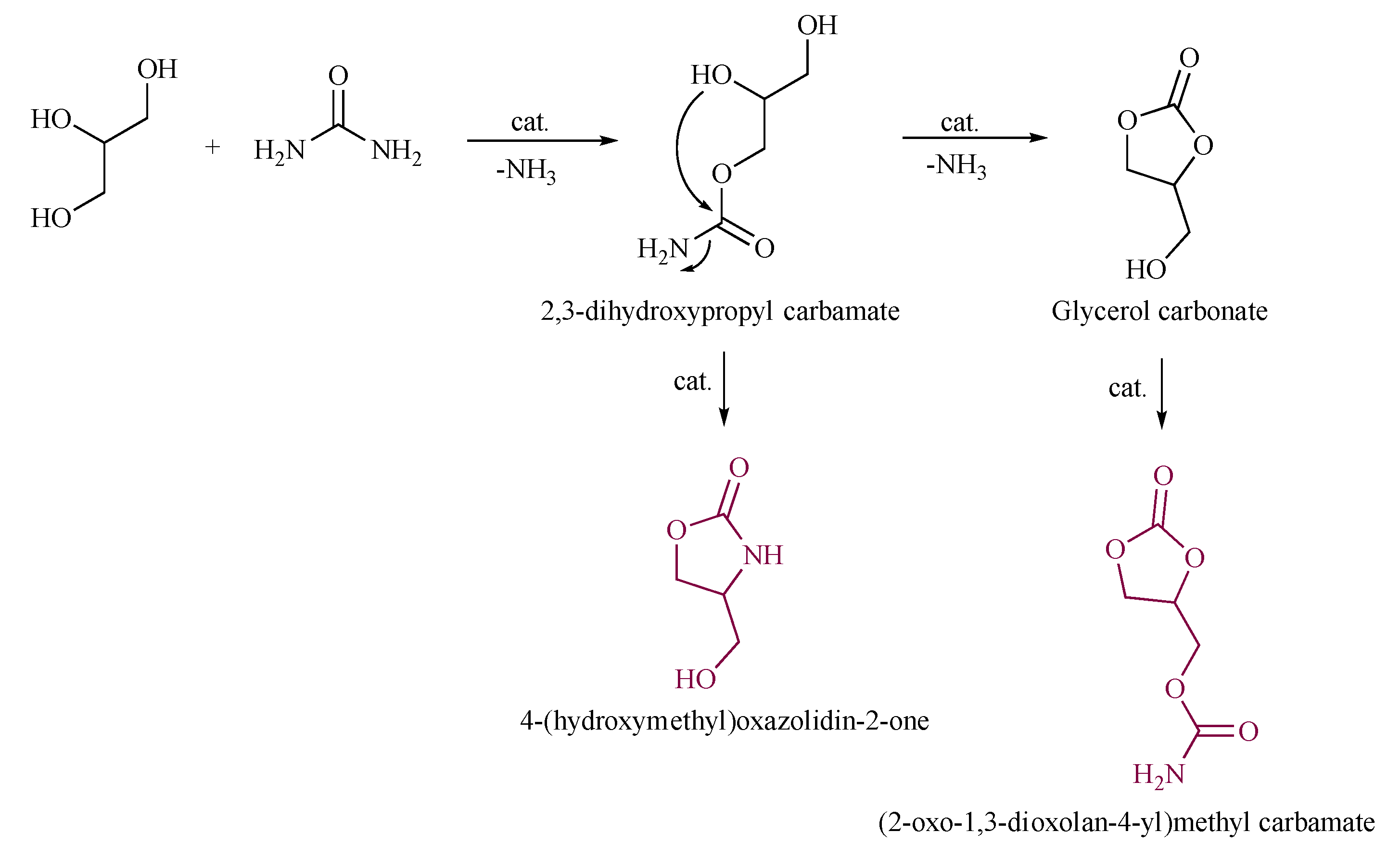
Scheme 1. Rubio-Marcos proposed reaction pathway of urea and glycerol.
Although it is difficult to establish a clear and general reaction mechanism theoretically, some researchers have used infrared spectroscopy and other methods to explore the reaction mechanism in depth. Calvino-Casilda et al. [11] showed through real-time attenuated total reflection infrared spectroscopy that the first step is a fast step, and the second step is a slow step, that is, the step that determines the reaction time. The reaction conditions must be strictly optimized to prevent the GC and urea from continuing to react to form glycerol carbonate carbamate (Scheme 1) [12].
3.1. Catalyst Application of Urea Route
The GC yield of the reaction system with the urea route is low, and a catalyst must be used to obtain a higher glycerol conversion rate [13]. Consequently, the design and selection of catalysts are particularly critical. The following catalysts bearing Lewis acidic sites seem to produce satisfying results with significant yields: manganese sulfate, zinc oxide, zinc sulfate, zinc chloride, metal oxides (CaO, La2O3, MgO, ZrO2, Al2O3), γ-zirconium phosphate, mixed oxides HTc-Zn derived from hydrotalcite, Co3O4/ZnO nanodispersion, gold-supported zeolite ZSM-5 catalyst, lanthanum oxide as a solid base catalyst, polymer-supported ionic liquids, and samarium-exchanged heteropolytungstate [4]. They are mainly divided into the following four categories of catalysts: metal salt, ionic liquid, solid acid or base, and zinc-based solid catalysts (Figure 2).
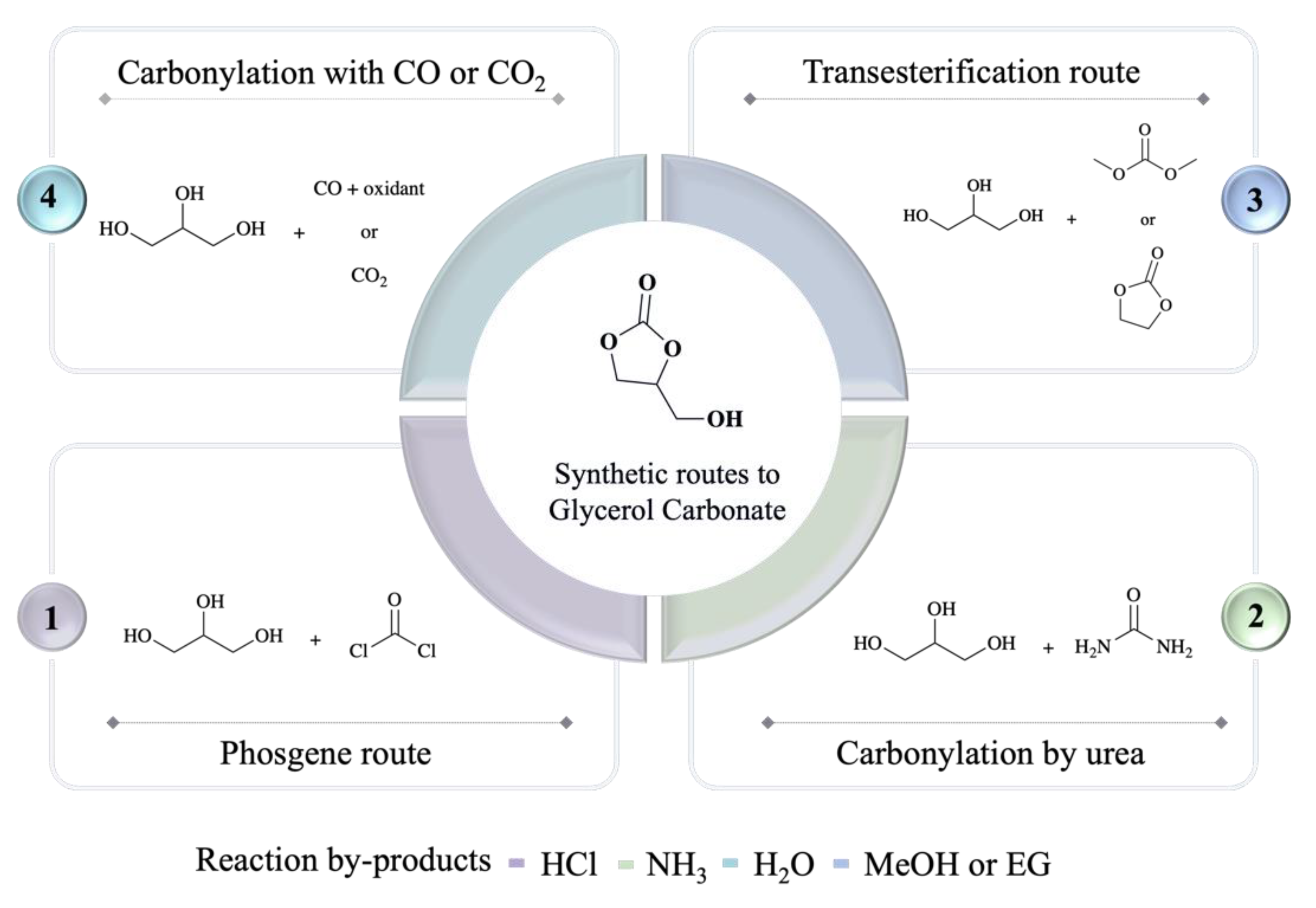
Figure 2. Main categories of catalysts in GC synthesis through urea route.
3.2. The Future of the Urea Route
The glycerolysis of urea represents an interesting synthetic procedure for GC that may have an industrial application. The good conversion of glycerol, the high selectivity, the easy separation of the catalyst and its full recovery, as well as the recovery of NH3 and the continuous separation of carbonate, make the methodology described very promising.
Among the reported catalysts for this route, the industrial application prospects of zinc-based solid catalysts and solid base catalysts are better [14][15]. At the same time, compared with homogeneous catalysts, heterogeneous catalysts have more research value because they are beneficial to product separation and catalyst recovery.
All in all, in the future development and design of high-efficiency heterogeneous catalysts, the goal should be to achieve the unity of high activity and high stability of the catalyst, and to clarify the role of Lewis acidic-alkaline sites on the catalyst surface in the activation reactants, thereby increasing the conversion rate of glycerol and the selectivity of GC
4. CO Oxidative Carbonylation Route
Carbonylation is a type of reaction in which carbonyl groups are introduced into the framework of organic compounds. This is an important method for preparing carbonyl compounds such as aldehydes and ketones [16]. Carbon monoxide is one of the most important carbonylation reagents. The carbonylation method is used to directly synthesize GC using CO, O2 and glycerol as raw materials under the action of a catalyst.
The reaction system is generally carried out under the conditions of 0.5–5 MPa and 70–170 °C. The reaction raw materials are cheap and easy to obtain, the reaction by-product is only water, and the atom utilization rate is high. It can also realize the synthesis of the target product in one step without intermediate reaction steps. Under certain reaction conditions, both CO2 and CO can be used as carbonylation reagents to react with glycerol to generate GC, but compared with CO2, CO has higher chemical activity. In the reaction of glycerol and CO to produce GC, the most common oxidant is oxygen, which can afford GC in high yield under relatively mild conditions [17].
Therefore, such a route realizes the sustainable conversion of cheap and easily available raw materials into high value-added chemicals.
4.1. The Future of CO Oxidative Carbonylation Route
All things considered, the above-mentioned experimental exploration provide a worthy point of view for future research on the oxidative carbonylation of glycerol to synthesize GC.
In summary, in the reaction process of this route, the most studied catalyst is the metal palladium catalyst, and, among them, the PdCl2(phen)-KI catalyst system had the best catalytic effect [18][19]. The advantage of this synthetic route is that GC can be generated under relatively mild conditions, the product is easy to separate, the selectivity of the product is high, and the yield is high. However, as CO has certain toxicity and explosiveness, its industrial application is restricted.
4.2. CO2 Direct Carbonylation Route
Also known as the CO2 conversion method, it refers to the direct reaction of CO2 and glycerol under certain conditions to produce GC.
Carbon dioxide is the main greenhouse gas, which is cheap and easy to obtain and non-toxic, while glycerol is a large by-product of the biodiesel industry. If the two are combined to synthesize high value-added chemicals, it will turn double waste into “treasure”, has extremely high environmental value. In addition, the atomic utilization rate of the reaction can reach 87% [20], and the only by-product of the reaction is water, which shows that the reaction system is highly compatible with the concept of atomic economy and green chemistry.
The biggest problem with this route is that it is difficult to break the carbon-oxygen double bond in the CO2 molecule, which makes the chemical properties of CO2 highly stable and difficult to activate.
The choice of suitable combinations of catalyst and dehydration agent is crucial to achieving feasible yields in the synthesis of GC from glycerol and carbon dioxide. Usually, quiet harsh conditions are necessary with temperatures ranging from 120 °C to 180 °C and pressures varying from 30 bar to 150 bar [21]. Nevertheless, the current research shows that the glycerol conversion rate and GC yield of this route are not high, and the production cost is relatively high.
In order to solve the dehydration problem of the reaction, it was attempted the direct synthesis of GC with glycerol and carbon dioxide under the combined action of 1,8-diazabicycloundec-7-ene/CH2Br2 [22]. This method used mild reaction conditions (70 °C, 1 MPa) and high yield of GC was obtained (86%). However, CH2Br2 is toxic and expensive, which hinders the industrial application of this method.
It was reported that the yield of GC could be enhanced when acetonitrile was used as solvent and dehydration reagent in the carbonylation of glycerol and CO2. Such as, Podila et al. [23] showed that dibutyltin oxides also catalyze the synthesis of GC, resulting in yields of 7% in presence of acetonitrile. Additionally, in the presence of acetonitrile, La2O3 in combination with ZnO or Cu resulted in yields of up to 15% as reported from other research groups [24][25][26].
Although the improved yield was obtained using acetonitrile as the dehydration agent [27], acetonitrile is easily hydrolyzed to acetic acid, which could etherify with glycerol to decrease the yield of GC. The dehydration agent is important for the carbonylation of alcohols with CO2. 2-Cyanopyridine was also reported to be an effective dehydration agent [28][29][30]. For example, Liu et al. [31] found it to be effective for the carbonylation of glycerol with CO2 directly catalyzed by the CeO2 catalyst. Additionally, as reported by Su et al. [32], it was shown that if 2-cyanopyridine is used as dehydration agent and promoter, a GC yield of 18.7% was obtained without any further additives. Recently, Qiao Zhang et al. [33] described the application of CaC2 as a dehydrating agent for the direct synthesis of carbamates from amines, CO2, and MeOH. Based on the use of CaC2, GC was successfully and directly synthesized from glycerol and CO2 under zinc catalysis and N-donor ligand, in 92% yield. CaC2 itself is also a sustainable chemical and promotes the transformation of CO2 into valuable products.
For developing a green methodology, McGregor et al., for the first time, demonstrated the catalytic activities of untreated biochar and biochar ash, catalytic material that can readily be produced from low-value biomass residues, in the synthesis of GC from the reaction of glycerol, acetonitrile, and CO2 [34].
It was provided novel insights into the behavior of dehydrating agents during the conversion of glycerol in the presence of carbon dioxide and a catalyst [35]. Particularly, they observed that conducting the reaction in the presence of adiponitrile resulted in a five-fold increase in GC yield when compared to acetonitrile, which is currently the most applied dehydrating agent.
In a very recent paper, Hu et al. [36] prepared a cobalt-based zeolitic imidazolate framework-67 (ZIF-67) as a catalyst for the carboxylation of glycerol in the presence of acetonitrile. The conversion, yield, and selectivity achieved with ZIF-67 were 32, 29, and 92%, respectively, conducting the reaction at 210 °C for 12 h. ZIF- 67 exhibited high crystallinity, a high specific surface area, good thermal stability as well as numerous Lewis basic sites, indicating its high catalytic activity.
In recent years, photocatalysis is becoming a hot field as introducing light into the thermally driven reaction system can improve the catalytic performance. He et al. [37] prepared a series of xLa2O2CO3–ZnO catalysts, which were used for the photo-thermal transformation of glycerol and CO2 into GC. The photo-thermal synergism as well as the cooperation between ZnO and La2O2CO3 contributed to its catalytic performance, thus achieving a glycerol conversion of 6.9% under the reaction conditions of 150 °C, 5.5 MPa CO2, and 20 mmol g−1 with a reaction time of 6 h when 20% La2O2CO3–ZnO was used as the catalyst.
4.3. The Future of CO2 Direct Carbonylation Route
Although many researchers have worked on this route, experiments have proved that the direct reaction between glycerol and CO2 is extremely difficult. This reaction is thermodynamically limited from the production of water during the process; therefore, in order to remove water and to shift the equilibrium towards GC, the use of suitable dehydrating agents has been proposed over the years. Nevertheless, most of the reagents employed to remove water also result in formation of other by-products.
In many experimental examples, the following similar difficulties and problems are encountered: the conversion of glycerol is too low, the carbon dioxide reaction activity is reduced, the reaction needs to be carried out under high pressure conditions, the dehydrating agents are not effective.
Therefore, even if the reaction itself is extremely attractive and important, its application is still very limited. Due to the limitation of thermodynamics, it is impossible to promote the reaction between glycerol and CO2 simply by developing a new high-efficiency catalyst. Moreover, the method of adding dehydrating agents to move the reaction to the right has very limited effect. In short, for the current research on this reaction system, the development of a more effective reaction system will be the main way to solve the problem.
5. Transesterification Route
In recent years, the transesterification of glycerol and organic carbonates to synthesize GC has attracted attention. The organic carbonates raw materials used are mainly ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC) and diethyl carbonate (DEC), while the catalysts are mainly inorganic or basic compounds, and lipase catalyst. In the alkaline catalytic environment, during the transesterification reaction, an excess of carbonate is generally added as both a reactant and a solvent, including the most widely used EC and DMC [2]. Figure 1 above depicts the main process of synthesizing GC through the transesterification reaction of EC or DMC with glycerol.
GC intermediates will be generated during the reaction, and the by-products of the reaction will be ethylene glycol (EG) or methanol, depending on the type of reactant carbonate. Li and Wang [20] studied the main differences between using EC and DMC as reactants and found that when EC is used as the reactant, the reaction equilibrium constant decreases with increasing temperature, while when DMC is used as the reactant, it is in contrast. For example, when using Al/Ca mixed oxide as the catalyst, the temperature adopted in the reaction between EG and glycerol is 35 °C [38], while to achieve the same GC conversion and selectivity, the temperature to be used in the reaction between DMC and glycerol should be higher than 80 °C [39]. In this way, it seems easier and more energy-efficient to synthesize GC by the reaction of EG and glycerol.
However, the separation process of the product should also be considered. From the physical properties of the compound, it is known that the boiling points of EC (261 °C) and EG (197 °C) are higher than the boiling points of DMC (90 °C) and methanol (64.5 °C). The separation of EC and EG requires more energy input. Therefore, from the perspective of energy saving, the advantages and disadvantages of the two reactions can offset each other [40].
5.1. Catalyst-Mediated Transesterification of EC with Glycerol
Ethylene carbonate is the simplest substance containing ODO groups, which can provide carbonate for the transesterification reaction. The reaction pathway is divided into the following two steps: the first step is to synthesize EC with titanium dioxide and ethylene oxide; the second step is through the transesterification of EC with glycerol to synthesize GC [41]. With EC as the reactant, the catalysts currently used in research include CaO, Al/Ca/Mg mixed oxides and hydrotalcite-like compounds, basic resins and molecular sieves, and also ionic liquids. The conversion rate after synthesis of GC is generally between 85–100%, the selectivity of GC is between 84–99% [41].
Climent et al. [40] selected hydrotalcite mixed metal oxides (Al/Mg and Al/Li) and alkaline oxides (MgO and CaO) as catalysts. The amount of catalyst was 0.5%, the reaction temperature was 35 °C, and finally a glycerol conversion of 98% was reached. They also showed that the strong alkaline Al/Ca-mixed oxide (AlCaMO) had the best catalytic activity. In the same year, Cho et al. [41] used ionic liquid as the catalyst, which was immobilized on the mesoporous material MCM41 (Mobil Composition of Matter No. 41, framework). The study showed that RNX-MCM41 with long alkyl chain had higher catalytic performance and its activity remained basically unchanged after it was used 3 times. Nevertheless, the method of synthesizing GC from glycerol and EC has the problem of not easily available raw materials, and the price is high. The by-product glycol produced in this reaction has a high boiling point making it difficult to separate from the product.
5.2. Catalyst-Mediated Transesterification of DMC with Glycerol
DMC is an environmentally friendly green chemical product, approved by European Union as a non-toxic substance that has become the most popular transesterification raw material in recent years. DMC is a solvent with a higher evaporation temperature and a faster evaporation rate. It has excellent solubility, can be used as a reactant and a solvent in the reaction system at the same time, no additional solvent is required. Not only does its reactivity meet the industry, but also its synthetic route is green and pollution-free [42][43]. However, the transesterification reaction of DMC with glycerol to afford GC is slow; hence, the catalyst plays a vital role in faster and selective production of GC. For this purpose, in recent years, the investigation of the reaction system of glycerol and DMC to synthesize GC, led to the search of different catalysts to be used in this reaction including alkali and alkaline-earth metal catalysts, mixed oxide catalysts, biological enzyme catalysts and ionic liquid catalysts [44][45][46][47][48][49].
5.3. The Future of the Transesterification Route
In view of the advantages and disadvantages of various types of catalysts, the best catalyst can be selected according to specific needs to suit the operating environment and economic conditions. For example, when considering economic benefits and production efficiency, you can choose the cheap alkaline-earth metal CaO, without considering its shortcomings of easy inactivation; when you need to obtain high purity and high value-added chemicals, you can choose high activity, stable but expensive catalysts, such as ILs, et cetera. At the same time, from the process point of view, the reactants are either not easy to obtain, or the synthesis cost is high, the process is complicated, and the reaction products are difficult to separate. Therefore, for the development of GC green synthesis, it is particularly important to seek a more optimized process for the reaction system.
References
- Sonnati, M.O.; Amigoni, S.; de Givenchy, E.P.T.; Darmanin, T.; Choulet, O.; Guittard, F. Glycerol carbonate as a versatile building block for tomorrow: Synthesis, reactivity, properties and applications. Green Chem. 2012, 15, 283–306.
- De Caro, P.; Bandres, M.; Urrutigoïty, M.; Cecutti, C.; Thiebaud-Roux, S. Recent Progress in Synthesis of Glycerol Carbonate and Evaluation of Its Plasticizing Properties. Front. Chem. 2019, 7, 308.
- Strain, F. Carbonate-Haloformate of Glycerol and Method of Producing Same. U.S. Patent 2446145, 27 July 1948.
- Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.-L.; Lam, M.K.; Chen, W.-H. Organic Carbonate Production Utilizing Crude Glycerol Derived as By-Product of Biodiesel Production: A Review. Energies 2020, 13, 1483.
- Dibenedetto, A.; Angelini, A. Chapter Two-Synthesis of Organic Carbonates. In Advances in Inorganic Chemistry; Aresta, M., van Eldik, R., Eds.; Academic Press: Amsterdam, The Netherlands, 2014; Volume 66, pp. 25–81. ISBN 9780124202214.
- Poliakoff, M.; Licence, P. Green chemistry. Nature 2007, 450, 810–812.
- Singh, G.; Pradhan, G.; Pradhan, S.; Sharma, Y.C. Transformation of Biodiesel waste Glycerol to Value added Glycerol Carbonate. Chem. Sci. Rev. Lett. 2020, 9, 1003–1013.
- Meessen, J.H.; Petersen, H. Urea. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000.
- Shukla, K.; Srivastava, V.C. Synthesis of organic carbonates from alcoholysis of urea: A review. Catal. Rev. 2017, 59, 1–43.
- Rubio-Marcos, F.; Calvino-Casilda, V.; Bañares, M.A.; Fernandez, J.F. Novel hierarchical Co3O4/ZnO mixtures by dry nanodispersion and their catalytic application in the carbonylation of glycerol. J. Catal. 2010, 275, 288–293.
- Calvino-Casilda, V.; Mul, G.; Fernández, J.; Rubio-Marcos, F.; Bañares, M.A. Monitoring the catalytic synthesis of glycerol carbonate by real-time attenuated total reflection FTIR spectroscopy. Appl. Catal. A Gen. 2011, 409-410, 106–112.
- Lopez-Sanchez, J.A.; Dimitratos, N.; Glanville, N.; Kesavan, L.; Hammond, C.; Edwards, J.K.; Carley, A.F.; Kiely, C.J.; Hutchings, G.J. Reactivity studies of Au–Pd supported nanoparticles for catalytic applications. Appl. Catal. A Gen. 2011, 391, 400–406.
- Zhang, H.; Li, H.; Wang, A.; Xu, C.; Yang, S. Progress of Catalytic Valorization of Bio-Glycerol with Urea into Glycerol Carbonate as a Monomer for Polymeric Materials Heng. Adv. Polym. Technol. 2020, 2020, 1–17.
- Lari, M.G.; de Moura, A.B.L.; Weimann, L.; Mitchell, S.; Mondelli, C.; Pérez-Ramírez, J. Design of a technical Mg-AI mixed oxide catalyst for the continuous manufacture of glycerol carbonate. J. Mat. Chem. A 2017, 5, 16200–16211.
- Zhang, P.; Liu, L.; Fan, M.; Dong, Y.; Jiang, P. The value-added utilization of glycerol for the synthesis of glycerol carbonate catalyzed with a novel porous ZnO catalyst. RSC Adv. 2016, 6, 76223–76230.
- Peng, J.-B.; Geng, H.-Q.; Wu, X.-F. The Chemistry of CO: Carbonylation. Chem 2018, 5, 526–552.
- Ozorio, L.P.; Mota, C.J.A. Direct Carbonation of Glycerol with CO2 Catalyzed by Metal Oxides. ChemPhysChem 2017, 18, 3260–3265.
- Doro, F.; Winnertz, P.; Leitner, W.; Prokofieva, A.; Müller, T.E. Adapting a Wacker-type catalyst system to the palladium-catalyzed oxidative carbonylation of aliphatic polyols. Green Chem. 2011, 13, 292–295.
- Pearson, D.M.; Conley, N.R.; Waymouth, R.M. Palladium-Catalyzed Carbonylation of Diols to Cyclic Carbonates. Adv. Synth. Catal. 2011, 353, 3007–3013.
- Li, J.; Wang, T. Chemical equilibrium of glycerol carbonate synthesis from glycerol. J. Chem. Thermodyn. 2011, 43, 731–736.
- Lukato, S.; Kasozi, G.N.; Naziriwo, B.; Tebandeke, E. Glycerol carbonylation with CO2 to form glycerol carbonate: A review of recent developments and challenges. Curr. Res. Green Sustain. Chem. 2021, 4, 100199.
- Na Lim, Y.; Lee, C.; Jang, H.-Y. ChemInform Abstract: Metal-Free Synthesis of Cyclic and Acyclic Carbonates from CO2 and Alcohols. ChemInform 2015, 46.
- Podila, S.; Plasseraud, L.; Cattey, H.; Ballivet-Tkatchenko, D.; Carrera, G.V.S.M.; Nunes Da Ponte, M.; Neuberg, S.; Behr, A. Synthesis of 1,2-glycerol carbonate from carbon dioxide: The role of methanol in fluid phase equilibrium. Indian J. Chem. 2012, 51, 1330–1338.
- Li, H.; Gao, D.; Gao, P.; Wang, F.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. The synthesis of glycerol carbonate from glycerol and CO2 over La2O2CO3–ZnO catalysts. Catal. Sci. Technol. 2013, 3, 2801–2809.
- Zhang, J.; He, D. Synthesis of glycerol carbonate and monoacetin from glycerol and carbon dioxide over Cu catalysts: The role of supports. J. Chem. Technol. Biotechnol. 2015, 90, 1077–1085.
- Zhang, J.; He, D. Surface properties of Cu/La2O3 and its catalytic performance in the synthesis of glycerol carbonate and monoacetin from glycerol and carbon dioxide. J. Colloid Interface Sci. 2014, 419, 31–38.
- Li, H.; Jiao, X.; Li, L.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y.; Zhang, B. Synthesis of glycerol carbonate by direct carbonylation of glycerol with CO2 over solid catalysts derived from Zn/Al/La and Zn/Al/La/M (M = Li, Mg and Zr) hydrotalcites. Catal. Sci. Technol. 2015, 5, 989–1005.
- Honda, M.; Tamura, M.; Nakagawa, Y.; Nakao, K.; Suzuki, K.; Tomishige, K. Organic carbonate synthesis from CO2 and alcohol over CeO2 with 2-cyanopyridine: Scope and mechanistic studies. J. Catal. 2014, 318, 95–107.
- Bansode, A.; Urakawa, A. Continuous DMC Synthesis from CO2 and Methanol over a CeO2 Catalyst in a Fixed Bed Reactor in the Presence of a Dehydrating Agent. ACS Catal. 2014, 4, 3877–3880.
- Unnikrishnan, P.; Darbha, S. Direct synthesis of dimethyl carbonate from CO2 and methanol over CeO2 catalysts of different morphologies. J. Chem. Sci. 2016, 128, 957–965.
- Liu, J.; Li, Y.; Zhang, J.; He, D. Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO2 preparation methods and reaction parameters. Appl. Catal. A Gen. 2016, 513, 9–18.
- Su, X.; Lin, W.; Cheng, H.; Zhang, C.; Wang, Y.; Yu, X.; Wu, Z.; Zhao, F. Metal-free catalytic conversion of CO2 and glycerol to glycerol carbonate. Green Chem. 2017, 19, 1775–1781.
- Zhang, Q.; Yuan, H.-Y.; Lin, X.-T.; Fukaya, N.; Fujitani, T.; Sato, K.; Choi, J.-C. Calcium carbide as a dehydrating agent for the synthesis of carbamates, glycerol carbonate, and cyclic carbonates from carbon dioxide. Green Chem. 2020, 22, 4231–4239.
- Collett, C.; Mašek, O.; Razali, N.; McGregor, J. Influence of Biochar Composition and Source Material on Catalytic Performance: The Carboxylation of Glycerol with CO2 as a Case Study. Catalysts 2020, 10, 1067.
- Razali, N.; McGregor, J. Improving Product Yield in the Direct Carboxylation of Glycerol with CO2 through the Tailored Selection of Dehydrating Agents. Catalysts 2021, 11, 138.
- Hu, C.; Yoshida, M.; Chen, H.-C.; Tsunekawa, S.; Lin, Y.-F.; Huang, J.-H. Production of glycerol carbonate from carboxylation of glycerol with CO2 using ZIF-67 as a catalyst. Chem. Eng. Sci. 2021, 235, 116451.
- Li, Y.; Liu, H.; Ma, L.; Liu, J.; He, D. Transforming glycerol and CO2 into glycerol carbonate over La2O2CO3–ZnO catalyst—A case study of the photo-thermal synergism. Catal. Sci. Technol. 2021, 11, 1007–1013.
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Maestro-Madurga, B.; Pesquera-Rodríguez, A.; Ramírez-López, C.; Lorenzo-Ibarreta, L.; Torrecilla-Soria, J.; Villarán-Velasco, M.C. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: Catalyst screening and reaction optimization. Appl. Catal. A Gen. 2009, 366, 315–324.
- Sahani, S.; Upadhyay, S.N.; Sharma, Y.C. Critical Review on Production of Glycerol Carbonate from Byproduct Glycerol through Transesterification. Ind. Eng. Chem. Res. 2020, 60, 67–88.
- Climent, M.J.; Corma, A.; De Frutos, P.; Iborra, S.; Noy, M.; Velty, A.; Concepción, P. Chemicals from biomass: Synthesis of glycerol carbonate by transesterification and carbonylation with urea with hydrotalcite catalysts. The role of acid–base pairs. J. Catal. 2010, 269, 140–149.
- Cho, H.J.; Kwon, H.M.; Jose, T.; Park, D.W. Synthesis of glycerol carbonate from ethylene carbonate and glycerol using im-mobilized ionic liquid catalysts. J. Ind. Eng. Chem. 2010, 16, 679–683.
- Lanjekar, K.; Rathod, V.K. Utilization of glycerol for the production of glycerol carbonate through greener route. J. Environ. Chem. Eng. 2013, 1, 1231–1236.
- Liu, P.; Derchi, M.; Hensen, E.J. Synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate over MgAl mixed oxide catalysts. Appl. Catal. A Gen. 2013, 467, 124–131.
- Gmehling, J.; Menke, J.; Krafczyk, J.; Fischer, J.; Fontaine, J.-C.; Kehiaian, H.V. Azeotropic Data for Binary Mixture. In Handbook of Chemistry and Physics; CRC Press LLC: Boca Raton, FL, USA, 2011; Volume 6.
- Sandesh, S.; Shanbhag, G.V.; Halgeri, A.B. Transesterification of Glycerol to Glycerol Carbonate Using KF/Al2O3 Catalyst: The Role of Support and Basicity. Catal. Lett. 2013, 143, 1226–1234.
- Bai, R.; Wang, Y.; Wang, S.; Mei, F.; Li, T.; Li, G. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate catalyzed by NaOH/γ-Al2O3. Fuel Process. Technol. 2013, 106, 209–214.
- Ramesh, S.; Debecker, D.P. Room temperature synthesis of glycerol carbonate catalyzed by spray dried sodium aluminate microspheres. Catal. Commun. 2017, 97, 102–105.
- Ramesh, S.; Devred, F.; Biggelaar, L.V.D.; Debecker, D.P. Hydrotalcites Promoted by NaAlO2 as Strongly Basic Catalysts with Record Activity in Glycerol Carbonate Synthesis. ChemCatChem 2018, 10, 1398–1405.
- Rittiron, P.; Niamnuy, C.; Donphai, W.; Chareonpanich, M.; Seubsai, A. Production of Glycerol Carbonate from Glycerol over Templated-Sodium-Aluminate Catalysts Prepared Using a Spray-Drying Method. ACS Omega 2019, 4, 9001–9009.
More
Information
Subjects:
Chemistry, Organic
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.6K
Entry Collection:
Organic Synthesis
Revisions:
6 times
(View History)
Update Date:
10 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




