Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mariusz Mojzych | + 2330 word(s) | 2330 | 2022-01-26 04:23:57 | | | |
| 2 | Amina Yu | -10 word(s) | 2320 | 2022-01-27 01:54:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mojzych, M. Heterocyclic Crown Ethers. Encyclopedia. Available online: https://encyclopedia.pub/entry/18867 (accessed on 12 January 2026).
Mojzych M. Heterocyclic Crown Ethers. Encyclopedia. Available at: https://encyclopedia.pub/entry/18867. Accessed January 12, 2026.
Mojzych, Mariusz. "Heterocyclic Crown Ethers" Encyclopedia, https://encyclopedia.pub/entry/18867 (accessed January 12, 2026).
Mojzych, M. (2022, January 27). Heterocyclic Crown Ethers. In Encyclopedia. https://encyclopedia.pub/entry/18867
Mojzych, Mariusz. "Heterocyclic Crown Ethers." Encyclopedia. Web. 27 January, 2022.
Copy Citation
Crown ethers are heterocyclic compounds present as cyclic oligomers in their simple form. These are extremely versatile compounds exhibiting higher binding affinity towards metal ions, including s-block and transition metal ions. For example, 18-crown-6 has a cavity that fits the size of 4f transition metal ions and has reflected exceptional attraction for complexation with the lanthanide ions.
crown ethers
iontophoresis
heterocyclic compounds
macrocyclic polyether
1. Introduction
Crown ethers (CEs) are macrocyclic polyethers and have three to twenty oxygen atoms alienated by two or more carbon atoms. These macromolecules can be either substituted or unsubstituted. This class of organic compounds has an interesting structure with a hydrophobic ring surrounding a hydrophilic cavity [1]. Crown ethers are heterocyclic compounds present as cyclic oligomers in their simple form. These are extremely versatile compounds exhibiting higher binding affinity towards metal ions, including s-block and transition metal ions [2]. For example, 18-crown-6 has a cavity that fits the size of 4f transition metal ions and has reflected exceptional attraction for complexation with the lanthanide ions [3]. Ethyleneoxy moiety (CH2CH2O-) is a crucial repeated unit of simple crown ether: repeated twice in dioxane and six times in 18-crown-6 [4].
These can be either substituted or unsubstituted and possess a hydrophobic ring surrounding a hydrophilic cavity, enabling them to form stable complexes with metal ions and contributing to host-guest chemistry [1][5]. Since their discovery, they have an outstanding capability to selectively coordinate ions, making them attractive for broad research applications [6]. The binding ability of crown ethers either with organic molecules or ions depends on their cavity size. They can carry different ions in a non-aqueous solvent. Crown ether also acting as a phase transfer catalyst. For example, 18-crown-6 shows binding ability with K+ ion, and 12-crown-4 tends to complex with Li+. Owing to this unique ability, CEs have broad applications in chemistry, biotechnology, and biochemistry. Alkali metal complexes with macrocyclic ligands mediate electron transfer processes.
where M is the alkali metal, e.g., potassium or sodium, and L is the complexant, e.g., 18-crown-6 as shown in Scheme 1 [7].
2M(S) + L→M+ L + M−
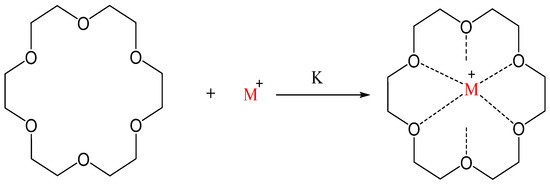
Scheme 1. Binding of M+ by 18-crown-6 ether; K is complex stability constant [8].
2. Biological and Pharmacological Analysis of Crown Ethers
Crown ethers are found to have synergistic biological and pharmacological potential, they are widely used in drug delivery. The activities include anti-cancer, anti-microbial, anti-inflammatory and drug delivery.
2.1. Antibacterial Potential
The Schiff bases containing bis-benzo-15-crown-5 and their sodium derivatives were examined using a well diffusion method against bacterial strains; Staphylococcus aureus, Shigella dysenteria type 2, Listeria monocytogenes, Escherichia coli, Salmonella typhi H, Staphylococcus epidermis, Brucella abortus, Micrococcus luteus, Bacillus cereus, and Pseudomonas putida while choosing DMF as a control. The Schiff bases with 5-methoxy groups have shown maximum activity against strains with a concentration of 103 µM [9]. Interestingly, the chelates (12, 13 and 14) containing Na are found more potent against bacterial strains than bis-benzo-15-crown-5 compounds as shown in Figure 1. The bactericidal activity was compared with commercial drugs; kanamycin K30, sulfamethoxazole SXT25, Amoxycillin AMP10, sulbactam SCF, and nystatin NYS100 to describe the potency of compounds [9].
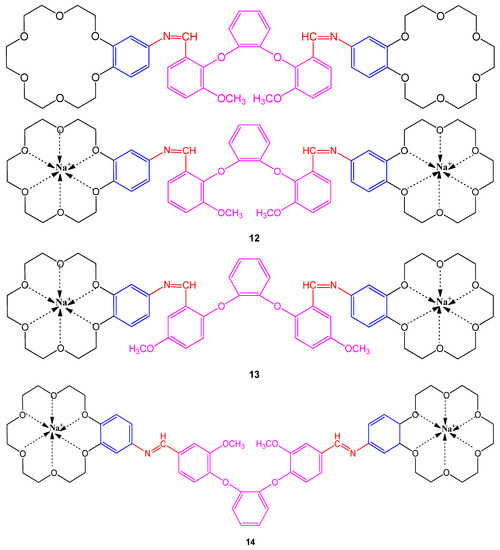
Figure 1. Structure of Schiff bases containing bis-benzo-15-crown-5 ether 12–14.
The novel chromene crown ethers and their respective sodium and potassium complexes were tested against strains; S. dysenteria type 2, S. epidermidis, P. vulgaris, K. pneumonia sp., Shigella dysenteria, and S. marcescens sp. Most of the derivatives were found to be moderately active against these pathogens. The compound 15 given in Figure 2. showed 15 mm zone of inhibition against Shigella dysenteria [10].
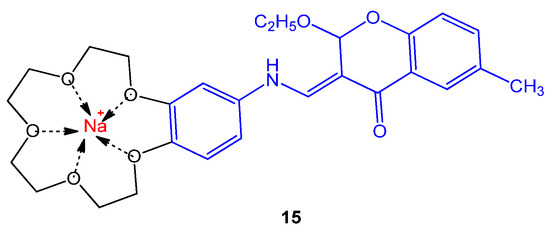
Figure 2. Structure of chromene crown ether complexed with the sodium ion 15.
2.2. Antifungal Potential
The crown ethers, their complexes of sodium moieties along with their alkali metal complexes, and other crown ether derivatives were investigated against fungal strain Candida albicans, revealed moderate to significant antifungal activity when compared with positive controls [9][10].
Four novel 4,4′-di(2,2′-benzimidazolyl)dibenzo18-crown-6 (16a–d) were investigated for their antifungal efficacy against Aspergillus sp. using DMF and bavistin as control and standard. To study the antifungal activity, agar well diffusion method was chosen. The compound 16d given in Figure 3. was found to be most active against fungal strains with maximum inhibitory activity [11].
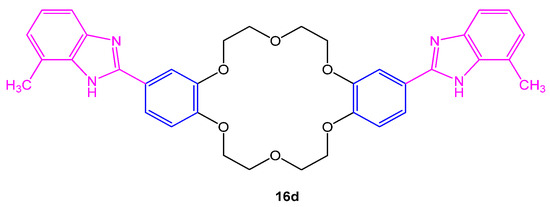
Figure 3. Structure of 4,4′-di(2,2′-benzimidazolyl)dibenzo18-crown-6 16d.
2.3. Anticancer Potential
Cytotoxic potential of aza-crown ether-squaramide conjugates (17a–d) were measured through MTT assay at the concentration of 50 µM choosing three cancer cell lines; human lung carcinoma (A549), human breast cancer (MCF-7) and human liver cancer (HepG2). The inhibitory concentration (IC50) in cell growth was compared with the doxorubicin drug. Results have shown that compounds 17b and 17d given in Figure 4. displayed moderate anti-proliferative activity as compared to others. Furthermore, the compounds enhanced cytotoxicity in HeLa cells in the presence of chloride or sodium ions that move across the cell membrane and promote cell apoptosis [12]. Another study reported in vitro anti-proliferative potential of the crown ether acyl derivatives in HBL-100, HeLa, SW1573, and WiDr human solid carcinomas, using cisplatin and etoposide as positive controls. The derivative showed comparable activity against WiDr cell line [13]. Two aza-crown ethers, N,N′-bis (dithiocarbamate)-1,10-diaza-18-crown-6 (L2−) were studied for their anticancer potential human cervix carcinoma cell line HeLa-229, the human ovarian carcinoma cell line A2780, and cisplatin-resistant mutant A2780 cis cells. The analysis revealed a new strategy to design metal-based drugs. The aza-crown ether Pt complex ligand increases affinity for antitumor activity, whereas the addition of Na or K salts further boosts the activity as compared to cisplatin, taken as reference drug [14].
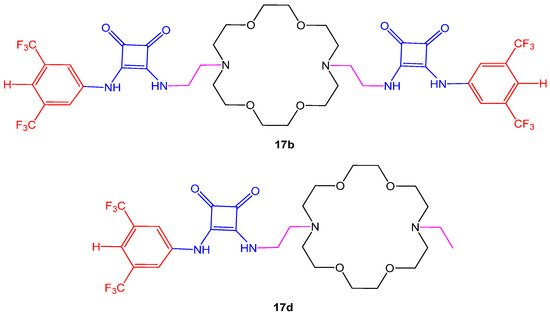
Figure 4. Structures of aza-crown ether-squaramide 17b, 17d.
2.4. Toxicology
The earthworm Eiseniafetida was exposed to 18-crown-6 compounds with variable concentrations in the dry soil to investigate the toxic effect of components towards growth, respiration, and burrowing behavior of the worm in soil. The experiments revealed a reduced rate of worm growth, and burrowing behavior indicated by decreased mass index of the worm from (96.3 ± 5.6 to 78.2 ± 6.4) measured in mean ± SD, as the concentration of 18-crown-6 increased from 0 to 200 mg kg−1 dry soil [15].
2.5. Amyloidogenesis Inhibitory Activity
The novel crown ether analogs were tested to evaluate their inhibitory potential against mutated transthyretin protein that promotes amyloidosis, forming amyloid fibril. Amyloid fibrils gather in specific tissues, including the eyes and the heart, and leave adverse effects. Anti-transthyretin amyloid genesis activities of crown ethers were observed using X-ray crystallography and binding assay using probes. Diflunisal, an anti-inflammatory drug was taken as a reference [16]. Among all, the 4′-carboxybenzo-18C6 (18) given in Figure 5. showed best anti amyloid fibril formation for transthyretin protein.
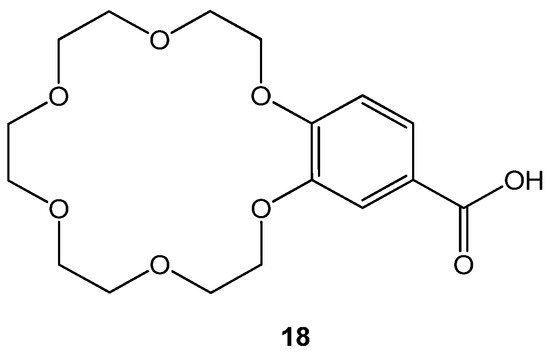
Figure 5. Structure of 4′-carboxybenzo-18-crown-6 ether 18.
2.6. Electrochemical Sensor
Crown ether-based sensors were constructed to determine serotonin in human serum. Crown ether was mixed with carbon nanotubes-ionic liquid crystal and at glassy carbon electrode surface (GC/(CNTs-ILC) allowed a stable host-guest inclusion complex between crown ethers and neurotransmitters. The crown cavity of 18-Crown-6 allows H-bond formation with the indole ring of serotonin. The sensor enables to examine drugs at a low cost [17]. Furthermore, potassium ion (K+) microsensors were made using electrochemical impedance spectroscopy (EIS) to understand potassium ions transport across the cellular membrane. The building block of sensors was 1-aza-18-crown-6 functionalized graphene oxide (Crown-GO). The crown ether part captures potassium ions, whereas graphene oxide provides the binding ability [18]. A study reports K+ chemical sensor development based on a self-assembled monolayer of 4-aminobenzo-18-crown-6 ether as selective ionophore that shows reproducible activity in disease diagnosis [19]. The 4-aminobenzo-18-crown-6 modified gold nanoparticles have been used by researchers to make the sensor to detect K+ in human urine samples through colorimetric assay. The K+ detection was compared with few other ions through UV-Vis and showed excellent anti-interference activity [20].
2.7. Disposable Potentiometric Sensor
Dibenzo 24-crown-8-ether as shown in Figure 6. based potentiometric sensors were built to determine Biperiden hydrochloride in urine and plasma. Biperiden is used to treat Parkinsonism. The findings demonstrated improved response time, lifetime, sensitivity, selectivity, and the possibility of miniaturization of the sensor compared with γ-cyclodextrins, calixarenes, and buckminsterfullerene C60 [21].
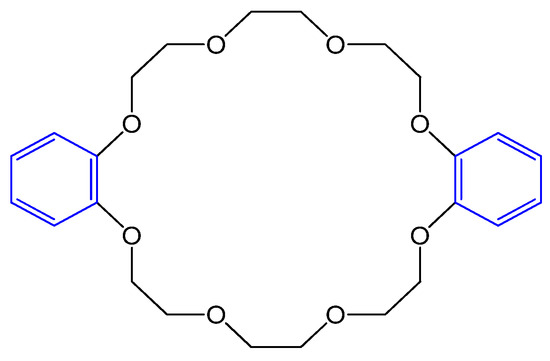
Figure 6. Structure of dibenzo-24-crown-8-ether.
2.8. Chiral Catalyst
Crown ethers are reported to have prime importance in performing the catalytic activity in many phase transfer reactions. The monosaccharide-based crown ethers shown in Figure 7. have been used in chacones epoxidation, leading to understanding the enantioselectivity of compounds that play a key role in drug designing [22].
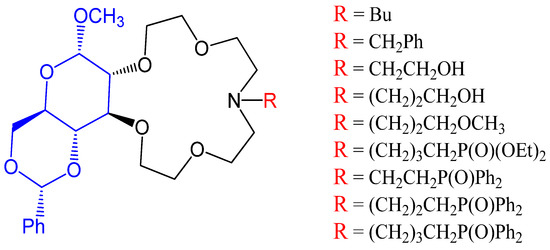
Figure 7 Monoaza-15-crown-5 lariat ether containing D-glucose.
2.9. Peptide Interaction and Self-Assembly
A neutral antimicrobial peptide holding crown ether side chain has been modified by interacting with cationic arginine to form a secondary structure, which interacts with the lipid membrane of the cell. The study finds its potential in the pharmaceutical industry [23]. Due to holding particular multi-cavity structures and diverse complexity, the iptycene-derived crown ether serves as first generation of synthetic hosts in molecular recognition. It can be a promising candidate for constructing self-assemblies. Hence, provide broader spectra of applications in biological and material sciences.
2.10. Crown Ethers as P-Glycoprotein Inhibitors
The effect of monoaza- and diaza-18-crown-6 ethers was studied as multidrug-resistant (MDR) reversal in model cell lines. The compounds were found to be more active P-glycoprotein (P-gp) inhibitors and increased apoptotic activity than verapamil, a commercially available drug. The activity was performed through ATPase activity showed that crown ethers inhibit P-gp without affecting their locality [24].
2.11. DNA Targeting
A G-quadruplex DNA (G4-DNA) probe with far-infrared luminescence property was synthesized and characterized by NMR and MS using modified aza-crown ether with triphenylaminequinoline derivative (TPAQD-ACE). The metal conjugate probes of TPAQD-ACE showed different binding abilities towards DNA, among which Ni and Fe presented maximum signaling at 640 nm in buffer. Furthermore, the cell staining results assured that Nickle metal ion probes bind more efficiently to DNA present in cells. The application has drawn significant attention of researchers in the biological field [25].
2.12. Enzyme Activation
The effect of crown ether ligands (19–27) shown in Figure 8. was studied on enzyme activities of hCA (human carbonic anhydrase) purified from erythrocytes using SDS-PAGE. All analogs activated the enzyme activity except 22 and 23 that interacted with the active part of the enzyme and inhibited the interaction between enzyme and substrate. The inhibitory potential of 22 and 23 was compared to Acetazolamide. Enzyme activity was increased with an increase of the ring cavity [26].
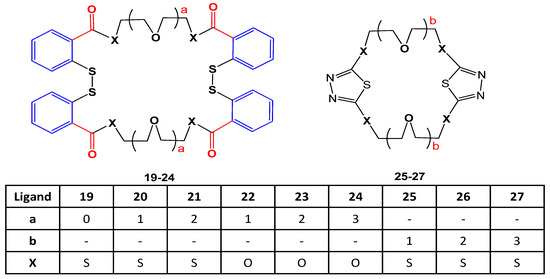
Figure 8. Structures of crown ethers (19–27).
2.13. Crown Ethers as Vesicles
In another study, Benzo-15-crown-5active polydiacetylene (PDA) based vesicle receptors were investigated to detect metal ions. The receptors showed a strong interaction with lead ions (Pb2+) visualized as color change from blue to red in buffer (pH = 7.2) and further characterized through UV spectroscopy. The maximum interaction of Pb2+-crown ethers on lipid interface makes crown ethers unique to detect biomolecules like proteins, sugars, and microbes [27].
2.14. Membrane Anchors
Lipid-nucleic acids (LiNAs) conjugates based on polyaza crown ethers were synthesized to investigate the fusion of liposomes to enhance content mixing within the cell. LiNAsget anchored into the outer liposomes leaflet and provide strong fusogens that may work as a key tool for fusing cell membranes [25].
2.15. Transfection Activity
The complex formation with DNA of two nitrogen-pivoted aza-crown ethers linked to the cholesteryl-fused ring system N-(cholesteryloxycarbonyl)aza-15-crown-5 and N-(cholesteryloxycarbonyl)aza-18-crown-6incorporated to liposomes were observed in the human embryonic kidney cell line (HEK293). The increased transfection activity revealed stable DNA-protective lipoplexes containing aza-crown ethers, attributed to perturbations of the endosomes and the loosely packed cargo plasmid DNA [28].
2.16. Crown Ethers-Tyrosine Kinase Inhibitors
Crown ethers (CEs) fused with quinazoline were studied as epidermal growth factor receptor (EGFR) inhibitors via in vitro tyrosine-kinase and phosphorylation assays. Some of these compounds are potent inhibitors of EGFR and give a broad spectrum of human tumors inhibition [29].
2.17. Fluorescent Chemosensor
Crown ether-acylhydrazone (L) based unique chemosensors were built in methanol solution to determine fluorescence of different metal ions. An unusual observation for Al3+ ions was seen in UV, a color change from pale blue to bright blue at 444 nm reveals an intense correlation of crown ethers and Al3+. Figure 9. shows the binding interaction between L and Al3+. These chemosensors are in progress to find applications in biochemistry [18].
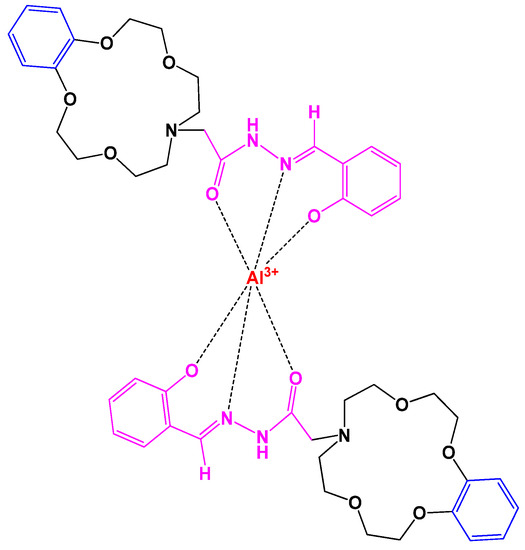
Figure 9. Crown complex showing the interaction between L and Al3+.
2.18. Ion Transport
Synthetic bucky ball-based molecular balls were designed linked with CEs that enable ion transport across the biological membrane. Generally, the molecular balls linked to small CEs showed high selectivity towards ion transport compared to those containing larger CEs [30].
2.19. Computational Study
Lithium-ion binding to 8-Crown-4 was investigated computationally to verify the ion binding using Spartan 10 software, and further optimization was examined using Gaussian 09. DFT computations showed two stable conformations of 8-Crown-4, the crown (Cr) and boat-chair (BC). The free energy level indicates that the BC conformer is intrinsically more stable than the Cr conformer. However, in nitromethane, the Cr conformer appeared more stable than the BC due to a 2.4 kcal mol–1difference in solvation energy [31]. A molecular docking study investigated the binding mode between TPAQD-ACE and the human G-quadruplex using AutoDock Vina. The results inferred that the introduction of metal ions to the compound enhances the fluorescent signal intensity of TPAQD-ACE and promotes the binding abilities of TPAQD-M-ACEs to G4 DNAs [32]. The Na+ and K+ selectivity of 18-Crown-6 ether, dibenzo-18-crown-6, and cryptans was compared through computational study. The results showed more affinity of compounds towards K+ than Na+ due to the solvent effect [33].
2.20. Antimicrobial Peptides
Antimicrobial peptides (AMPs) are constituted of numerous species, immune systems and minimize the development of bacterial resistance. Different factors such as length, hydrophobicity, amphiphilicity, flexibility, and the net charge on AMPS control its selectivity and potency. This 14-residue peptide as shown below in Figure 10. exhibits moderate permeability across EYPC vesicles and contains four 21-crown-7 modified phenylalanines with 10 leucines. The peptide 12-residue exhibits high permeability where an increase in length up to 13–16 residues enhances activity showing smaller ring size may decrease peptide activity [34].
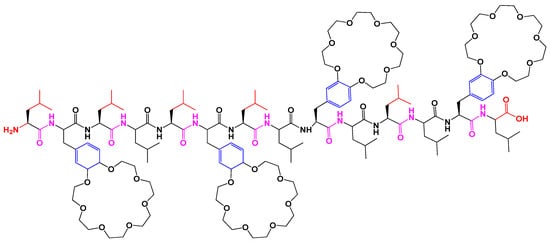
Figure 10. Structure of 14-residue model peptide.
2.21. Covalent Organic Frameworks (COFs)
COFs periodic structure potential for catalysis, energy storage, ions conductor, gas separation, and sorption. Py-B24C8-COF andPy-B18C6-COF exhibit capture capacity capture Cs+ and K+, respectively. COFs offer’s platform for precisely designing ions absorbents [35]. Olefin Chiral COFs(CCOFs 17-R and 18-R) exhibit higher enantioselectivity than their reduced Chiral COFs structures when used as fluorescence sensors to detect chiral amino alcohols [36].
References
- Ethers, A.; Basok, S.S.; Schepetkin, I.A.; Khlebnikov, A.I.; Lutsyuk, A.F.; Kirichenko, T.I.; Kirpotina, L.N.; Pavlovsky, V.I.; Leonov, K.A.; Vishenkova, D.A.; et al. Synthesis, Biological Evaluation, and Molecular Modeling of Aza-Crown Ethers. Molecules 2021, 26, 2225.
- Davis, F.; Higson, S. Crown Ethers, Cryptands and Other Compounds; John Wiley and Sons: Hoboken, NJ, USA, 2011.
- Alabdaly, B.I. Synthesis, Characterization and Antibacterial Activity of New Complexes of Some Lanthanide Ions with Benzo 18-Crown-6 and 221-Cryptand. IOSR J. Appl. Chem. 2013, 6, 32–39.
- Ijeri, V.; Vocanson, F.; Martelet, C.; Jaffrezic-renault, N.; Lyon, E.C. De Capacitive Sensing of Amino Acids Using Caliraxene-Coated Silicon Transducers. Electroanalysis. 2007, 19, 510–514.
- Bukhzam, A.; Bader, N. Crown Ethers: Their Complexes and Analytical Applications. J. Appl. Chem. 2014, 3, 237–244.
- Steed, J.W. First- and second-sphere coordination chemistry of alkali metal crown ether complexes. Coord. Chem. Rev. 2001, 215, 171–221.
- Jedliński, Z. Novel Electron-Transfer Reactions Mediated by Alkali Metals Complexed by Macrocyclic Ligand. Acc. Chem. Res. 1998, 31, 55–61.
- Ushakov, E.N.; Alfimov, M.V.; Gromov, S.P. Crown Ether Based Optical Molecular Sensors and Photocontrolled Crown Ether-Based Optical Molecular Sensors and Photocontrolled Ionophores. J. Macroheterocyc. 2010, 3, 189–200.
- Hayvalı, Z.; Güler, H.; Öğütcü, H.; Sarı, N. Novel bis-crown ethers and their sodium complexes as antimicrobial agent: Synthesis and spectroscopic characterizations. Med. Chem. Res. 2014, 23, 3652–3661.
- Şahin Gül, D.; Ogutcu, H.; Hayvalı, Z. Investigation of photophysical behaviours and antimicrobial activity of novel benzo-15-crown-5 substituted coumarin and chromone derivatives. J. Mol. Struct. 2020, 1204, 127569.
- Klenc, J.; Raux, E.; Barnes, S.; Sullivan, S.; Duszynska, B.; Bojarski, A.J.; Strekowski, L. Synthesis of 4-Substituted 2-(4-Methylpiperazino) pyrimidines and Quinazoline Analogs as Serotonin 5-HT 2A Receptor Ligands. J. Heterocycl. Chem. 2009, 46, 1259–1265.
- Yu, X.H.; Cai, X.J.; Hong, X.Q.; Tam, K.Y.; Zhang, K.; Chen, W.H. Synthesis and biological evaluation of aza-crown ether-squaramide conjugates as anion/cation symporters. Future Med. Chem. 2019, 11, 1091–1106.
- Febles, M.; Montalvão, S.; Crespín, G.D.; Norte, M.; Padrón, J.M.; Tammela, P.; Fernández, J.J.; Daranas, A.H. Synthesis and biological evaluation of crown ether acyl derivatives. Bioorganic Med. Chem. Lett. 2016, 26, 5591–5593.
- Arenaza-Corona, A.; Couce-Fortúnez, M.D.; De Blas, A.; Morales-Morales, D.; Santillan, R.; Höpfl, H.; Rodríguez-Blas, T.; Barba, V. Further Approaches in the Design of Antitumor Agents with Response to Cell Resistance: Looking toward Aza Crown Ether-dtc Complexes. Inorg. Chem. 2020, 59, 15120–15134.
- Du, Y.; Rao, P.; Li, Y.; Qiu, J.; Qiu, W.; Tang, H.; Potter, M.A. Toxicological responses of the earthworm Eisenia fetida to 18-crown-6 under laboratory conditions. Bull. Environ. Contam. Toxicol. 2014, 93, 452–455.
- Yokoyama, T.; Mizuguchi, M. Crown Ethers as Transthyretin Amyloidogenesis Inhibitors. J. Med. Chem. 2019, 62, 2076–2082.
- Atta, N.F.; Ahmed, Y.M.; Galal, A. Electrochemical Determination of Neurotransmitters at Crown Ether Modified Carbon Nanotube Composite: Application for Sub-nano-sensing of Serotonin in Human Serum. Electroanalysis 2019, 31, 1204–1214.
- Zhang, Q.; Jiao, Q.; Zhang, Q.; Liu, Z. A novel crown ether-acylhydrazone turn-on fluorescent chemosensor for Al3+ ion. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2019, 211, 1–8.
- Kumbhat, S.; Singh, U. A potassium-selective electrochemical sensor based on crown-ether functionalized self assembled monolayer. J. Electroanal. Chem. 2018, 809, 31–35.
- Qiu, J.; Zhang, Y.; Dong, C.; Huang, Y.; Sun, L.; Ruan, H.; Wang, H.; Li, X.; Wu, A. Rapid colorimetric detection of potassium ions based on crown ether modified Au NPs sensor. Sens. Actuators B Chem. 2019, 281, 783–788.
- Khaled, E.; Hassan, H.N.A.; Ahmed, M.A.; El-Attar, R.O. Crown Ether/Carbon Nanotubes Based Biperiden Disposable Potentiometric Sensor. Electroanalysis 2017, 29, 975–982.
- Bako, P.; Keglevich, G.; Rapi, Z. Asymmetric Phase Transfer Reactions Catalyzed by Chiral Crown Ethers Derived from Monosaccharides. Lett. Org. Chem. 2010, 7, 645–656.
- Paquet-Côté, P.A.; Fillion, M.; Provencher, M.È.; Otis, F.; Dionne, J.; Cardinal, S.; Collignon, B.; Bürck, J.; Lagüe, P.; Ulrich, A.S.; et al. Crown ether modified peptide interactions with model membranes. Supramol. Chem. 2019, 31, 159–171.
- Guberović, I.; Marjanović, M.; Mioč, M.; Ester, K.; Martin-Kleiner, I.; Šumanovac Ramljak, T.; Mlinarić-Majerski, K.; Kralj, M. Crown ethers reverse P-glycoprotein-mediated multidrug resistance in cancer cells. Sci. Rep. 2018, 8, 14467.
- Löffler, P.M.G.; Hansen, A.H.; Ries, O.; Jakobsen, U.; Rabe, A.; Sørensen, K.T.; Glud, K.; Vogel, S. Lipidated Polyaza Crown Ethers as Membrane Anchors for DNA-Controlled Content Mixing between Liposomes. Sci. Rep. 2019, 9, 13856.
- Akkemik, E.; Cicek, B.; Camadan, Y.; Calisir, U.; Onbasioglu, Z. The determination of the carbonic anhydrases activators in vitro effect of mixed donor crown ethers. J. Biochem. Mol. Toxicol. 2018, 32, e22032.
- Pan, X.; Wang, Y.; Jiang, H.; Zou, G.; Zhang, Q. Benzo-15-crown-5 functionalized polydiacetylene-based colorimetric self-assembled vesicular receptors for lead ion recognition. J. Mater. Chem. 2011, 21, 3604–3610.
- Sewbalas, A.; Islam, R.U.; Van Otterlo, W.A.L.; De Koning, C.B.; Singh, M.; Arbuthnot, P.; Ariatti, M. Enhancement of transfection activity in HEK293 cells by lipoplexes containing cholesteryl nitrogen-pivoted aza-crown ethers. Med. Chem. Res. 2013, 22, 2561–2569.
- Hu, S.; Xie, G.; Zhang, D.X.; Davis, C.; Long, W.; Hu, Y.; Wang, F.; Kang, X.; Tan, F.; Ding, L.; et al. Synthesis and biological evaluation of crown ether fused quinazoline analogues as potent EGFR inhibitors. Bioorganic Med. Chem. Lett. 2012, 22, 6301–6305.
- Li, N.; Chen, F.; Shen, J.; Zhang, H.; Wang, T.; Ye, R.; Li, T.; Loh, T.P.; Yang, Y.Y.; Zeng, H. Buckyball-Based Spherical Display of Crown Ethers for de Novo Custom Design of Ion Transport Selectivity. J. Am. Chem. Soc. 2020, 142, 21082–21090.
- Van der Ham, A.; Hansen, T.; Lodder, G.; Codée, J.D.C.; Hamlin, T.A.; Filippov, D.V. Computational and NMR Studies on the Complexation of Lithium Ion to 8-Crown-4. ChemPhysChem. 2019, 20, 2103–2109.
- You, D.; Liu, L.; Yang, Q.; Wu, X.; Li, S.; Li, A. A far-red aza-crown ether fluorescent probe for selective G-quadruplex DNA targeting. Dye. Pigment. 2020, 176, 108222.
- Gholiee, Y.; Salehzadeh, S. The solvent effect on selectivity of four well-known cryptands and crown ethers toward Na+ and K+ cations; A computational study. J. Mol. Liq. 2020, 309, 113149.
- Paquet-Côté, P.A.; Paradis, J.P.; Auger, M.; Voyer, N. Crown ether modified peptides: Length and crown ring size impact on membrane interactions. Biochim. Biophys. Acta-Biomembr. 2020, 1862, 183261.
- An, S.; Xu, Q.; Ni, Z.; Hu, J.; Peng, C.; Zhai, L.; Guo, Y.; Liu, H. Construction of Covalent Organic Frameworks with Crown Ether Struts. Angew. Chem.-Int. Ed. 2021, 9959–9963.
- Yuan, C.; Fu, S.; Yang, K.; Hou, B.; Liu, Y.; Jiang, J.; Cui, Y. Crystalline C-C and C=C Bond-Linked Chiral Covalent Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 369–381.
More
Information
Subjects:
Chemistry, Organic; Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.5K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
27 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




