Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jelonia Tasha Rumph | + 2032 word(s) | 2032 | 2022-01-26 05:21:21 | | | |
| 2 | Dean Liu | Meta information modification | 2032 | 2022-01-27 01:48:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rumph, J. Environmental Contaminants and Disparities in Women's Health. Encyclopedia. Available online: https://encyclopedia.pub/entry/18859 (accessed on 08 February 2026).
Rumph J. Environmental Contaminants and Disparities in Women's Health. Encyclopedia. Available at: https://encyclopedia.pub/entry/18859. Accessed February 08, 2026.
Rumph, Jelonia. "Environmental Contaminants and Disparities in Women's Health" Encyclopedia, https://encyclopedia.pub/entry/18859 (accessed February 08, 2026).
Rumph, J. (2022, January 26). Environmental Contaminants and Disparities in Women's Health. In Encyclopedia. https://encyclopedia.pub/entry/18859
Rumph, Jelonia. "Environmental Contaminants and Disparities in Women's Health." Encyclopedia. Web. 26 January, 2022.
Copy Citation
Environmental contaminants generally fall into three categories: persistent organic pollutants (POPs), endocrine-disrupting chemicals (EDCs), and heavy metals.
women’s health
environmental contaminants
pollution
health disparities
1. Introduction
POPs are carbon-based chemicals that are not easily metabolized and exhibit an extended half-life of 10+ years. Because of their ability to bioaccumulate in adipose tissue, they can biomagnify within the food chain; thus, the body burden of toxicants tends to increase with age in both humans and animals [1]. POPs are produced by both natural and anthropogenic processes, though most POPs of concern are produced intentionally for commercial use, as shown in Figure 1 [2].
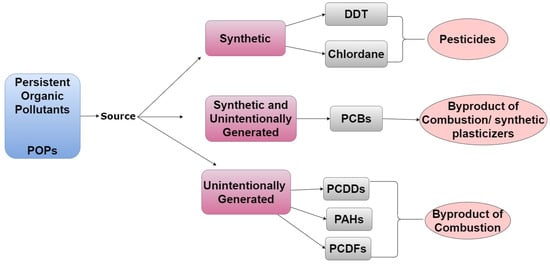
Figure 1. A tree diagram listing emission sources of POPs, representative examples of chemicals from each source, and how/why they are in the environment.
Many POPs can disrupt the endocrine system and are therefore also classified as EDCs. The endocrine system is a collection of hormone-secreting glands that are critical to regulating developmental, metabolic, and reproductive processes [3]. EDCs frequently act as hormone agonists or antagonists and influence the endocrine system by activating, blocking, or by altering normal hormone activity via interactions with nuclear receptors or altering metabolism [4]. Steroid hormones and their respective target organs are exquisitely sensitive to interference by EDCs and these chemicals can have wide-ranging effects on human health. Humans can be exposed to EDCs through residential, agricultural, pharmaceutical, and industrial activities, as shown in Figure 2 [5].
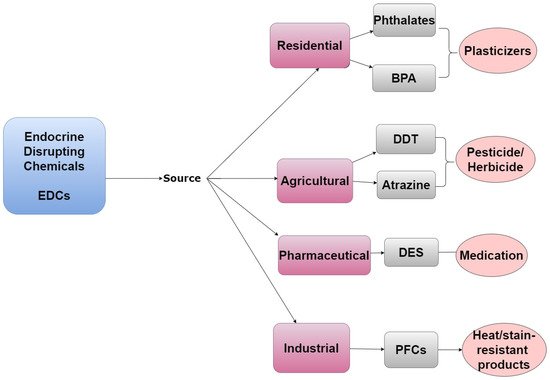
Figure 2. A tree diagram listing the emission sources of EDCs, representative examples of chemicals from each source, and how/why they are in the environment.
Naturally occurring heavy metals are normally present in the environment as trace elements; however, their accumulation can lead to toxicity—making these compounds environmental contaminants. Although heavy metals are not always classified as EDCs, some have endocrine-disrupting properties. For example, cobalt and cadmium have both been shown to exhibit estrogen-like activity in the absence of estradiol [6][7][8]. Additional metals with endocrine-disrupting properties include arsenic, lead, mercury, chromium, copper, nickel, cadmium, and tin [8][9]. Thus, while heavy metals are naturally occurring and have both geogenic and atmospheric sources their use in industrial, pharmaceutical, and agricultural processes can lead to their excess accumulation in the environment, as demonstrated by Figure 3 [10].
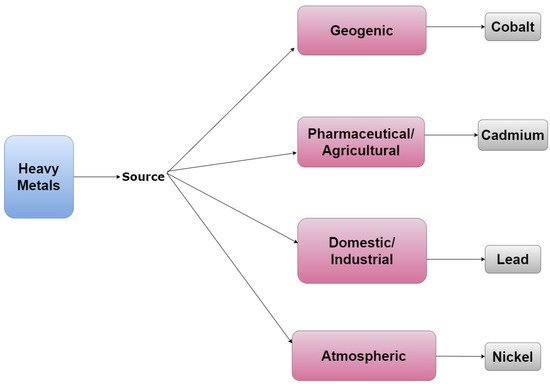
Figure 3. A tree diagram that lists representative examples of heavy metals and their emission sources.
2. Examples of POPs
Pesticides are well-characterized synthetic POPs. Dichlorodiphenyltrichloroethane (DDT), a pesticide that was widely used in the United States from 1946 to 1972, acts as an estrogen agonist [11]. However, its metabolite, dichlorobiphenyl dichloroethylene (DDE), is an androgen antagonist [12]. Chlordane is another pesticide and POP that was in commercial and residential use in the United States from 1948 to 1988 but was banned due to health concerns. Perfluorinated compounds (PFCs) are examples of POPs that are still used today to create heat-resistant and non-stick kitchenware [13] despite concerns that they can disrupt pregnancy and have been suggested to be carcinogenic [14].
Unintentionally generated POPs include polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs), polycyclic aromatic hydrocarbons (PAHs), and polychlorinated dibenzofurans (PCDFs). Although PCBs were previously widely produced for commercial use, intentional manufacturing of these compounds has now been banned in most countries. Nevertheless, they are still released by some industrial processes as well as incineration of household and commercial waste [15]. From the 1950s until 1977, PCBs were synthesized to create microwave ovens, air conditioners, and electric cables [15][16]. PCDDs are byproducts of pesticide manufacture and processes utilizing chlorine bleaching. PCDDs are also produced by volcanic eruptions and forest fires. The generation of PCDFs is associated with the synthesis and incineration of products containing PCBs [17]. Lastly, PAHs are byproducts of cigarette smoke, barbecuing, and grilling [18][19].
3. Examples of EDCs
Bisphenol A (BPA) is a high-volume EDC with broad residential use. It is used to make plastics for food and beverage storage, and it is a component of epoxy resins that were used to form the lining of food cans and baby bottles in previous years. It was determined that BPA can leach from these and other containers and accumulate in food. Although the use of BPA in food containers has now been banned in most countries, this compound remains in production and can be found in a variety of consumer products. BPA was previously considered to be a weak estrogen; however, more recent studies indicate that this compound exhibits similar potency to estradiol in some cellular contexts [20][21][22]. For this reason, BPA was replaced by bisphenol S (BPS) in most countries in the early 2000s [21][23]. Unfortunately, BPS appears to have similar adverse health effects as those associated with BPA [24]. Phthalates are another widely used residential EDC with a short half-life in humans. They are used to make plastic flexible and more durable. Phthalates are also incorporated into personal care products including soap and hair spray due to their binding and solvent properties [25].
Pharmaceutical EDCs include diethylstilbestrol (DES), a synthetic estrogen that was prescribed to pregnant women to prevent miscarriages and preterm birth from 1938 to 1971 [26]. DES was also injected into livestock to increase meat production [27]. DES was banned as a therapy for pregnant women following the clinical manifestations of its endocrine-disrupting properties. Clinical manifestation of in utero exposure to DES included increased incidence of vaginal cancers in reproductive-age daughters as well as adverse effects in mothers and sons [28]. Shortly after DES was banned as a pharmaceutical, the United States Food and Drug Administration also banned its use in meat production [29].
Agricultural EDCs include the pesticide DDT, a POP described above. Atrazine is an agricultural EDC that currently remains in use despite having been found to influence the female reproductive system by dysregulating the hypothalamic-pituitary–ovarian axis [30]. Industrial EDCs include PCB-153 which was previously used to create dielectric insulating fluid. PCB-153 is no longer synthesized today, but the chemical persists in the environment and is the most prevalent PCB found in the human body [2]. Industrial EDCs also include PFCs, which are also considered POPs.
4. Examples of Heavy Metals
Cobalt is an example of a geogenic heavy metal, whereas cadmium and copper are considered pharmaceutical compounds [31][32]. Domestic and industrial heavy metals include lead [10] while atmospheric heavy metals include nickel and copper. Cadmium and nickel are also considered agricultural heavy metals [33].
Although heavy metals, POPs, and EDCs have overlapping characteristics, each group also has unique distinctions as noted in Figure 4. Members of each of these groups of chemicals have also been shown to influence the endocrine system and are well-positioned to influence the development of reproductive diseases in women. Since the literature and environmental justice movements suggest that minorities are more likely to live in areas with known environmental contamination [34][35], herein, researchers will review the current literature to identify potential associations between environmental contaminant exposure and the development of diseases known to exhibit racial disparities among women.
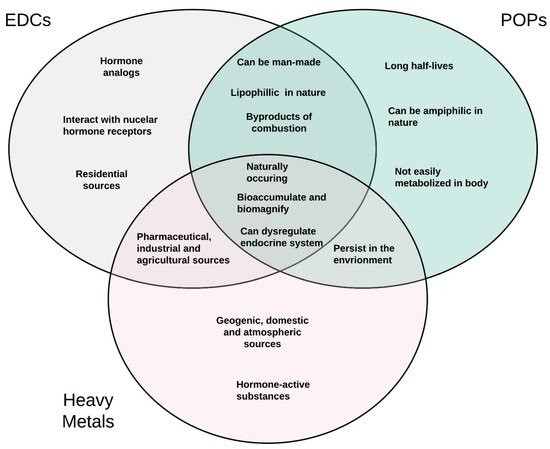
Figure 4. A three-way Venn diagram displaying commonalities and differences in the characteristics of POPs, EDCs, and heavy metals.
5. Risk Factors Associated with Human Exposure to Environmental Contaminants
A plethora of factors influence a person’s risk of exposure to environmental contaminants. These factors include socioeconomic status, occupation, diet, and personal habits. Although race is not a risk factor for environmental exposures per se, certain minority groups are more likely to fall into social groups (e.g., low socioeconomic status) that are at increased risk of exposure.
5.1. Socioeconomic Status, Occupation, and Geographic Locale
Previous studies suggest that socioeconomic status—which is correlated to occupation and geographic locale—plays a significant role in the risk of exposure to environmental contaminants [36]. In the United States, low-income residents, which are disproportionally minorities, have a more pronounced exposure to particulate matter-emitting facilities [37][38]. Furthermore, it has been reported that Black and Hispanic women living in Chicago were more likely to reside in areas with higher ambient concentrations of heavy metals including cadmium, mercury, and lead compared to white women [39].
The risk of exposure to environmental contaminants also varies by occupation. United States military personnel, which includes a disproportionally high percent of Black and Hispanic Americans, are at increased risk of exposure to environmental contaminants [40]. Additional occupations that may lead to disproportionate exposure to environmental contaminants include firefighters, miners, farmers, and industrial workers [41]. Furthermore, Ash et al. reported that individuals in low-paying jobs, which are more likely to be Black or Hispanic, were subjected to higher incidences of exposure than their counterparts with higher-paying occupations [42].
Furthermore, low socioeconomic status is positively correlated to high exposure to air pollution globally [43]. A study conducted in Italy supported the correlation between socioeconomic status and atmospheric pollution, reporting that low socioeconomic status increased the risk of exposure to particulate matter and nitrogen dioxide—each of which is a public health concern [44]. Citizens of developing countries are also at heightened risk of exposure to air pollution. However, it is predicted that much of the pollution experienced in developing countries is generated indoors as opposed to industrialized countries where pollution is generated from activities such as fossil fuel burning [45]. For example, in some developing nations such as sub-Saharan Africa, approximately 83% of the population relies on cooking methods that generate indoor pollution (e.g., solid fuel burning). In these settings, women are typically responsible for cooking and are therefore more susceptible to being exposed to this form of indoor pollution [46].
Peruvian and Guatemalan women are susceptible to exposure to PAHs and other harmful compounds through cooking methods as well [47]. Additionally, indoor exposure to benzo(a)pyrene (BaP) from cooking oil fumes has been reported to range from 19 to 23 μg/m3 in Taiwan [48]. Similarly, in rural Burundi, indoor BaP emission from wood combustion is estimated to be approximately 100 μg/m3 [49]. Socioeconomic status, occupation, and geographic location each influence one’s risk of exposure to environmental contaminants. Unfortunately, persons of color are disproportionally affected by factors that increase the risk of environmental contaminant exposure compared to whites.
5.2. Diet
As stated above, POPs, EDCs, and heavy metals have the potential to bioaccumulate and biomagnify within the food chain [50]. Thus, dietary habits can influence the body’s burden of environmental contaminants. Although data tracking dietary environmental contaminant exposure are scarce, a group in India recently reported that meat and dairy products are often traced with environmental contaminants [51]. Law et al. reported that chicken skin and fish from China had higher levels of environmental contaminants compared to beef and pork [52]. Food packaging, which is prevalent in industrialized countries, is also a major source of environmental contaminants because EDCs/POPs can leach into food [53]. Therefore, the food-borne risk of exposure to environmental contaminants may vary depending on the country of origin, food preferences, and food storage practices.
5.3. Use of Personal Care Products
Individuals that use personal care and beauty products are also susceptible to excess chemical exposures because these products frequently contain environmental contaminants [54]. Zota et al. reported that chemical exposures from beauty products vary by race and Black women were more likely to use products that contain EDCs. The group reported that Black women were at risk of being exposed to heavy metals, parabens, and phthalates through beauty products such as hair relaxers and vaginal douches [55]. Using a nationally representative sample of reproductive-aged women, Branch et al. found that Black women in the United States douched more frequently than other races. The group also found that women who douched had a 150% higher exposure to diethyl phthalate [56]. Studies suggest that hair care products used by Black women are more likely to contain placental-derived extracts, typically from cows or sheep, which have endocrine-disrupting activity. James-Todd et al. reported 49.4% (African American) and 26.4% (African-Caribbean) of Black women used hair products that contain placental extracts compared to 7.7% of white women [57].
The use of hair products that contain placental extracts or other EDCs among Black women has also been linked to altered reproductive development. Tiwary et al. reported that the use of hair products containing placental extracts on Black daughters between the ages of 14 and 93 months led to the premature development of breast and pubic hair. However, after the use of these products ceased, their sexual development regressed [58]. The group also reported that among military personnel, non-whites were four times more likely to use hair products containing EDCs and placental extracts compared to whites. However, women of all races/ethnicities were more likely to use these products compared to men [59]. Together, these studies suggest that the environmental contaminants found in hair products can negatively impact the endocrine system, potentially contributing to racial health disparities and sex-related differences in disease occurrence [55][58][59][60].
References
- Bonefeld-Jørgensen, E.C.; Ghisari, M.; Wielsøe, M.; Bjerregaard-Olesen, C.; Kjeldsen, L.S.; Long, M. Biomonitoring and hormone-disrupting effect biomarkers of persistent organic pollutants in vitro and ex vivo. Basic Clin. Pharmacol. Toxicol. 2014, 115, 118–128.
- National Toxicology Program. NTP technical report on the toxicology and carcinogenesis studies of 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153) (CAS No. 35065-27-1) in female Harlan Sprague-Dawley rats (Gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2006, 529, 4–168.
- Rumph, J.T.; Stephens, V.R.; Archibong, A.E.; Osteen, K.G.; Bruner-Tran, K.L. Environmental Endocrine Disruptors and Endometriosis. Adv. Anat. Embryol. Cell Biol. 2020, 232, 57–78.
- Combarnous, Y.; Nguyen, T.M.D. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics 2019, 7, 5.
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine Disrupting Chemicals: Effects on Endocrine Glands. Front. Endocrinol. 2019, 10, 178.
- Ye, S.; Chung, H.W.; Jeong, K.; Sung, Y.A.; Lee, H.; Park, S.Y.; Kim, H.; Ha, E.H. Blood cadmium and volume of uterine fibroids in premenopausal women. Ann. Occup. Environ. Med. 2017, 29, 22.
- Jackson, L.W.; Zullo, M.D.; Goldberg, J.M. The association between heavy metals, endometriosis and uterine myomas among premenopausal women: National Health and Nutrition Examination Survey 1999–2002. Hum. Reprod. 2008, 23, 679–687.
- Johnstone, E.B.; Louis, G.M.; Parsons, P.J.; Steuerwald, A.J.; Palmer, C.D.; Chen, Z.; Sun, L.; Hammoud, A.O.; Dorais, J.; Peterson, C.M. Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: Findings from the ENDO Study. Reprod. Toxicol. 2014, 49, 27–32.
- Martin, M.B.; Reiter, R.; Pham, T.; Avellanet, Y.R.; Camara, J.; Lahm, M.; Pentecost, E.; Pratap, K.; Gilmore, B.A.; Divekar, S.; et al. Estrogen-like activity of metals in MCF-7 breast cancer cells. Endocrinology 2003, 144, 2425–2436.
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164.
- Jaga, K. What are the implications of the interaction between DDT and estrogen receptors in the body? Med. Hypotheses 2000, 54, 18–25.
- Kelce, W.R.; Stone, C.R.; Laws, S.C.; Gray, L.E.; Kemppainen, J.A.; Wilson, E.M. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature 1995, 375, 581–585.
- Corsini, E.; Luebke, R.W.; Germolec, D.R.; DeWitt, J.C. Perfluorinated compounds: Emerging POPs with potential immunotoxicity. Toxicol. Lett. 2014, 230, 263–270.
- Zlatnik, M.G. Endocrine-Disrupting Chemicals and Reproductive Health. J. Midwifery Women’s Health 2016, 61, 442–455.
- Faroon, O.; Ruiz, P. Polychlorinated biphenyls: New evidence from the last decade. Toxicol. Ind. Health 2016, 32, 1825–1847.
- Dhakal, K.; Gadupudi, G.S.; Lehmler, H.J.; Ludewig, G.; Duffel, M.W.; Robertson, L.W. Sources and toxicities of phenolic polychlorinated biphenyls (OH-PCBs). Environ. Sci. Pollut. Res. Int. 2018, 25, 16277–16290.
- Hutzinger, O.; Choudhry, G.G.; Chittim, B.G.; Johnston, L.E. Formation of polychlorinated dibenzofurans and dioxins during combustion, electrical equipment fires and PCB incineration. Environ. Health Perspect. 1985, 60, 3–9.
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 145, 5–15.
- Hamidi, E.N.; Hajeb, P.; Selamat, J.; Abdull Razis, A.F. Polycyclic Aromatic Hydrocarbons (PAHs) and their Bioaccessibility in Meat: A Tool for Assessing Human Cancer Risk. Asian Pac. J. Cancer Prev. 2016, 17, 15–23.
- Rathee, M.; Malik, P.; Singh, J. Bisphenol A in dental sealants and its estrogen like effect. Indian J. Endocrinol. Metab. 2012, 16, 339–342.
- Hartle, J.C.; Navas-Acien, A.; Lawrence, R.S. The consumption of canned food and beverages and urinary Bisphenol A concentrations in NHANES 2003–2008. Environ. Res. 2016, 150, 375–382.
- Kendig, E.L.; Buesing, D.R.; Christie, S.M.; Cookman, C.J.; Gear, R.B.; Hugo, E.R.; Kendziorski, J.A.; Ungi, K.R.; Williams, K.; Belcher, S.M. Estrogen-like disruptive effects of dietary exposure to bisphenol A or 17α-ethinyl estradiol in CD1 mice. Int. J. Toxicol. 2012, 31, 537–550.
- Gao, H.; Yang, B.-J.; Li, N.; Feng, L.-M.; Shi, X.-Y.; Zhao, W.-H.; Liu, S.-J. Bisphenol A and hormone-associated cancers: Current progress and perspectives. Medicine 2015, 94, e211.
- McDonough, C.M.; Xu, H.S.; Guo, T.L. Toxicity of bisphenol analogues on the reproductive, nervous, and immune systems, and their relationships to gut microbiome and metabolism: Insights from a multi-species comparison. Crit. Rev. Toxicol. 2021, 51, 283–300.
- Parlett, L.E.; Calafat, A.M.; Swan, S.H. Women’s exposure to phthalates in relation to use of personal care products. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 197–206.
- Schechter, T.; Finkelstein, Y.; Koren, G. Pregnant “DES daughters” and their offspring. Can. Fam. Physician Med. Fam. Can. 2005, 51, 493–494.
- Jukes, T.H. Diethylstilbestrol in beef production: What is the risk to consumers? Prev. Med. 1976, 5, 438–453.
- Herbst, A.L.; Ulfelder, H.; Poskanzer, D.C. Adenocarcinoma of the vagina: Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. 1971, 284, 878–881.
- Vogel, S.A. The politics of plastics: The making and unmaking of bisphenol a “safety”. Am. J. Public Health 2009, 99 (Suppl. S3), S559–S566.
- Wirbisky, S.E.; Freeman, J.L. Atrazine Exposure and Reproductive Dysfunction through the Hypothalamus-Pituitary-Gonadal (HPG) Axis. Toxics 2015, 3, 414–450.
- Nessa, F.; Khan, S.A.; Abu Shawish, K.Y.I. Lead, Cadmium and Nickel Contents of Some Medicinal Agents. Indian J. Pharm. Sci. 2016, 78, 111–119.
- Liu, G.; Wang, J.; Liu, X.; Liu, X.; Li, X.; Ren, Y.; Wang, J.; Dong, L. Partitioning and geochemical fractions of heavy metals from geogenic and anthropogenic sources in various soil particle size fractions. Geoderma 2018, 312, 104–113.
- Kim, C.-H.; Yoo, D.-C.; Kwon, Y.-M.; Han, W.-S.; Kim, G.-S.; Park, M.-J.; Kim, Y.S.; Choi, D. A study on characteristics of atmospheric heavy metals in subway station. Toxicol. Res. 2010, 26, 157–162.
- Landrigan, P.J.; Rauh, V.A.; Galvez, M.P. Environmental justice and the health of children. Mt. Sinai J. Med. 2010, 77, 178–187.
- Pasetto, R.; Mattioli, B.; Marsili, D. Environmental Justice in Industrially Contaminated Sites: A Review of Scientific Evidence in the WHO European Region. Int. J. Environ. Res. Public Health 2019, 16, 998.
- Maantay, J. Mapping environmental injustices: Pitfalls and potential of geographic information systems in assessing environmental health and equity. Environ. Health Perspect. 2002, 110 (Suppl. S2), 161–171.
- Mikati, I.; Benson, A.F.; Luben, T.J.; Sacks, J.D.; Richmond-Bryant, J. Disparities in Distribution of Particulate Matter Emission Sources by Race and Poverty Status. Am. J. Public Health 2018, 108, 480–485.
- Li, Z.; Konisky, D.M.; Zirogiannis, N. Racial, ethnic, and income disparities in air pollution: A study of excess emissions in Texas. PLoS ONE 2019, 14, e0220696.
- Kaushiva, A.; Kresovich, J.; Erdal, S.; Rauscher, G. Abstract D096: Disparities in toxic heavy metal burden and breast cancer risk: Findings from the Metropolitan Chicago Breast Cancer Registry. Cancer Epidemiol. Biomark. Prev. 2020, 29 (Suppl. S2), D096.
- Geretto, M.; Ferrari, M.; De Angelis, R.; Crociata, F.; Sebastiani, N.; Pulliero, A.; Au, W.; Izzotti, A. Occupational Exposures and Environmental Health Hazards of Military Personnel. Int. J. Environ. Res. Public Health 2021, 18, 5395.
- Filippidou, E.-C.; Tsacheva, N. 007. Occupational exposure to chemical agents and its impact on the respiratory system. J. Thorac. Dis. 2015, 7 (Suppl. S1), AB007.
- Ash, M.; Boyce, J.K. Racial disparities in pollution exposure and employment at US industrial facilities. Proc. Natl. Acad. Sci. USA 2018, 115, 10636–10641.
- Hajat, A.; Hsia, C.; O’Neill, M.S. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Curr. Environ. Health Rep. 2015, 2, 440–450.
- Mannocci, A.; Ciarlo, I.; D’Egidio, V.; Del Cimmuto, A.; de Giusti, M.; Villari, P.; La Torre, G. Socioeconomic Deprivation Status and Air Pollution by PM10 and NO2: An Assessment at Municipal Level of 11 Years in Italy. J. Environ. Public Health 2019, 2019, 2058467.
- Mannucci, P.M.; Franchini, M. Health Effects of Ambient Air Pollution in Developing Countries. Int. J. Environ. Res. Public Health 2017, 14, 1048.
- Bede-Ojimadu, O.; Orisakwe, O.E. Exposure to Wood Smoke and Associated Health Effects in Sub-Saharan Africa: A Systematic Review. Ann. Glob. Health 2020, 86, 32.
- Weinstein, J.R.; Asteria-Peñaloza, R.; Diaz-Artiga, A.; Davila, G.; Hammond, S.K.; Ryde, I.T.; Meyer, J.N.; Benowitz, N.; Thompson, L.M. Exposure to polycyclic aromatic hydrocarbons and volatile organic compounds among recently pregnant rural Guatemalan women cooking and heating with solid fuels. Int. J. Hyg. Environ. Health 2017, 220, 726–735.
- Chiang, T.A.; Wu, P.F.; Ko, Y.C. Identification of carcinogens in cooking oil fumes. Environ. Res. 1999, 81, 18–22.
- Viau, C.; Hakizimana, G.; Bouchard, M. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int. Arch. Occup. Environ. Health 2000, 73, 331–338.
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 2345283.
- Sharma, B.M.; Bharat, G.K.; Chakraborty, P.; Martiník, J.; Audy, O.; Kukučka, P.; Přibylová, P.; Kukreti, P.K.; Sharma, A.; Kalina, J.; et al. A comprehensive assessment of endocrine-disrupting chemicals in an Indian food basket: Levels, dietary intakes, and comparison with European data. Environ. Pollut. 2021, 288, 117750.
- Law, A.Y.S.; Wei, X.; Zhang, X.; Mak, N.K.; Cheung, K.C.; Wong, M.H.; Giesy, J.P.; Wong, C.K.C. Biological analysis of endocrine-disrupting chemicals in animal meats from the Pearl River Delta, China. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 93–100.
- Muncke, J. Exposure to endocrine disrupting compounds via the food chain: Is packaging a relevant source? Sci. Total Environ. 2009, 407, 4549–4559.
- Dodson, R.E.; Nishioka, M.; Standley, L.J.; Perovich, L.J.; Brody, J.G.; Rudel, R.A. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect. 2012, 120, 935–943.
- Zota, A.R.; Shamasunder, B. The environmental injustice of beauty: Framing chemical exposures from beauty products as a health disparities concern. Am. J. Obstet. Gynecol. 2017, 217, 418.e1–418.e6.
- Branch, F.; Woodruff, T.J.; Mitro, S.D.; Zota, A.R. Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001–2004. Environ. Health 2015, 14, 57.
- James-Todd, T.; Senie, R.; Terry, M.B. Racial/ethnic differences in hormonally-active hair product use: A plausible risk factor for health disparities. J. Immigr. Minor. Health 2012, 14, 506–511.
- Tiwary, C.M. Premature sexual development in children following the use of estrogen- or placenta-containing hair products. Clin. Pediatr. 1998, 37, 733–739.
- Tiwary, C.M.; Ward, J.A. Use of hair products containing hormone or placenta by US military personnel. J. Pediatr. Endocrinol. Metab. 2003, 16, 1025–1032.
- James-Todd, T.; Connolly, L.; Preston, E.V.; Quinn, M.R.; Plotan, M.; Xie, Y.; Gandi, B.; Mahalingaiah, S. Hormonal activity in commonly used Black hair care products: Evaluating hormone disruption as a plausible contribution to health disparities. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 476–486.
More
Information
Subjects:
Toxicology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
27 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




