
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hamza Mechchate | + 1493 word(s) | 1493 | 2022-01-05 07:42:03 | | | |
| 2 | Lindsay Dong | + 897 word(s) | 2390 | 2022-01-26 02:37:01 | | |
Video Upload Options
Fruits vinegar (FsV) is a healthy drink wealthy in bioactive compounds that provide several beneficial properties. It contains a cocktail of bioactive ingredients including polyphenolic acids, organic acids, tetramethylperazine, and melanoidins. Acetic acid is the most abundant organic acid and chlorogenic acid is the major phenol in apple vinegar. The administration of fruits vinegar could prevent diabetes, hypercholesterolemia, oxidative stress, cancer, and boost immunity as well as provide a remarkable antioxidant ability.
1. Introduction
2. Chemical Characteristics of FsV
| Variety | Country | Method of Vinegarmaking | Methods | Bioactive Compounds Identified | References |
|---|---|---|---|---|---|
| Grape vinegar | Turkey | Artisanal and industrial | HPLC-DAD | Gallic acid (16.36–18.23 mg/L), catechin (13.76–27.50 mg/L), epicatechin (4.96–8.20 mg/L), caffeic acid (6.30–10.30 mg/L), chlorogenic acid (0.16–3.73 mg/L), syringic acid (0.33–0.70 mg/L), p-coumaric acid (0.23–0.56 mg/L), and ferulic acid (0.06–0.35 mg/L) | [4] |
| Grape vinegar | Industrial | HPLC-PDA | Gallic acid (6 ± 2 mg/100 mL) and p-hydroxybenzoic acid (0.90 ± 0.05 mg/100 mL) | [7] | |
| Apple vinegar | Gallic acid (0.8 ± 0.04 mg/100 mL), p-hydroxybenzoic acid (0.2 ± 0.1 mg/100 mL), catechin (2.4 ± 0.1 mg/100 mL), syringic acid (0.12 ± 0.02 mg/100 mL), caffeic acid (0.40 ± 0.01 mg/100 mL), and p-coumaric acid (0.08 ± 0.01 mg/100 mL) | ||||
| Apple vinegar | Artisanal | HPLC-DAD | Gallic acid (61.24 ± 2.21 mg/L), chlorogenic acid (347.70 ± 31.94 mg/L), catechin (68.20 mg/L), and caffeic acid (17.21 ± 0.33 mg/L) | [28] | |
| Pomegranate vinegar | Gallic acid (67.80 ± 2.88 mg/L), catechin (47 ± 1.10 mg/L), and caffeic acid (13.41 ± 0.60 mg/L) | ||||
| Aromatic vinegar * | China | Artisanal | HPLC | Gallic acid, p-hydroxybenzoic acid, vanillic acid, catechin, caffeic acid, chlorogenic acid, syringic acid, ethyl gallate, p-coumaric acid, ferulic acid, sinapic acid, and rutin. | [29] |
| Grape vinegar | Turkey | Industrial | LC-DAD-ESI-MS/MS | Gallic acid (7.45–21.84 mg/L), tyrosol (11.54–17.68 mg/L), protocatechuic acid (7.21–11.05 mg/L), caftaric acid (1.76–15.83 mg/L), cholorogenic acid (0.09–1.77 mg/L), coutaric acid (0–1.95 mg/L) caffeic acid (0.11–2.58 mg/L), ferulic acid (0.01–0.21 mg/L), fertaric acid (0.03–0.83 mg/L), vanilic acid (0–2.58 mg/L), p-coumaric acid (0.02–0.45 mg/L), syringic acid (1.24–9.04 mg/L), procyanidin B2 (0.09–3.11 mg/L), catechin (3.73–27.11 mg/L), epicatechin (0.57–15.13 mg/L), quercetin-3-O-galactoside (0.04–0.39 mg/L), kaempferol-3-O-rutinoside (0–0.04 mg/L), rutin (0.02–0.20 mg/L), isorhamnetin-3-O-glucoside (0.05–0.09 mg/L), and quercetin (0.06–0.69 mg/L). |
[8] |
| Apple vinegar | Gallic acid (0.47–2.57 mg/L), protocatechuic acid (1.15–6.35 mg/L), cholorogenic acid (2.96–16.29 mg/L), caffeic acid (0.19–1.77 mg/L), vanilic acid (0.63–3.42 mg/L), p-coumaric acid (0.13–0.81 mg/L), procyanidin B2 (0.12–1.35 mg/L), catechin (0.14–0.95 mg/L), epicatechin (0.04–1.36 mg/L), luteolin-3-O-rutinoside (0.30–1.98 mg/L), isorhamnetin-3-O-rutinoside (0.10–0.63 mg/L), isorhamnetin-3-O-glucoside (0.08–0.48 mg/L), kaempferol-3-O-glucoside (0.03–0.20 mg/L), quercetin-3-O-rhamnoside (0.20–3.41 mg/L), quercetin (0.20–1.41 mg/L), rutin (0.04–0.29 mg/L), luteolin (0.27–1.63 mg/L), apigenin0.02–0.13 mg/L), phloretin (0.59–7.86 mg/L), and phloridzin (7.64–44.35 mg/L). | ||||
| Apple vinegar | Japan | Industrial | LC-MS | Chlorogenic acid (3.1–19.6 mg/100 mL), 4-p-coumaric acid (0–0.21 mg/100 mL), isomer of p-coumaroyquinic acid (0–1.3 mg/100 mL), 5-hydroxymethylfurfural (2.7–4.1 mg/100 mL), protocatechic acid (0–0.41 mg/100 mL), p-hydroxybenzoic acid (0–0.77 mg/100 mL), caffeic acid (0–0.76 mg/100 mL), isomer of chlorogenic acid (0–3.1 mg/100 mL), and p-coumaric acid (0–0.21 mg/100 mL) | [30] |
| Persimmon vinegar | China | Artisanal | HPLC | Gallic acid (22.91 ± 1.22 mg/L), (+/−)-catechin hydrate (0.16 ± 0.89 mg/L), chlorogenic acid (0.06 ± 0.12 mg/L), caffeic acid (0.04 ± 0.06 mg/L), p-coumaric acid (0.03 ± 0.21 mg/L), trans-ferulic acid (0.02 ± 0.11 mg/L), (-)-epicatechin gallate (0.13 ± 0.09 mg/L), and phloridzin (0.38 ± 0.12 mg/L) | [31] |
| Apple vinegar | Gallic acid (0.35 ± 0.02 mg/L), vanillic acid (0.06 ± 0.04 mg/L), chlorogenic acid (6.56 ± 0.43 mg/L), caffeic acid (3.03 ± 0.02 mg/L), p-coumaric acid (0.33 ± 0.28 mg/L), trans-ferulic acid (0.24 ± 0.07 mg/L), (-)-epicatechin gallate (0.77 ± 0.34), and phloridzin (1.76 ± 0.34 mg/L). | ||||
| Kiwifruit vinegar | Gallic acid (9.67 ± 0.59 mg/L), (+/−)-catechin hydrate (1.47 ± 0.34 mg/L), vanillic acid (1.77 ± 0.23 mg/L), chlorogenic acid (3.12 ± 0.21 mg/L), caffeic acid (0.04 ± 0.05 mg/L), p-coumaric acid (0.34 ± 0.01 mg/L), trans-ferulic acid (0.01 ± 0.03 mg/L), and phloridzin (0.49 ± 0.02 mg/L) | ||||
| Apple vinegar | Brazil | Industrial | HPLC-PDA | Phloretin-2′-β-d-glucoside (4.81–15.55 mg/L), 5-caffeoylquinic acid (20.62–26.85 mg/L), caffeic acid (0.51–3.87 mg/L), p-coumaric acid (1.16–2.03 mg/L), quercetin-3-rutinoside (2.69–4.65 mg/L), quercetin-3-d-galactoside (0.73–9.75 mg/L), quercetin-3-β-d-glucoside (1.58–3.45 mg/L), quercetin-3-d-xyloside (1.62–2.54 mg/L), quercetin-O-α-l-arabinofuranoside (0.85–1.34 mg/L), and quercetin-3-O-rhamnoside (1.13–3.37 mg/L). | [32] |
| Apple vinegar | China | Industrial | HPLC-PDA | Chlorogenic acid (0.11–10.91 µg/mL), protocatechuic acid (0.08–1.54 µg/mL), and p-coumaric acid (0.10–0.17 µg/mL | [5] |
| Red wine vinegar | Gallic acid (4.10–9.99 µg/mL), protocatechuic acid (0.47–1.38 µg/mL), p-coumaric acid (0.81–1.39 µg/mL), and caffeic acid (1.48–1.73 µg/mL) | ||||
| White wine vinegar | Protocatechuic acid (0.16–0.32 µg/mL), p-coumaric acid (0–0.18 µg/mL), caffeic acid (0–0.32 µg/mL), and ferulic acid (0–0.31 µg/mL) | ||||
| Balsamic vinegar | Gallic acid (7.50–12.56 µg/mL), protocatechuic acid (0–3.29 µg/mL), p-coumaric acid (1.17–1.97 µg/mL), and caffeic acid (0–3.58 µg/mL) | ||||
| Sour cherry vinegar | Turkey | Industrial | HPLC | Gallic acid (160–170 mg/mL), chlorogenic acid (45–55 mg/mL), p-coumaric acid (17–23 mg/mL), caffeic acid (3.5–4 mg/mL), ferulic acid (1.3–4.6 mg/mL), catechin (0.7–1 mg/mL), and epicatechin (1.7–3.5 mg/mL) | [33] |
| Palm vinegar | Thailand | Artisanal | LC-MS | Gallic acid (14.14 ± 0.07 µg/mL), catechin (8.61 ± 0.32 µg/mL), rutin (6.67 ± 0.03 µg/mL), isoquercetin (11.27 ± 0.12 µg/mL), and quercetin (10.33 ± 0.16 µg/mL) | [34] |
| Brow beer vinegar | Italy | Industrial | HPLC-DAD-ESI(+)-MS | Protocatechuic acid O-glucoside (7.42 ± 0.03 mg/L), 3-caffeoylquinic acid (40.01 ± 1.13 mg/L), (4-Hydroxyphenyl) acetic acid (11.84 ± 0.02 mg/L), 4-vinylguaiacol (10.22 ± 0.04 mg/L), Catechin 7 O-glucoside (8.84 ± 0.02 mg/L), 4-hydroxybenzoic acid (38.23 ± 0.05 mg/L), (3-hydroxyphenyl)acetic acid (18.95 ± 0.04 mg/L), catechin 5 O-glucoside (7.24 ± 0.06 mg/L), coumaric acid O-glucoside (4.90 ± 0.05 mg/L), cerulic acid O-glucoside (4.33 ± 0.02 mg/L), gallic acid (5.72 ± 0.04 mg/L), vanilic acid O-glucoside (10.25 ± 0.03 mg/L), gallocatechin (7.66 ± 0.10 mg/L), sinapic acid O-glucoside (14.03 ± 0.12 mg/L), catechin O-diglucoside (8.41 ± 0.04 mg/L), kaempferol O-glucoside (6.28 ± 0.04 mg/L), feruloylquinic acid (6.60 ± 0.15 mg/L), chlorogenic acid (18.30 ± 0.02 mg/L), (+)-catechin (7.89 ± 0.04 mg/L), (−)-epicatechin (7.78 ± 0.12 mg/L), caffeic acid (10.58 ± 0.08 mg/L), sinapic acid (15.5 ± 0.06 mg/L), apigenin O-glucoside (6.15 ± 0.02 mg/L), quercetin O-glucoside (7.05 ± 0.06 mg/L), cohumulone I (4.44 ± 0.02 mg/L), cohumulone II (6.58 ± 0.10 mg/L), 8-prenylnaringenin (2.33 ± 0.02 mg/L), 6-prenylnaringenin (1.86 ± 0.02 mg/L), humulone (5.62 ± 0.08 mg/L), and isohumulone (4.14 ± 0.03 mg/L) | [35] |
| Pineapple vinegar | Industrial | UHPLC-QTOF-MS | Catechol, peonidin, (+)-catechin 3-O-gallate, m-coumaric acid, 7,3’,4’-trihydroxyflavone, 4-vinylsyringol, ferulic acid, mullein, genistin, 3,4-dihydroxyphenylglycol, 4-ethylcatechol, 6-prenylnaringenin, gallic acid, kaempferol 3-O-xylosyl-glucoside, 6,8-Dihydroxykaempferol, spinacetin 3-O-glucosyl-(1-6)-[apiosyl(1-2)]-glucoside, and malvidin 3-O-arabinoside | [36] | |
| Cherry vinegar | Spain | Industrial | UPLC-DAD | Gallic acid (2.08–2.99 mg/L), HMF (6.96–9.48 mg/L), protocatechuic acid (2.12–2.43 mg/L), caftaric acid (2.05–2.81 mg/L), furoic acid (2.46–16.53 mg/L), protocatechualdehyde (0.046–0.263 mg/L), cis-p-Coutaric acid (1.83–2.25 mg/L), trans-p-Coutaric acid (1.15–1.55 mg/L), tyrosol (24.6–28.9 mg/L), catequin (0.165–0.334 mg/L), caffeic acid (0.184–0.308 mg/L), vanillic acid (2.66–3.44 mg/L), syringic acid (2.16–5.44 mg/L), vanillin (1.05–2.97 mg/L), cis-p-coumaric acid (0.174–0.481 mg/L), syringaldehyde (0.50–5.12 mg/L), coniferyl aldehyde (0.959–2.85 mg/L), and sinapaldehyde (16.1–19.1 mg/L) | [37] |
| Sugarcane vinegar | China | Industrial | UPLC-MS | Benzoic acid (1.027 ± 0.07 mg/L), ferulic acid (1.1240.063 mg/L), quinic acid (0.031 ± 0.002 mg/L), chlorogenic acid (1.217 ± 0.063 mg/L), apigenin (0.004 ± 0 mg/L), kaempferol (0.003 ± 0.0001 mg/L), caffeic acid (0.005 ± 0.0001 mg/L), luteolin (0.005 ± 0.0001 mg/L), and p-coumaric acid (0.027 ± 0.0001 mg/L) | [38] |
| Citrus vinegar | Italy | Industrial | UPLC-UV | Gallic acid (2.62–5.63 mg/L), neochlorogenic acid (2.69–5.83 mg/L), chlorogenic acid (2.95–58.51 mg/L), vanillic acid (0.47–3.64 mg/L), caffeic acid (1.39–3.64 mg/L), epicatechin (0–2.91 mg/L), procyanidin (0–9.43 mg/L), rutin (1.76–146.3 mg/L), quercetin (0.23–8.62 mg/L), eriocitrin (0.27–13.20 mg/L), neoeriocitrin (53.41–513.30 mg/L), narirutin (3.05–18.24 mg/L), naringin (61.19–700.56 mg/L), hesperidin (12.15–92.12 mg/L), neohesperidin (63.51–366.93 mg/L), didymin (1.73–9.82 mg/L), and hesperetin (0–15.54 mg/L) | [39] |
3. Beneficial Properties of FsV
3.1. Antihyperglycemic Effect
Investigations of the antihyperglycemic activity of FsV were started in the late 80s. In fact, the research conducted by [43] has demonstrated that the co-administration of 2% acetic acid with meals (starch intake) decreased significantly glycemia. Subsequently, multiple studies also found that the administration of apple vinegar reduced blood glucose levels. This effect may be due in part to the stimulation of glucose uptake and enhancement of the action of insulin in skeletal muscle [44]. These beneficial effects have been attributed to acetic acid which acts via MAPK pathway (Figure 1).
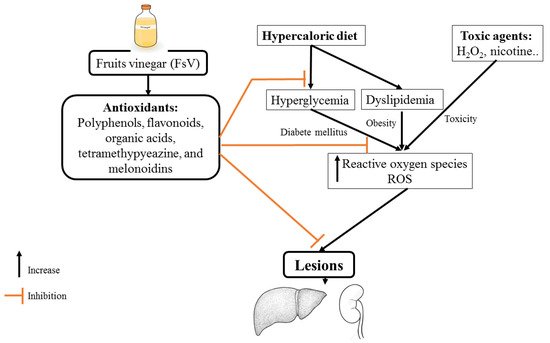
3.2. Antihyperlipidemic Effect
Vinegar is used in traditional medicine to treat dyslipidemia, which promotes the development of cardiovascular diseases [48]. The administration of apple vinegar during 8 weeks ameliorates lipid profile (cholesterol, low-density lipoprotein (LDL), and triglycerides) [48]. Additionally, mice fed with a hyper-fat diet and treated with synthetic acetic acid vinegar or nipa vinegar reduced total cholesterol, triglycerides, LDL, and leptin levels [49].
3.3. Antimicrobial Effect
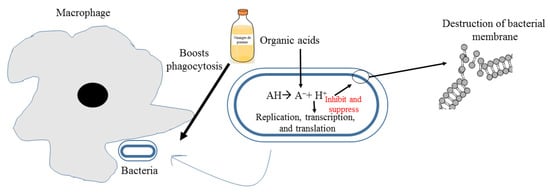
3.4. Antioxidant Effect
3.5. Anti-Inflammatory Effect
3.6. Other Effects
References
- Ramírez-Guzmán, K.N.; Torres-León, C.; Martinez-Medina, G.A.; de la Rosa, O.; Hernández-Almanza, A.; Alvarez-Perez, O.B.; Araujo, R.; González, L.R.; Londoño, L.; Ventura, J. Traditional Fermented Beverages in Mexico. In Fermented Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 605–635.
- Robledo-Márquez, K.; Ramírez, V.; González-Córdova, A.F.; Ramírez-Rodríguez, Y.; García-Ortega, L.; Trujillo, J. Research Opportunities: Traditional Fermented Beverages in Mexico. Cultural, Microbiological, Chemical, and Functional Aspects. Food Res. Int. 2021, 147, 110482.
- Bounihi, A.; Bitam, A.; Bouazza, A.; Yargui, L.; Koceir, E.A. Fruit Vinegars Attenuate Cardiac Injury via Anti-Inflammatory and Anti-Adiposity Actions in High-Fat Diet-Induced Obese Rats. Pharm. Biol. 2017, 55, 43–52.
- Budak, H.N.; Guzel-Seydim, Z.B. Antioxidant Activity and Phenolic Content of Wine Vinegars Produced by Two Different Techniques. J. Sci. Food Agric. 2010, 90, 2021–2026.
- Liu, Q.; Tang, G.-Y.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants 2019, 8, 78.
- Zhou, J.; Li, L.; Yue, Q.; Zhang, Q. Determination of Organic Acids in Apple Vinegar by Ion Exclusion Chromatography. China Brew. 2005, 12. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZNGZ200512018.htm (accessed on 22 April 2021).
- Bakir, S.; Toydemir, G.; Boyacioglu, D.; Beekwilder, J.; Capanoglu, E. Fruit Antioxidants during Vinegar Processing: Changes in Content and In Vitro Bio-Accessibility. Int. J. Mol. Sci. 2016, 17, 1658.
- Kelebek, H.; Kadiroğlu, P.; Demircan, N.B.; Selli, S. Screening of Bioactive Components in Grape and Apple Vinegars: Antioxidant and Antimicrobial Potential. J. Inst. Brew. 2017, 123, 407–416.
- Chen, H.; Chen, T.; Giudici, P.; Chen, F. Vinegar Functions on Health: Constituents, Sources, and Formation Mechanisms. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1124–1138.
- Zhu, Z.; Zhang, Y.; Wang, W.; Huang, Z.; Wang, J.; Li, X.; Sun, S. Structural Characterisation and Antioxidant Activity of Melanoidins from High-Temperature Fermented Apple. Int. J. Food Sci. Technol. 2020, 56, 2471–2480.
- Chen, J.-C.; Chen, Q.-H.; Guo, Q.; Ruan, S.; Ruan, H.; He, G.-Q.; Gu, Q. Simultaneous Determination of Acetoin and Tetramethylpyrazine in Traditional Vinegars by HPLC Method. Food Chem. 2010, 122, 1247–1252.
- Wu, J.; Zhao, H.; Du, M.; Song, L.; Xu, X. Dispersive Liquid–Liquid Microextraction for Rapid and Inexpensive Determination of Tetramethylpyrazine in Vinegar. Food Chem. 2019, 286, 141–145.
- Budak, N.H.; Kumbul Doguc, D.; Savas, C.M.; Seydim, A.C.; Kok Tas, T.; Ciris, M.I.; Guzel-Seydim, Z.B. Effects of Apple Cider Vinegars Produced with Different Techniques on Blood Lipids in High-Cholesterol-Fed Rats. J. Agric. Food Chem. 2011, 59, 6638–6644.
- Raspor, P.; Goranovič, D. Biotechnological Applications of Acetic Acid Bacteria. Crit. Rev. Biotechnol. 2008, 28, 101–124.
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, R757–R764.
- Ousaaid, D.; Laaroussi, H.; Bakour, M.; ElGhouizi, A.; Aboulghazi, A.; Lyoussi, B.; ElArabi, I. Beneficial Effects of Apple Vinegar on Hyperglycemia and Hyperlipidemia in Hypercaloric-Fed Rats. Available online: https://www.hindawi.com/journals/jdr/2020/9284987/ (accessed on 4 November 2020).
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic Acid Upregulates the Expression of Genes for Fatty Acid Oxidation Enzymes in Liver to Suppress Body Fat Accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986.
- Sakakibara, S.; Yamauchi, T.; Oshima, Y.; Tsukamoto, Y.; Kadowaki, T. Acetic Acid Activates Hepatic AMPK and Reduces Hyperglycemia in Diabetic KK-A (y) Mice. Biochem. Biophys. Res. Commun. 2006, 344, 597–604.
- Yamashita, H.; Maruta, H.; Jozuka, M.; Kimura, R.; Iwabuchi, H.; Yamato, M.; Saito, T.; Fujisawa, K.; Takahashi, Y.; Kimoto, M. Effects of Acetate on Lipid Metabolism in Muscles and Adipose Tissues of Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2009, 73, 570–576.
- Lafraxo, H.; Bakour, M.; Laaroussi, H.; El Ghouizi, A.; Ousaaid, D.; Aboulghazi, A.; Lyoussi, B. The Synergistic Beneficial Effect of Thyme Honey and Olive Oil against Diabetes and Its Complications Induced by Alloxan in Wistar Rats. Evid.-Based Complement. Alternat. Med. 2021, 2021, 9949056.
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Teixeira, J.A.; Lyoussi, B. Effect of Antioxidant-Rich Propolis and Bee Pollen Extracts against d-Glucose Induced Type 2 Diabetes in Rats. Food Res. Int. 2020, 138, 109802.
- Patrignani, F.; D’Alessandro, M.; Vannini, L.; Lanciotti, R. Use of Functional Microbial Starters and Probiotics to Improve Functional Compound Availability in Fermented Dairy Products and Beverages. In Sustainability of the Food System; Elsevier: Amsterdam, The Netherlands, 2020; pp. 167–180.
- Xia, T.; Zhang, B.; Duan, W.; Zhang, J.; Wang, M. Nutrients and Bioactive Components from Vinegar: A Fermented and Functional Food. J. Funct. Foods 2020, 64, 103681.
- De Jong, C.; Hazelwood, L.A.; Dijkstra, A.; Pepin, L. Use of the Micro-Scale Platform for High Throughput Screening of Flavor Characteristics in Strains (Yeast/LAB) for Alcoholic Beverages. In Flavour Science; Elsevier: Amsterdam, The Netherlands, 2014; pp. 355–359. ISBN 978-0-12-398549-1.
- Lee, J.H.; Choi, K.H.; Kim, S.H.; Park, K.S.; Park, S.H.; Kim, J.S.; Kang, S.A.; Cheong, C.; Jang, K.H. Physicochemical Characteristics and Electric Conductivity of Various Fruit Wines. Int. Food Res. J. 2013, 20, 2987–2993.
- Launholt, T.L.; Kristiansen, C.B.; Hjorth, P. Safety and Side Effects of Apple Vinegar Intake and Its Effect on Metabolic Parameters and Body Weight: A Systematic Review. Eur. J. Nutr. 2020, 59, 2273–2289.
- Xia, T.; Zhang, J.; Yao, J.; Zhang, B.; Duan, W.; Zhao, C.; Du, P.; Song, J.; Zheng, Y.; Wang, M. Shanxi Aged Vinegar Protects against Alcohol-Induced Liver Injury via Activating Nrf2-Mediated Antioxidant and Inhibiting TLR4-Induced Inflammatory Response. Nutrients 2018, 10, 805.
- Aykın, E.; Budak, N.H.; Güzel-Seydim, Z.B. Bioactive Components of Mother Vinegar. J. Am. Coll. Nutr. 2015, 34, 80–89.
- Duan, W.; Xia, T.; Zhang, B.; Li, S.; Zhang, C.; Zhao, C.; Song, J.; Wang, M. Changes of Physicochemical, Bioactive Compounds and Antioxidant Capacity during the Brewing Process of Zhenjiang Aromatic Vinegar. Molecules 2019, 24, 3935.
- Nakamura, K.; Ogasawara, Y.; Endou, K.; Fujimori, S.; Koyama, M.; Akano, H. Phenolic Compounds Responsible for the Superoxide Dismutase-like Activity in High-Brix Apple Vinegar. J. Agric. Food Chem. 2010, 58, 10124–10132.
- Ren, M.; Wang, X.; Tian, C.; Li, X.; Zhang, B.; Song, X.; Zhang, J. Characterization of Organic Acids and Phenolic Compounds of Cereal Vinegars and Fruit Vinegars in China. J. Food Process. Preserv. 2017, 41, e12937.
- Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A.; Alberti, A.; Zielinski, A.A.F. A New Approach to the Use of Apple Pomace in Cider Making for the Recovery of Phenolic Compounds. LWT 2020, 126, 109316.
- Özen, M.; Özdemir, N.; Filiz, B.E.; Budak, N.H.; Kök-Taş, T. Sour Cherry (Prunus cerasus L.) Vinegars Produced from Fresh Fruit or Juice Concentrate: Bioactive Compounds, Volatile Aroma Compounds and Antioxidant Capacities. Food Chem. 2020, 309, 125664.
- Chatatikun, M.; Kwanhian, W. Phenolic Profile of Nipa Palm Vinegar and Evaluation of Its Antilipidemic Activities. Evid.-Based Complement. Alternat. Med. 2020, 2020, 6769726.
- Mudura, E.; Coldea, T.E.; Socaciu, C.; Ranga, F.; Pop, C.R.; Rotar, A.M.; Pasqualone, A. Brown Beer Vinegar: A Potentially Functional Product Based on Its Phenolic Profile and Antioxidant Activity. J. Serb. Chem. Soc. 2018, 83, 19–30.
- Roda, A.; Lucini, L.; Torchio, F.; Dordoni, R.; De Faveri, D.M.; Lambri, M. Metabolite Profiling and Volatiles of Pineapple Wine and Vinegar Obtained from Pineapple Waste. Food Chem. 2017, 229, 734–742.
- Jiménez-Sánchez, M.; Durán-Guerrero, E.; Rodríguez-Dodero, M.C.; Barroso, C.G.; Castro, R. Use of Ultrasound at a Pilot Scale to Accelerate the Ageing of Sherry Vinegar. Ultrason. Sonochem. 2020, 69, 105244.
- Chen, G.-L.; Zheng, F.-J.; Lin, B.; Lao, S.-B.; He, J.; Huang, Z.; Zeng, Y.; Sun, J.; Verma, K.K. Phenolic and Volatile Compounds in the Production of Sugarcane Vinegar. ACS Omega 2020, 5, 30587–30595.
- Giuffrè, A.M.; Zappia, C.; Capocasale, M.; Poiana, M.; Sidari, R.; Di Donna, L.; Bartella, L.; Sindona, G.; Corradini, G.; Giudici, P.; et al. Vinegar Production to Valorise Citrus bergamia By-Products. Eur. Food Res. Technol. 2019, 245, 667–675.
- Ozturk, I.; Caliskan, O.; Tornuk, F.; Ozcan, N.; Yalcin, H.; Baslar, M.; Sagdic, O. Antioxidant, Antimicrobial, Mineral, Volatile, Physicochemical and Microbiological Characteristics of Traditional Home-Made Turkish Vinegars. LWT Food Sci. Technol. 2015, 63, 144–151.
- Chen, J.; Tian, J.; Ge, H.; Liu, R.; Xiao, J. Effects of Tetramethylpyrazine from Chinese Black Vinegar on Antioxidant and Hypolipidemia Activities in HepG2 Cells. Food Chem. Toxicol. 2017, 109, 930–940.
- El-Sayed, T.S.; Nour El-Deen, M.M.; Rokaya, M.E.; Sherif, M.M. Evaluation of the Antibacterial Effect of Apple Vinegar as a Root Canal Irrigant Using Endovac Irrigation System. Al-Azhar Dent. J. Girls 2019, 6, 53–59.
- Ebihara, K.; Nakajima, A. Effect of Acetic Acid and Vinegar on Blood Glucose and Insulin Responses to Orally Administered Sucrose and Starch. Agric. Biol. Chem. 1988, 52, 1311–1312.
- Mitrou, P.; Petsiou, E.; Papakonstantinou, E.; Maratou, E.; Lambadiari, V.; Dimitriadis, P.; Spanoudi, F.; Raptis, S.A.; Dimitriadis, G. Vinegar Consumption Increases Insulin-Stimulated Glucose Uptake by the Forearm Muscle in Humans with Type 2 Diabetes. J. Diabetes Res. 2015, 2015, 175204.
- Solieri, L.; Giudici, P. Vinegars of the World. In Vinegars of the World; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–16.
- Brighenti, F.; Castellani, G.; Benini, L.; Casiraghi, M.C.; Leopardi, E.; Crovetti, R.; Testolin, G. Effect of Neutralized and Native Vinegar on Blood Glucose and Acetate Responses to a Mixed Meal in Healthy Subjects. Eur. J. Clin. Nutr. 1995, 49, 242–247.
- Ogawa, N.; Satsu, H.; Watanabe, H.; Fukaya, M.; Tsukamoto, Y.; Miyamoto, Y.; Shimizu, M. Acetic Acid Suppresses the Increase in Disaccharidase Activity That Occurs during Culture of Caco-2 Cells. J. Nutr. 2000, 130, 507–513.
- Bahesheti, Z.; Chan, Y.H.; Nia, H.S.; Hajihosseini, F.; Nazari, R.; Shaabani, M. Influence of Apple Cider Vinegar on Blood Lipids. Life Sci. J. 2012, 9, 2431–2440.
- Beh, B.K.; Mohamad, N.E.; Yeap, S.K.; Ky, H.; Boo, S.Y.; Chua, J.Y.H.; Tan, S.W.; Ho, W.Y.; Sharifuddin, S.A.; Long, K. Anti-Obesity and Anti-Inflammatory Effects of Synthetic Acetic Acid Vinegar and Nipa Vinegar on High-Fat-Diet-Induced Obese Mice. Sci. Rep. 2017, 7, 6664.
- Ousaaid, D.; Imtara, H.; Laaroussi, H.; Lyoussi, B.; Elarabi, I. An Investigation of Moroccan Vinegars: Their Physicochemical Properties and Antioxidant and Antibacterial Activities. Available online: https://www.hindawi.com/journals/jfq/2021/6618444/ (accessed on 10 February 2021).
- Yagnik, D.; Serafin, V.; Shah, A.J. Antimicrobial Activity of Apple Cider Vinegar against Escherichia Coli, Staphylococcus Aureus and Candida Albicans; Downregulating Cytokine and Microbial Protein Expression. Sci. Rep. 2018, 8, 1732.
- Hindi, N.K. In Vitro Antibacterial Activity of Aquatic Garlic Extract, Apple Vinegar and Apple Vinegar-Garlic Extract Combination. Am. J. Phytomed. Clin. Ther. 2013, 1, 42–51.
- Hindi, N.K.K.; Al-Mahdi, Z.K.A.; Chabuck, Z.A.G. Antibacterial Activity of the Aquatic Extractof Fresh, Dry Powder Ginger, Apple Vinegar Extract of Fresh Ginger and Crud Oil of Ginger (Zingiberofficinale) against Different Types of Bacteria in Hilla City, Iraq. Prostate 2014, 3, 6.
- Yagnik, D.; Ward, M.; Shah, A.J. Antibacterial Apple Cider Vinegar Eradicates Methicillin Resistant Staphylococcus aureus and Resistant Escherichia coli. Sci. Rep. 2021, 11, 1854.
- Alakomi, H.-L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic Acid Permeabilizes Gram-Negative Bacteria by Disrupting the Outer Membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005.
- Brul, S.; Coote, P. Preservative Agents in Foods: Mode of Action and Microbial Resistance Mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17.
- Hirshfield, I.N.; Terzulli, S.; O’Byrne, C. Weak Organic Acids: A Panoply of Effects on Bacteria. Sci. Prog. 2003, 86, 245–269.
- Wang, H.; Liu, J.; Liu, Z. Effect of Enzymatic Digestion, Chemical and Boiled Water Extraction Techniques on Apparent Antioxidant Bioactivities of Apple Peel. J. Food Meas. Charact. 2019, 13, 959–966.
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, Production, Composition and Health Benefits of Vinegars: A Review. Food Chem. 2017, 221, 1621–1630.
- Wakuda, T.; Azuma, K.; Saimoto, H.; Ifuku, S.; Morimoto, M.; Arifuku, I.; Asaka, M.; Tsuka, T.; Imagawa, T.; Okamoto, Y. Protective Effects of Galacturonic Acid-Rich Vinegar Brewed from Japanese Pear in a Dextran Sodium Sulfate-Induced Acute Colitis Model. J. Funct. Foods 2013, 5, 516–523.
- Tasdemir, S.S.; Sanlier, N. An Insight into the Anticancer Effects of Fermented Foods: A Review. J. Funct. Foods 2020, 75, 104281.
- Hashimoto, M.; Obara, K.; Ozono, M.; Furuyashiki, M.; Ikeda, T.; Suda, Y.; Fukase, K.; Fujimoto, Y.; Shigehisa, H. Separation and Characterization of the Immunostimulatory Components in Unpolished Rice Black Vinegar (Kurozu). J. Biosci. Bioeng. 2013, 116, 688–696.
- Kohchi, C.; Inagawa, H.; Nishizawa, T.; Yamaguchi, T.; Nagai, S.; Soma, G.-I. Applications of Lipopolysaccharide Derived from Pantoea Agglomerans (IP-PA1) for Health Care Based on Macrophage Network Theory. J. Biosci. Bioeng. 2006, 102, 485–496.
- Mimura, A.; Suzuki, Y.; Toshima, Y.; Yazaki, S.; Ohtsuki, T.; Ui, S.; Hyodoh, F. Induction of Apoptosis in Human Leukemia Cells by Naturally Fermented Sugar Cane Vinegar (Kibizu) of Amami Ohshima Island. Biofactors 2004, 22, 93–97.
- Seki, T.; Morimura, S.; Shigematsu, T.; Maeda, H.; Kida, K. Antitumor Activity of Rice-Shochu Post-Distillation Slurry and Vinegar Produced from the Post-Distillation Slurry via Oral Administration in a Mouse Model. Biofactors 2004, 22, 103–105.
- Kondo, S.; Tayama, K.; Tsukamoto, Y.; Ikeda, K.; Yamori, Y. Antihypertensive Effects of Acetic Acid and Vinegar on Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 2001, 65, 2690–2694.
- Ohnami, K. Effects of Kurosu on the Blood Pressure of the Spontaneously Hypertension Rats. Kiso Rinsho 1985, 19, 237–241.
- Na, L.; Chu, X.; Jiang, S.; Li, C.; Li, G.; He, Y.; Liu, Y.; Li, Y.; Sun, C. Vinegar Decreases Blood Pressure by Down-Regulating AT1R Expression via the AMPK/PGC-1α/PPARγ Pathway in Spontaneously Hypertensive Rats. Eur. J. Nutr. 2016, 55, 1245–1253.




