Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Jesús Láinez-Ramos-Bossini | + 2295 word(s) | 2295 | 2022-01-29 09:02:09 | | | |
| 2 | Jason Zhu | Meta information modification | 2295 | 2022-01-30 07:45:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Láinez-Ramos-Bossini, A.J. CT Imaging Findings of Gastric Volvulus. Encyclopedia. Available online: https://encyclopedia.pub/entry/19019 (accessed on 14 January 2026).
Láinez-Ramos-Bossini AJ. CT Imaging Findings of Gastric Volvulus. Encyclopedia. Available at: https://encyclopedia.pub/entry/19019. Accessed January 14, 2026.
Láinez-Ramos-Bossini, Antonio Jesús. "CT Imaging Findings of Gastric Volvulus" Encyclopedia, https://encyclopedia.pub/entry/19019 (accessed January 14, 2026).
Láinez-Ramos-Bossini, A.J. (2022, January 29). CT Imaging Findings of Gastric Volvulus. In Encyclopedia. https://encyclopedia.pub/entry/19019
Láinez-Ramos-Bossini, Antonio Jesús. "CT Imaging Findings of Gastric Volvulus." Encyclopedia. Web. 29 January, 2022.
Copy Citation
Gastric volvulus (GV) is a life-threatening emergency condition that prompts emergent surgical management. Although many cases of GV have been described in the literature, its pathophysiology is still poorly understood. Here, we provide imaging evidence supporting that the pathophysiology of many GVs is based on caudal re-descent of hiatal hernia into the abdominal cavity
gastric volvulus
computed tomography
back-and-forth stomach
1. Introduction
Gastric volvulus (GV) is a rare complication secondary to twisting of the stomach more than 180° around its own axis, either transversally or longitudinally, resulting in a closed-loop obstruction [1][2][3][4]. The first reports of this entity date back to the end of the 19th century [5]. Since then, numerous case reports and series have been published, contributing to a better understanding of GV. Although sociodemographic data are limited [6], no significant differences in its incidence have been described in terms of sex or race, and it is more frequent in the fifth decade of life [2][7][8][9]. Because the clinical presentation of GV is nonspecific, imaging examinations are required for appropriate diagnosis. Multi-detector computerized tomography (MDCT) scanners offer excellent temporal and spatial resolution with multiplanar reformatting capability, high image quality and diagnostic reliability. Accordingly, MDCT is currently considered the ‘gold standard’ in the diagnosis of GV [10][11].
Several classification systems of GV have been described in the literature, emphasizing different aspects such as the degree of rotation (partial, <180° vs. total, >180°), etiology (primary vs. secondary), or time from onset of symptoms (acute vs. chronic) [12]. However, the most relevant classification of GV, which was described as early as 1912 [13] and completed in 1940 by Singleton [14][15], is topographic. This classification is based on the main axis of rotation and differentiates two main types of GV, namely organoaxial and mesenteroaxial. The former group is more frequent [16] and characterized by a longitudinal axis of rotation (imaginary line that joins the gastroesophageal and antropyloric junctions), so that the greater curvature lies above the lesser curvature. The latter group is defined by rotation around the axial axis of the stomach (imaginary line joining the greater and lesser curvatures), so that the gastroesophageal junction (GEJ) lies below the gastroduodenal junction. There is also a less frequent (incidence around 2%) form of GV in which rotation occurs in both rotational axes [16]. Although the topographic classification is useful, imaging findings are often confusing and difficult to interpret, leading to over- and under-diagnosis of GV [6]. Moreover, they provide little information regarding the underlying pathophysiological mechanism.
2. Pathophysiology of Acute Gastric Volvulus
2.1. Association between GV and PH
In current series, all cases of GV originated from HHs containing the gastric fundus or the entire stomach. This is contradictory to previous reports, which have consistently reported that most GV cases are associated with PHs [16][17][18][19]. Despite the presumed importance of this type of hernia in the pathophysiology of GV, there are very few insights on this aspect in recent literature. Paradoxically, excellent descriptions on the role of PH in GV can be found in old works by Culver [20] and by Gerson and Lewicki [21]. The former wrote an exhaustive description on the process that leads to GV through a case series of four patients with identical mechanisms. According to Culver, PH in GV develops through a diaphragmatic defect that is contiguous but not continuous with the esophageal hiatus. Herniation of the cardias into the chest through the said defect would result in the fundus and antrum eventually being located in the thoracic cavity. Re-herniation of the antrum into the abdomen through the diaphragmatic defect would then cause a space compromise, leading to GV after peristaltic movements originating from the herniated antrum. Although this theory reproduces a plausible mechanism based on the imaging findings of current series, no diaphragmatic defect was observed in any of current patients, neither during imaging examinations nor intraoperatively. Thus, Culver’s theory is not fully supported by the findings present in current series.
Twelve years after the study by Culver, Gerson and Lewicki [21] shed more light on this topic. They published a series including two cases through which they elucidated the more likely pathophysiologic mechanism involved in GV, namely subdiaphragmatic redescent of the fundus with fundic distension and crowding of the hernial orifice. To current knowledge, this is the only existing publication in which the pathophysiologic theory of GV is supported by consistent imaging examinations. However, it should be noted that all imaging exams used by these authors were based on barium swallow studies (i.e., not multiplanar imaging), and that they did not provide information on the type of HH before the acute presentation. Remarkably, these authors did not make the mechanism underlying GV contingent upon PH.
The question arises: why is PH so frequently associated with GV? PH is defined by stomach herniation with the gastroesophageal junction (GEJ) remaining in its normal anatomical position (i.e., the gastric fundus herniates into the mediastinum) [22]. Although not explicit in this definition, it is virtually assumed that the GEJ never moved from its normal location. However, in the case of a caudal re-herniation of a previously herniated stomach, the GEJ is pulled downward, mimicking a PH. This explains the common misinterpretation of previously published series and is supported by the imaging findings in current series, in which all cases of GV occurred in previous sliding (i.e., not paraesophageal) HHs.
Despite the illustrative and pedagogical nature of the studies by Culver and by Gerson and Lewicki, the underlying mechanisms of GV seem to have had no significant impact on the current understanding of GV. In fact, very few publications have discussed this theory and only in a marginal manner (e.g., [21]). Nevertheless, when analyzing images presented in most publications reporting GVs, it seems that this mechanism is fairly common. Finally, researchers would like to emphasize that, regardless of the type of HH associated with GV, the development of the latter mainly depends on the lack of sufficient abdominal anchorage of the stomach. This point has been largely discussed in previous works, which highlighted that the stomach is a highly mobile organ whose mobility increases when the supporting ligaments become loose or detached, initiating a cascade of events that may lead to GV [15][23].
2.2. Terminological Inconsistencies: Upside-Down Stomach, Chronic GV, Organoaxial GV
On the other hand, researchers would like to emphasize another controversial issue which concerns the concept of ‘upside-down stomach’ (UDS). Some of the sliding HHs that were present in current series would correspond to UDSs according to the definitions given by some authors, since there was a complete stomach herniation in the mediastinum with an inverted position of the lesser and greater curvatures. However, in current opinion, the term UDS has been used in a particularly inconsistent manner in the literature, being frequently interchanged with other terms, including ‘chronic GV’, ‘organoaxial GV’, and even ‘paraesophageal hernia’. For instance, Umemura et al. [22] pointed out that the UDS is usually caused by organoaxial volvulus, referencing a study by Gryglewski et al. [24], in which they present a case of ‘incarcerated UDS’ that is quite reminiscent of the cases in current series. According to some authors, UDS is a type of mixed (i.e., type 3) HH, whilst for others it is a different type of hernia [25]. Other authors have described it as a ‘type of large paraesophageal hernia’ [26]. In the above-mentioned article by Umemura et al. it is stated that organoaxial volvulus ‘is often called UDS’. Similarly, al Daoud et al. [18] stated that ‘paraesophogeal hernias (…) show up as an inverted stomach’, which coincides with the description by Carter et al., who refer to PH directly as UDS [15]. To top it all off, some authors have discussed the concept of ‘chronic GV’ and have posed the debate on whether chronic intrathoracic VG is -an end-stage of the evolution of a sliding hernia or an extension of a true type II paraesophageal hernia’, which remains unresolved to date [27]. For these reasons, researchers classified all patients in current series as having sliding hiatal hernias, regardless of the degree of torsion in their thoracic cavity.
These are just but a few examples that illustrate the obvious terminological inconsistencies and heterogeneous definitions of these concepts. However, beyond the terminological debates, one might wonder: should the finding of an entire herniated stomach result in surgical correction to prevent the development of GV? In this regard, radiologists should be aware of current controversies regarding the management of large HHs. It is crucial to distinguish between symptomatic PH and asymptomatic or minimally symptomatic HH. According to Collet et al. [28], only patients with symptomatic hernias should undergo surgery, and prophylactic repair to prevent acute incarceration should only be undertaken in patients younger than 75 in good condition. In addition, authors such as Andolfi et al. [29] have suggested that asymptomatic patients younger than 50 should also be considered for surgery, provided that a comprehensive review of the risks, benefits and alternatives available is thoroughly discussed with the patients. In conclusion, current opinions seem to advocate offering elective surgery to all symptomatic patients and to asymptomatic individuals at low operative risk. Nevertheless, more studies are needed to address the unanswered questions regarding the optimal management of large PHs [30].
2.3. Pathophysiology of GV: The ‘Back-and-Forth Stomach’
Based on the previous literature and on the imaging findings in current series, researchers propose a simplified mechanism explaining the development of GV from an HH. First, a sliding HH originates (Figure 1A), which progressively increases in size until it includes a large part or all of the stomach (including the antrum) in the thorax (Figure 1B,C). At this site it usually undergoes an axial rotation, predisposing the fundus to re-herniate into the abdomen, although horizontal rotation is not always present. Finally, there is a downward re-herniation of the fundus into the abdominal cavity through the esophageal hiatus, giving rise to obstruction due to inability to drain its content, and thus triggering acute GV (Figure 1D). The re-herniation of the fundus could be secondary to a sudden increase in intrathoracic pressure. It is logical to think that the three cases in current series in which an HH containing only the fundus was demonstrated probably evolved into a hernia containing the entire stomach since the antrum was located above the diaphragm in the acute presentation. In fact, the mean time from diagnosis of HH to the development of GV was similar in the group of patients in whom the hernia was demonstrated to contain only the fundus (x = 6.3 years, SD = 3.1) in comparison with those containing the entire stomach (x = 3.5 years, SD = 3.1). This supports the hypothesis that patients with HH containing the fundus eventually developed a complete HH prior to the development of GV, but this should be confirmed in future studies.
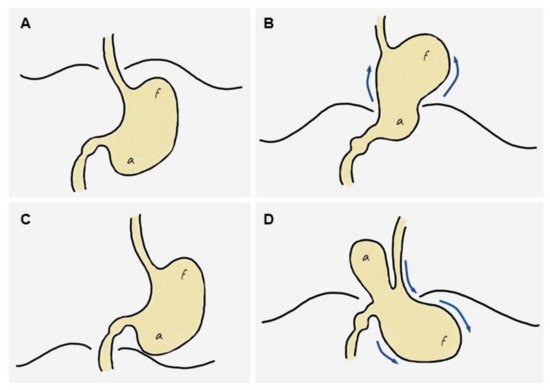
Figure 1. Diagram of the stages leading to the ‘back-and-forth’ stomach. (A) Normal stomach. (B) A portion of the cardias/fundus slides upwards into the mediastinum, leading to a sliding hiatal hernia, which increases progressively over time to eventually include most or all of the stomach in the mediastinum (including the antrum). (C) The entire stomach is located in the mediastinum and may rotate horizontally, predisposing the fundus to re-herniate into the abdomen. (D) A downward re-herniation of the fundus into the abdominal cavity through the esophageal hiatus occurs; the antrum normally lies above the diaphragm. The inability of the fundus content to be drained through the hernia neck leads to acute obstruction, i.e., gastric volvulus.
This pathogenesis gives rise to a highly specific CT semiology, characterized by the presence of the antrum lying above the diaphragm and the fundus below (usually dilated in the absence of NGT aspirate). Researchers believe that these findings should be interpreted unequivocally as a sign of GV in the clinical context of acute epigastric or low chest pain.
3. Clinical Features, Laboratory Abnormalities and Outcomes of GV in Current Series
Regarding the presenting symptoms, these depend on the rapidity of onset, degree of gastric rotation, amount of obstruction and final position of the stomach [1][8][12][31][32][33]. Current series is in agreement with previous studies [9][34], with epigastric or lower thoracic abdominal pain associated with vomiting being the most frequent clinical presentation and common in all cases. Borchardt’s triad, which includes the inability to pass the NGT, was found in three cases (42.9%), a significantly lower percentage than that described in classic series (70%) [7]. This could suggest that this type of GV allows better passage of the NGT than other subtypes and is in agreement with previous studies [20].
Abnormalities in blood parameters described in the literature are very broad and varied, and they include elevated aldolase, CK [35], amylase, alkaline phosphatase (secondary to a kinking of the common bile duct when the duodenum is rotated) [36] and elevated white blood cell count [20]. The findings in current series show leukocytosis with neutrophilia and discrete increase in CRP values in all cases.
Regarding patient management, all cases in current series were treated surgically by laparotomy, gastropexy and/or partial resection of the stomach, with or without fundoplication. These procedures represent the most traditional management of the acute episode [29][33], although other techniques have been described (e.g., gastrojejunostomy, Opolzer’s procedure, Tanner’s procedure) [19]. Endoscopic decompression and gastrostomy, described in elderly [1] and high-surgical-risk [37] patients as a conservative method of management, was not performed in any of current patients. Of note, these conservative strategies are not without risk since there is a significant risk of perforation [7], and cases of GV have even been reported in patients with gastrostomy [9][38]. All the approaches were performed by laparotomy, although the use of laparoscopy has been described in the literature since 2004 [39] in high-risk elderly patients [36][37], and successful outcomes have been reported using endoscopic techniques [40]. Currently, some authors consider it a safe and acceptable approach [33]. Finally, mortality associated with acute GV is variable. Classic series report mortality rates of 30–50% [17], while more recent series estimates are around 15–20% [27]. In current series, only one of the patients died during the postoperative period, which makes up 14.2% mortality, in agreement with mortality estimates.
References
- Okeny, P.K.; Abbassi, O.; Warsi, A. Second-look laparostomy for perforated gangrenous gastric volvulus to prevent total gastrectomy. BMJ Case Rep. 2018, 2018, bcr2017223060.
- Rashid, F.; Thangarajah, T.; Mulvey, D.; Larvin, M.; Iftikhar, S.Y. A review article on gastric volvulus: A challenge to diagnosis and management. Int. J. Surg. 2010, 8, 18–24.
- Peterson, C.M.; Anderson, J.S.; Hara, A.K.; Carenza, J.W.; Menias, C.O. Volvulus of the gastrointestinal tract: Appearances at multimodality imaging. Radiographics 2009, 29, 1281–1293.
- Millet, I.; Orliac, C.; Alili, C.; Guillon, F.; Taourel, P. Computed tomography findings of acute gastric volvulus. Eur. Radiol. 2014, 24, 3115–3122.
- Berti, A. Singolare attortigliamento dell’esofago col duodeno sequito da rapida morte. Gazz. Med. Ital. 1866, 9, 139–141.
- Mazaheri, P.; Ballard, D.H.; Neal, K.A.; Raptis, D.A.; Shetty, A.S.; Raptis, C.A.; Mellnick, V.M. CT of gastric volvulus: Interobserver reliability, radiologists’ accuracy, and imaging findings. Am. J. Roentgenol. 2019, 212, 103–108.
- Chau, B.; Dufel, S. Gastric volvulus. Emerg. Med. J. 2007, 24, 446–447.
- Verde, F.; Hawasli, H.; Johnson, P.T.; Fishman, E.K. Gastric volvulus: Unraveling the diagnosis with MPRs. Emerg. Radiol. 2019, 26, 221–225.
- Cavanagh, Y.; Carlin, N.; Yuridullah, R.; Shaikh, S. Acute Gastric Volvulus Causing Splenic Avulsion and Hemoperitoneum. Case Rep. Gastrointest. Med. 2018, 2018, 2961063.
- Coulier, B.; Ramboux, A. Acute obstructive gastric volvulus diagnosed by helical CT. JBR-BTR 2002, 85, 43.
- Singham, S.; Sounness, B. Mesenteroaxial volvulus in an adult: Time is of the essence in acute presentation. Biomed. Imaging Interv. J. 2009, 5, e18.
- Shivanand, G.; Seema, S.; Srivastava, D.N.; Pande, G.; Sahni, P.; Prasad, R.; Ramachandra, N. Gastric volvulus: Acute and chronic presentation. Clin. Imaging 2003, 27, 265–268.
- Von Haberer, H. Volvulus des Magens bei Carcinom. Dtsch. Z. Chir. 1912, 115, 497–532.
- Singleton, A. Chronic gastric volvulus. Radiology 1940, 34, 53–61.
- Carter, R.; Brewer, L.A.; Hinshaw, D.B. Acute gastric volvulus. A study of 25 cases. Am. J. Surg. 1980, 140, 99–106.
- Wastell, C.; Ellis, H. Volvulus of the stomach a review with a report of 8 cases. Br. J. Surg. 1971, 58, 557–562.
- Teague, W.J.; Ackroyd, R.; Watson, D.I.; Devitt, P.G. Changing patterns in the management of gastric volvulus over 14 years. Br. J. Surg. 2000, 87, 358–361.
- Al Daoud, F.; Daswani, G.S.; Perinjelil, V.; Nigam, T. Acute Organoaxial gastric volvulus: A massive problem with a twist-case report. Int. J. Surg. Case Rep. 2017, 41, 366–369.
- Tanner, N.C. Chronic and recurrent volvulus of the stomach with late results of “colonic displacement”. Am. J. Surg. 1968, 4, 505–515.
- Culver, G.J.; Pirson, H.S.; Bean, B.C. Mechanism of Obstruction in Para-Esophageal Diaphragmatic Hernias. JAMA J. Am. Med. Assoc. 1962, 181, 933–938.
- Gerson, D.E.; Lewicki, A.M. Intrathoracic stomach: When does it obstruct? Radiology 1976, 119, 257–264.
- Umemura, A.; Suto, T.; Fujiwara, H.; Ikeda, K.; Nakamura, S.; Hayano, M.; Nitta, H.; Takahara, T.; Hasegawa, Y.; Katagiri, H.; et al. Cardiopulmonary impairments caused by a large hiatal hernia with organoaxial gastric volvulus showing upside-down stomach: A case report. Am. J. Case Rep. 2019, 20, 1530–1535.
- Dalgaard, J. Volvulus of the stomach. Acta Clin. Scand. 1952, 103, 131–153.
- Gryglewski, A.; Kuta, M.; Pasternak, A.; Opach, Z.; Walocha, J.; Richter, P. Hiatal hernia with upside-down stomach. Management of acute incarceration: Case presentation and review of literature. Folia Med. Crac. 2016, 56, 61–66.
- Schiergens, T.S.; Thomas, M.N.; Hüttl, T.P.; Thasler, W.E. Management of acute upside-down stomach. BMC Surg. 2013, 13, 55.
- Sakran, N.; Nevo, H.; Hadar, N.; Raziel, A.; Hershko, D. Laparoscopic Repair of a Large Paraesophageal Hernia with Migration of the Stomach into the Mediastinum Creating an Upside-Down Stomach. Case Rep. Surg. 2017, 2017, 7428195.
- Katkhouda, N.; Mavor, E.; Achanta, K.; Friedlander, M.H.; Grant, S.W.; Essani, R.; Mason, R.J.; Foster, M.; Mouiel, J. Laparoscopic repair of chronic intrathoracic gastric volvulus. Surgery 2000, 128, 784–790.
- Collet, D.; Luc, G.; Chiche, L. Management of large para-esophageal hiatal hernias. J. Visc. Surg. 2013, 150, 395–402.
- Andolfi, C.; Jalilvand, A.; Plana, A.; Fisichella, P.M. Surgical Treatment of Paraesophageal Hernias: A Review. J. Laparoendosc. Adv. Surg. Technol. A 2016, 26, 778.
- Dellaportas, D.; Papaconstantinou, I.; Nastos, C.; Karamanolis, G.; Theodosopoulos, T. Large Paraesophageal Hiatus Hernia: Is Surgery Mandatory? Chirurgia 2018, 113, 765–771.
- Chiechi, M.V.; Hamrick-Turner, J.; Abbitt, P.L. Gastric herniation and volvulus: CT and MR appearance. Gastrointest. Radiol. 1992, 17, 99–101.
- Lee, H.Y.; Park, J.H.; Kim, S.G. Chronic gastric volvulus with laparoscopic gastropexy after endoscopic reduction: A case report. J. Gastric Cancer 2015, 15, 147–150.
- Palanivelu, C.; Rangarajan, M.; Shetty, A.R.; Senthilkumar, R. Laparoscopic suture gastropexy for gastric volvulus: A report of 14 cases. Surg. Endosc. Other Interv. Technol. 2007, 21, 863–866.
- Jacob, C.E.; LoPasso, F.P.; Zilberstein, B.; Bresciani, C.J.C.; Kuga, R.; Cecconello, I.; Gama-Rodrigues, J.J. Gastric volvulus: A review of 38 cases. ABCD Arq. Bras. Cir. Dig. 2009, 22, 96–100.
- Woon, C.Y.L.; Chung, A.Y.F.; Low, A.S.C.; Wong, W.K. Delayed diagnosis of intermittent mesenteroaxial volvulus of the stomach by computed tomography: A case report. J. Med. Case Rep. 2008, 2, 343.
- Godshall, D.; Mossallam, U.; Rosenbaum, R. Gastric volvulus: Case report and review of the literature. J. Emerg. Med. 1999, 17, 837–840.
- Jeong, S.-H.; Ha, C.-Y.; Lee, Y.-J.; Choi, S.-K.; Hong, S.-C.; Jung, E.-J.; Ju, Y.-T.; Jeong, C.-Y.; Ha, W.-S. Acute gastric volvulus treated with laparoscopic reduction and percutaneous endoscopic gastrostomy. J. Korean Surg. Soc. 2013, 85, 47–50.
- Schrag, S.P.; Sharma, R.; Jaik, N.P.; Seamon, M.J.; Lukaszczyk, J.J.; Martin, N.D.; Hoey, B.A.; Stawicki, S.P. Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J. Gastrointest. Liver Dis. 2007, 16, 407–418.
- Naim, H.J.; Smith, R.; Gorecki, P.J. Emergent Laparoscopic Reduction of Acute Gastric Volvulus with Anterior Gastropexy. Surg. Laparosc. Endosc. Percutaneous Technol. 2003, 13, 389–391.
- Jamil, L.H.; Huang, B.L.; Kunkel, D.C.; Jayaraman, V.; Soffer, E.E. Successful gastric volvulus reduction and gastropexy using a dual endoscope technique. Case Rep. Med. 2014, 2014, 136381.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Gastrointestinal Disease
Revisions:
2 times
(View History)
Update Date:
30 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




