Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thiago Viana Miranda Lima | + 1250 word(s) | 1250 | 2021-12-14 09:18:29 | | | |
| 2 | Catherine Yang | Meta information modification | 1250 | 2022-01-26 01:52:25 | | | | |
| 3 | Catherine Yang | Meta information modification | 1250 | 2022-01-26 03:15:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Viana Miranda Lima, T. Radionuclide Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/18761 (accessed on 06 March 2026).
Viana Miranda Lima T. Radionuclide Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/18761. Accessed March 06, 2026.
Viana Miranda Lima, Thiago. "Radionuclide Development" Encyclopedia, https://encyclopedia.pub/entry/18761 (accessed March 06, 2026).
Viana Miranda Lima, T. (2022, January 25). Radionuclide Development. In Encyclopedia. https://encyclopedia.pub/entry/18761
Viana Miranda Lima, Thiago. "Radionuclide Development." Encyclopedia. Web. 25 January, 2022.
Copy Citation
In the field of radionuclide development, the continual changing of clinically used radionuclides, which is sometimes influenced by instrumentation technology but also driven by availability, patient safety and clinical questions. Some areas, such as tumour imaging, have faced challenges when changing radionuclides based on availability, when this produced undesirable clinical findings with the introduction of unclear focal uptakes and unspecific uptakes.
nuclear medicine
radionuclide
development
1. Drive for Radionuclide Development
The initial success story of theragnostics in nuclear medicine began with iodine radioisotopes (I-131, I-123 and I-124). Radionuclides of the same element allowed the preparation of chemically identical radiopharmaceuticals for diagnosis and therapy, enabling the concept of radiotheragnostics in the truest sense [1]. In this regard, scandium and terbium are of particular interest, as they present several radioisotopes which may be of value for clinical translation [1][2][3].
Scandium has attracted the attention of researchers and nuclear physicians alike, due to the existence of matched radioisotopes which could have a possible theragnostic application [4][1][2][3][5][6]. Sc-43 and Sc-44 are promising for PET imaging, with image quality comparable to the more common clinically used radionuclides, such as F-18 and Ga-68 [7][8]. Sc-47 is a β– emitter suitable for therapeutic purposes and also produces γ-ray emissions that are useful for SPECT imaging. The application of Sc-43/Sc-44 (T1/2 = 3.9 and 4.0 h, respectively) for PET would be advantageous when comparing it to the most-employed radiometal currently, Ga-68 (T1/2 = 68 min); the almost four-fold longer half-lives of Sc-43/Sc-44 would enable the shipment of Sc-43/Sc-44-radiopharmaceuticals to distant PET centres [1]. In addition, images could be acquired over longer periods. Finally, the stable co-ordination of scandium with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) can allow the application of the same targeting agents as are subsequently used for therapeutic applications [1][9]. Sc-43/Sc-44 may, therefore, be employed for diagnosis, as well as for planning and monitoring targeted radionuclide therapy with Lu-177 and Y-90. The exact matched therapeutic counterpart Sc-47 would be even more appealing, as it can enable the concept of using chemically identical radiopharmaceuticals with the same kinetic properties for diagnosis and therapy [1]. Currently, Sc-47 cannot be produced in the quantity required for clinical application.
Terbium is unique in that it represents radioisotopes for all four modalities in nuclear medicine [1][10]. Tb-155 (T1/2 = 5.3 d) emits γ-radiation for SPECT imaging, and Tb-152 (T1/2 = 17.5 h) decays by the emission of positrons that are useful for PET. The decay of Tb-161 (T1/2 = 6.9 d) is characterized by the emission of low-energy β— particles and γ-rays, similar to Lu-177, but, additionally, comprises a significant number of Auger/conversion electrons (~12 e−/decay). It is, therefore, a promising candidate for therapeutic purposes [1][11][12][13]. Auger electron emitters have very attractive properties for cancer therapy, since their nanometre–micrometre range results in a high LET, which is potent for causing lethal damage in cancer cells [14]. Tb-149 (T1/2 = 4.1 h) decays by the emission of α-particles, potentially allowing its use for α-therapy [1][15]. Since terbium belongs to the group of lanthanides, stable coordination is feasible with DOTA, a macrocyclic chelator that is commonly used for the chelation of Lu-177. The production of Tb radioisotopes and their chemical (lanthanide) separation are not trivial processes, which is why Tb radioisotopes have not yet been translated to a clinical routine [1].
Additionally, the differences in instrumentation have also driven the introduction and reappearance of different radionuclides. Although there is increased availability of PET devices, SPECT devices are still present in large numbers, and they have evolved to match the quantitative capabilities of PET and pave the way towards more clinical routines using SPECT devices [16]. Other than clinical aspects, some of the main needs faced by radionuclide development include overcoming shortages in production [17][18], availability and distribution, particularly for clinics situated far away from manufacturing sites (Figure 1).
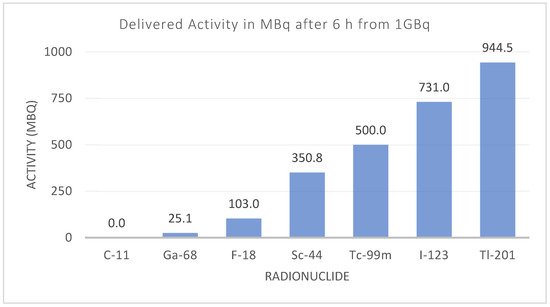
Figure 1. Activity for different diagnostic radionuclides six hours after production. The effect of the physical half-life can limit the radionuclide’s availability and distribution, depending on the number of facilities producing that radionuclide.
2. The Revolving World of the Radionuclide
The hunt for the “perfect” radionuclide for any given clinical indication is almost impossible. A “perfect” nuclide can be identified in combination with the chelator in question to be used for a specific application. Should one have an antibody to be applied for example, with a longer biological half-life, a radionuclide with a longer half-life will be preferred. The nuclide must be able to easily combine with the chelator and remain stable over the required period of time. Examples of a small chelator which is effective in medical application include DOTATOC and DOTATATE, which has been of particular use in the diagnosis and treatment of neuroendocrine tumours (NET). The use of Ga-68 for diagnosis and Lu-177 for therapy has ensured the popularity of these two radiometals in nuclear medicine. The 68Ge/68Ga generator was developed in the 1960s but has only recently escalated in popularity, particularly due to the development of the prostate-specific membrane antigen (PSMA). PSMA is a transmembrane glycoprotein which is overexpressed in prostate cancer cells. A number of radiolabelled PSMA probes have been developed, including the most widely-used: [68Ga]Ga-PSMA-11. It is well known that [68Ga]Ga-PSMA-11 PET/CT is superior to both conventional imaging [19] and choline-based PET/CT for evaluating prostate cancer patients, primarily in the context of biochemical failure but also for staging purposes [20][21][22][23][24][25][26][27][28][29][30][31][32]. [18F]F-PSMA-1007 is a novel PSMA-based radiopharmaceutical that has several advantages over [68Ga]Ga-PSMA-11. 18F-labeled agents enable large-scale production, allowing for a larger number of patient studies, as compared with the limited quantity achieved by the generator-produced Ga-68. In addition, the longer physical half-life of F-18 (T1/2 = 109 min) allows for its central production and distribution to satellite centres. F-18 also benefits from higher spatial resolution in comparison with Ga-68, due to its lower positron energy (average β+ energy = 250 keV), reducing the distance travelled by the positron before its annihilation and the production of the imaged gammas [33][34]. From a therapeutic perspective, Lu-177 currently holds sway as the most popular radiometal, with its ability to label to somatostatin analogues effectively working in its favour.
A possible challenge in the proposal of new radionuclides, is the effect of different biological and chemical clearances and uptakes that might be introduced. In the context of PSMA, this has been reported for unclear focal uptakes in the lymph nodes, ganglia [35] and unspecific bone uptake for F-18 [36]. The recent application of tracers that act as fibroblast-activation-protein inhibitors (FAPI) with Ga-68, again sees this radionuclide in the limelight, as it has been shown to be effective in the PET/CT imaging of 28 cancers, with fast and high tumour uptake [37].
One field that has seen many evolution steps in both radionuclide and instrumentation is nuclear cardiology. During the 1950s, the link between potassium uptake and blood flow, as well as structural and functional integrity, was demonstrated [38]. Since Tl-201 and Rb-82 have biological properties similar to potassium, they became the go-to nuclides for identifying patients with anginal chest pain and epicardial coronary artery narrowing [38][39][40]. With the arrival of Tc-99m, there was a natural shift driven by availability, instrumentation and patient exposure (Tl-201 has a much greater half-life, 73 h, compared to that of Tc-99m, 6 h) for similar quantities of injected radioactivity.
Perfusion imaging with PET is increasingly available in larger nuclear medicine departments worldwide, replacing or complementing conventional myocardial perfusion scintigraphy (MPS) in SPECT. As a result, this has seen the return of Rb-82 and the arrival of other PET tracers including [15O]H2O, [13N]NH3 and [18F]Fluridipaz [41]. This resurrection was only achievable with improvements in quantification accuracy, which was especially the case for Rb-82, due to its non-pure positron emissions [42][43].
References
- Müller, C.; Domnanich, K.A.; Umbricht, C.A.; Van Der Meulen, N.P. Scandium and terbium radionuclides for radiotheranostics: Current state of development towards clinical application. Br. J. Radiol. 2018, 91, 20180074.
- Huclier-Markai, S.; Alliot, C.; Kerdjoudj, R.; Mougin-Degraef, M.; Chouin, N.; Haddad, F. Promising Scandium Radionuclides for Nuclear Medicine: A Review on the Production and Chemistry up to In Vivo Proofs of Concept. Cancer Biother. Radiopharm. 2018, 33, 316–329.
- Mikolajczak, R.; Huclier-Markai, S.; Alliot, C.; Haddad, F.; Szikra, D.; Forgacs, V.; Garnuszek, P. Production of scandium radionuclides for theranostic applications: Towards standardization of quality requirements. EJNMMI Radiopharm. Chem. 2021, 6, 19.
- Domnanich, K.A.; Eichler, R.; Müller, C.; Jordi, S.; Yakusheva, V.; Braccini, S.; Behe, M.; Schibli, R.; Türler, A.; van der Meulen, N.P. Production and separation of 43Sc for radiopharmaceutical purposes. EJNMMI Radiopharm. Chem. 2017, 2, 14.
- Müller, C.; Bunka, M.; Haller, S.; Köster, U.; Groehn, V.; Bernhardt, P.; Van Der Meulen, N.; Türler, A.; Schibli, R. Promising prospects for 44Sc-/47Sc-based theragnostics: Application of 47Sc for radionuclide tumor therapy in mice. J. Nucl. Med. 2014, 55, 1658–1664.
- van der Meulen, N.P.; Bunka, M.; Domnanich, K.A.; Müller, C.; Haller, S.; Vermeulen, C.; Türler, A.; Schibli, R. Cyclotron production of 44Sc: From bench to bedside. Nucl. Med. Biol. 2015, 42, 745–751.
- Lima, T.V.M.; Gnesin, S.; Strobel, K.; Pérez, M.D.S.; Roos, J.E.; Müller, C.; van der Meulen, N.P. Fifty shades of scandium: Comparative study of pet capabilities using sc-43 and sc-44 with respect to conventional clinical radionuclides. Diagnostics 2021, 11, 1826.
- Lima, T.V.M.; Gnesin, S.; Nitzsche, E.; Ortega, P.G.; Müller, C.; van der Meulen, N.P. First Phantom-Based Quantitative Assessment of Scandium-44 Using a Commercial PET Device. Front. Phys. 2020, 8, 241.
- Majkowska-Pilip, A.; Bilewicz, A. Macrocyclic complexes of scandium radionuclides as precursors for diagnostic and therapeutic radiopharmaceuticals. J. Inorg. Biochem. 2011, 105, 313–320.
- Müller, C.; Zhernosekov, K.; Köster, U.; Johnston, K.; Dorrer, H.; Hohn, A.; Van Der Walt, N.T.; Türler, A.; Schibli, R. A unique matched quadruplet of terbium radioisotopes for PET and SPECT and for α- and β--radionuclide therapy: An in vivo proof-of-concept study with a new receptor-targeted folate derivative. J. Nucl. Med. 2012, 53, 1951–1959.
- Champion, C.; Quinto, M.A.; Morgat, C.; Zanotti-Fregonara, P.; Hindié, E. Comparison between three promising β-emitting radionuclides, 67Cu, 47Sc and 161Tb, with emphasis on doses delivered to minimal residual disease. Theranostics 2016, 6, 1611–1618.
- Bernhardt, P.; Benjegård, S.A.; Kölby, L.; Johanson, V.; Nilsson, O.; Ahlman, H.; Forssell-Aronsson, E. Dosimetric comparison of radionuclides for therapy of somatostatin receptor-expressing tumors. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 514–524.
- Lehenberger, S.; Barkhausen, C.; Cohrs, S.; Fischer, E.; Grünberg, J.; Hohn, A.; Köster, U.; Schibli, R.; Türler, A.; Zhernosekov, K. The low-energy β—And electron emitter 161Tb as an alternative to 177Lu for targeted radionuclide therapy. Nucl. Med. Biol. 2011, 38, 917–924.
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27.
- Beyer, G.-J.; Čomor, J.J.; Daković, M.; Soloviev, D.; Tamburella, C.; Hagebø, E.; Allan, B.; Dmitriev, S.N.; Zaitseva, N.G.; Starodub, G.Y.; et al. Production routes of the alpha emitting 149 Tb for medical application Medical radionuclide production/Therapeutic radionuclides/Spallation production/Heavy ion induced nuclear reaction/149 Tb. Radiochim. Acta 2002, 90, 247–252.
- Bailey, D.L.; Willowson, K.P. An evidence-based review of quantitative SPECT imaging and potential clinical applications. J. Nucl. Med. 2013, 54, 83–89.
- Einstein, A.J. Breaking America’s Dependence on Imported Molybdenum. JACC Cardiovasc. Imaging 2009, 2, 369–371.
- Dilsizian, V.; Narula, J. Seeking Remedy for Molly’s Woe. Time for a Thallium Pill? JACC Cardiovasc. Imaging 2009, 2, 375–377.
- Werner, P.; Neumann, C.; Eiber, M.; Wester, H.J.; Schottelius, M. Tc-PSMA-I&S-SPECT/CT: Experience in prostate cancer imaging in an outpatient center. EJNMMI Res. 2020, 10, 45.
- Hirmas, N.; Leyh, C.; Sraieb, M.; Barbato, F.; Schaarschmidt, B.M.; Umutlu, L.; Nader, M.; Wedemeyer, H.; Ferdinandus, J.; Rischpler, C.; et al. 68 Ga-PSMA-11 PET/CT Improves Tumor Detection and Impacts Management in Patients with Hepatocellular Carcinoma. J. Nucl. Med. 2021, 62, 1235–1241.
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. Pet imaging with a gallium-labelled psma ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495.
- Maurer, T.; Gschwend, J.E.; Rauscher, I.; Souvatzoglou, M.; Haller, B.; Weirich, G.; Wester, H.J.; Heck, M.; Kübler, H.; Beer, A.J.; et al. Diagnostic efficacy of 68Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J. Urol. 2016, 195, 1436–1443.
- Afshar-Oromieh, A.; Avtzi, E.; Giesel, F.L.; Holland-Letz, T.; Linhart, H.G.; Eder, M.; Eisenhut, M.; Boxler, S.; Hadaschik, B.A.; Kratochwil, C.; et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 197–209.
- Van Leeuwen, P.J.; Emmett, L.; Ho, B.; Delprado, W.; Ting, F.; Nguyen, Q.; Stricker, P.D. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017, 119, 209–215.
- Herlemann, A.; Wenter, V.; Kretschmer, A.; Thierfelder, K.M.; Bartenstein, P.; Faber, C.; Gildehaus, F.J.; Stief, C.G.; Gratzke, C.; Fendler, W.P. 68Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Regions Prior to Lymph Node Dissection in Patients with Prostate Cancer. Eur. Urol. 2016, 70, 553–557.
- Öbek, C.; Doğanca, T.; Demirci, E.; Ocak, M.; Kural, A.R.; Yıldırım, A.; Yücetaş, U.; Demirdağ, Ç.; Erdoğan, S.M.; Kabasakal, L. The accuracy of 68Ga-PSMA PET/CT in primary lymph node staging in high-risk prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1806–1812.
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20.
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.P.; Kübler, H.; Haberhorn, U.; Eisenhut, M.; et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J. Nucl. Med. 2015, 56, 668–674.
- Morigi, J.J.; Stricker, P.D.; Van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J. Nucl. Med. 2015, 56, 1185–1190.
- Uprimny, C. 68 Ga-PSMA-11 PET/CT: The rising star of nuclear medicine in prostate cancer imaging? Wien. Med. Wochenschr. 2019, 169, 3–11.
- Fendler, W.P.; Calais, J.; Eiber, M.; Flavell, R.R.; Mishoe, A.; Feng, F.Y.; Nguyen, H.G.; Reiter, R.E.; Rettig, M.B.; Okamoto, S.; et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 856–863.
- Kuten, J.; Fahoum, I.; Savin, Z.; Shamni, O.; Gitstein, G.; Hershkovitz, D.; Mabjeesh, N.J.; Yossepowitch, O.; Mishani, E.; Even-Sapir, E. Head-to-head comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard. J. Nucl. Med. 2020, 61, 527–532.
- Kesch, C.; Kratochwil, C.; Mier, W.; Kopka, K.; Giesel, F.L. 68Ga or 18F for prostate cancer imaging? J. Nucl. Med. 2017, 58, 687–688.
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688.
- Kroenke, M.; Mirzoyan, L.; Horn, T.; Peeken, J.C.; Wurzer, A.; Wester, H.J.; Makowski, M.; Weber, W.A.; Eiber, M.; Rauscher, I. Matched-Pair Comparison of 68Ga-PSMA-11 and 18F-rhPSMA-7 PET/CT in Patients with Primary and Biochemical Recurrence of Prostate Cancer: Frequency of Non-Tumor-Related Uptake and Tumor Positivity. J. Nucl. Med. 2021, 62, 1082–1088.
- Grünig, H.; Maurer, A.; Thali, Y.; Kovacs, Z.; Strobel, K.; Burger, I.A.; Müller, J. Focal unspecific bone uptake on -PSMA-1007 PET: A multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4483–4494.
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805.
- Dilsizian, V.; Taillefer, R. Journey in evolution of nuclear cardiology: Will there be another quantum leap with the f-18-labeled myocardial perfusion tracers? JACC Cardiovasc. Imaging 2012, 5, 1269–1284.
- Prokop, E.K.; Strauss, H.W.; Shaw, J.; Pitt, B.; Wagner, H.N. Comparison of regional myocardial perfusion determined by ionic potassium 43 to that determined by microspheres. Circulation 1974, 50, 978–984.
- Pohost, G.M.; Zir, L.M.; Moore, R.H.; McKusick, K.A.; Guiney, T.E.; Beller, G.A. Differentiation of transiently ischemic from infarcted myocardium by serial imaging after a single dose of Thallium 201. Circulation 1977, 55, 294–302.
- Sciagrà, R.; Lubberink, M.; Hyafil, F.; Saraste, A.; Slart, R.H.J.A.; Agostini, D.; Nappi, C.; Georgoulias, P.; Bucerius, J.; Rischpler, C.; et al. EANM procedural guidelines for PET/CT quantitative myocardial perfusion imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1040–1069.
- Harnett, D.T.; Hazra, S.; Maze, R.; Mc Ardle, B.A.; Alenazy, A.; Simard, T.; Henry, E.; Dwivedi, G.; Glover, C.; deKemp, R.A.; et al. Clinical performance of Rb-82 myocardial perfusion PET and Tc-99m-based SPECT in patients with extreme obesity. J. Nucl. Cardiol. 2019, 26, 275–283.
- Armstrong, I.S.; Memmott, M.J.; Tonge, C.M.; Arumugam, P. The impact of prompt gamma compensation on myocardial blood flow measurements with rubidium-82 dynamic PET. J. Nucl. Cardiol. 2018, 25, 596–605.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
26 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





