
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Samiru Sudharaka Wickramasuriya | + 3742 word(s) | 3742 | 2022-01-24 10:34:34 | | | |
| 2 | Lindsay Dong | Meta information modification | 3742 | 2022-01-25 03:25:52 | | |
Video Upload Options
“Gut health” refers to the physical state and physiological function of the gastrointestinal tract and in the livestock system; this topic is often focused on the complex interacting components of the intestinal system that influence animal growth performance and host-microbial homeostasis.
1. Introduction
The importance of gut health in animals goes beyond the physical barrier of the gut [1]. Although substantial scientific findings on gut health-related topics have been published, the understanding of its composition, importance, regulation, or interactions is still at the rudimentary stage. Nonetheless, the fact that gut health has been a matter of contention in scientific research reflects its importance in various aspects of animal nutrition and production. The term “gut health” encompasses broader areas of the physical state and physiological function of the many parts of the gastrointestinal (GI) tract [2]. Bischoff [3], while proposing the definition of gut health, used the definition as outlined by the WHO and defined the “gut health in human medicine” as a state of physical and mental well-being in the absence of GI complaints that require the consultation of a doctor, in the absence of indications or risks of bowel diseases, and the absence of confirmed bowel diseases. This definition of gut health in human medicine may be applicable to animals as well, including poultry, due to the similarity in structure, physiology, and function of the gut, despite quantitative and qualitative differences that may exist between animals and humans. Kogut and Arsenault [4] defined gut health as “the absence/prevention/avoidance of disease so that the animal is able to perform its physiological functions in order to withstand exogenous and endogenous stressors”. Later, Celi et al. [5] proposed the definition of gut health as “a steady-state, where the microbiome and the intestinal tract exist in symbiotic equilibrium, and where the welfare and performance of the animal are not constrained by intestinal dysfunction”. These definitions show that gut health is a holistic term and point out the importance of complex interactions of various components of the gut interacting with the host animal and the environment to maintain homeostasis. Indeed, Jha et al. [6] revised the early definition by Conway [7] on gut health being the function of three major components (i.e., diet, the mucosa, and the commensal microbiota); they suggested that gut health is holistically the function of four major components, including diet (i.e., nutrition), mucosa, microbiome, and the immune system, in a holistic way. Thus, understanding the four major components is a prerequisite to defining gut health and will also expand our knowledge of their functions in determining gut health. Further, a wide range of intrinsic and extrinsic factors [5] affecting gut health (e.g., gut components) need to be better elucidated.
2. Structure and Physiology
2.1. Chicken Gut and Its Relation to Gut Health
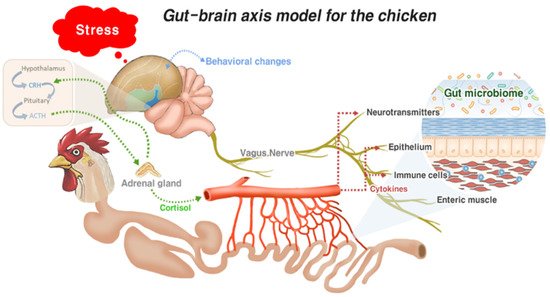
2.2. Gut–Brain Axis and Gut Health
3. Intestinal Immune System in Gut Health
Since the gut is constantly challenged by the antigenic substances from the diet and the dynamic ecosystem of gut microbiota populations, inflammation, which is an important part of innate and adaptive immune responses to protect the host against antigenic challenges, plays a critical role in the healing process and dictates the status of the gut health. Although inflammation is a vital response to infections for the survival of the host, the balance of factors that promote and regulate inflammatory responses needs to be better understood. In livestock, inflammation that is initiated by diet or external stressors often causes dysbiosis, with unwanted consequences on growth performance and gut health [26]. Thus, studies on effective methods for chicken production are directed towards balancing pro- and anti-inflammatory responses to prevent overzealous chronic inflammatory responses that can be detrimental to gut health. The main limitation of an anti-inflammatory approach in chickens is their vulnerability to infections, particularly in young chickens, since their immune systems are not fully developed. Gut inflammation is initiated by gut resident immune cells, mostly innate immune cells, including APCs and heterophils, that express pattern recognition receptors (PRRs). In the chicken gut, various toll-like receptors (TLRs), including TLR1, 2, 3, 4, 5, 7, and 15, are expressed [27], and the activation of TLRs induces downstream signaling pathways, such as mitogen-activated protein kinase (MAPK) and nuclear factor kappa beta (NF-κB). This results in a protective immune response supported by the recruitment of immune cells and the expression of associated proinflammatory mediators and AMPs. The process of inflammation consumes nutrients, which are routed from productive purposes, thus reducing the appetite in poultry. Therefore, it is critical to find a point of balance between inflammation and anti-inflammation for a healthy gut in poultry.
GALT plays a major role in the gut immune response, inducing a full spectrum of innate and adaptive immune responses that vary depending on the nature of the stressors, including pathogens. The common features of the host immune response aim to limit the infection. The mucus layers, with varying thicknesses and compositions, trap invasive bacteria via the luminal flow, as well as providing lubrication, and colonization sites, nutrients for commensal bacteria, and a transport system between the gut contents and epithelial linings [28]. The optimum mucus layer thickness is represented as the number of goblet cells secreting mucins. Mucins can be either neutral or acidic, and the latter protects against bacterial translocation [29]. Abnormal mucus layer thickness is associated with enteric infection and poor performance [30]. Besides the mucus layer, AMPs and secretory IgA (sIgA) are integral components of the gut immune system. AMPs are expressed by a variety of cells, including Paneth cells. Defensins are the most studied AMPs in chickens; the chicken genome encodes for 14 β-defensin genes, also known as gallinacins, but no α-defensins [31]. NK-lysin, a homolog of human granulysin, has also been reported to play a role in gut infection by protozoan parasites such as Eimeria spp. [32][33][34]. They possess broad-spectrum antimicrobial activities against Gram-negative and Gram-positive bacteria, fungi, viruses, and protozoa [35]. They are also involved in the modulation of immune responses and determination of the microbiota composition [31]. IgA is secreted from the plasma cells present in the lamina propria; they can neutralize pathogens and facilitate their removal from the GI tract and play a role in intestinal homeostasis, including establishment, maintenance, and the control of commensal microbes [36]. Since lamina propria IgA-positive cells and intestinal IgA levels are both significantly reduced in germ-free animals, they could play a potential role in the gut microbiota [37]. Several studies have been conducted on the relationship between gut microbiota and the host immune response. The mucus secreted from goblet cells can act as a source of nutrients for the resident microbiota, which can be used to inhibit the expansion of other bacteria [38]. Controlled inflammation requires gut microbiota to regulate the gut immune response either towards inflammation or tolerance [39].
4. Microbiota in Chicken Gut Health
5. Intestinal Infections and Their Impact on Gut Health
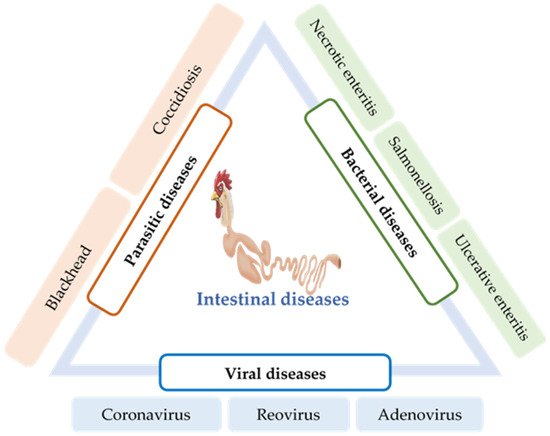
5.1. Parasitic Diseases
Coccidiosis is a common intestinal disease caused by several species of the protozoan parasite of the genus Eimeria, which invades the intestinal lining of chickens. It is one of the major intestinal infectious diseases of poultry that causes severe economic losses worldwide [58][59]. Coccidiosis is initiated when chickens ingest the environmentally resistant Eimeria oocysts. These Eimeria spp. characteristically infect a specific site of the intestinal tract, resulting in severe inflammation and necrosis in the submucosa, although the mechanism of site-specificity of Eimeria is not well-known [60][61]. For every oocyst ingested, innumerable parasites are reproduced in the intestine and transmitted to the entire poultry house via contaminated feces. The invasion of these Eimeria sporozoites into the intestinal tract and their subsequent intracellular development damages the intestinal epithelium, obstructing nutrient absorption and immune suppression of the host chicken for their survival. Consequentially, poor growth performance and higher mortality have been reported in chickens [62].
Blackhead (Histomoniasis) is a chicken gut disease caused by the single-celled protozoa Histomonas meleagridis. Although it is relatively common in captive-raised game birds and turkeys [63], it is also found in several other avian species, including grouse, quail, and pheasants [64]. The pathogenesis of H. meleagridis begins with the colonization of the caecum of the birds, thereafter leading to severe intestinal inflammation and necrosis [65]. Histomonas parasites penetrate the blood vessels and reach the liver via the portal veins [63]. Moreover, unusual lesions have also been reported in other visceral organs of turkey, such as the kidneys, lungs, and bursa of Fabricius [66]. In chickens, lesions are mostly found in the caecum, with less or no necrosis in other organs [67].
5.2. Bacterial Diseases
NE is an enteric disease of poultry caused by C. perfringens, and coccidiosis is its primary risk factor. C. perfringens is a spore-forming, anaerobic Gram-positive bacilli that is widely distributed in freshwater or soil. It is also found as a member of the normal intestinal flora of birds [68]. C. perfringens strains are classified into five toxin types (A–E), according to the toxins they produce [69]. B-like toxin (NetB) produced by C. perfringens is identified as the causative agent of NE. However, a simple infection is not sufficient to provoke the disease. Increased consumption of barley, wheat, or other poorly digestible proteins and coinfection by Eimeria spp. are various predisposing factors that trigger the disease [70][71].
Ulcerative enteritis (UE) is one of the most common acute bacterial diseases in quail caused by the bacterium Clostridium colinum. Several predisposing factors, such as coccidiosis, chicken infectious anemia virus (circovirus), Gumboro disease, and stress conditions, may increase the incidence of UE and subsequent mortality [72]. Intestinal lesions are characterized by multiple ulcers throughout the tract, including the duodenum, jejunum, ileum, and cecum; peritonitis and multifocal necrotizing hepatitis are also observed in many cases [73].
5.3. Viral Diseases
The poultry coronavirus, also known as the infectious bronchitis virus (IBV), is a highly contagious, acute disease-causing agent of poultry. High mortality is the major concern of coronavirus infection. However, poor growth performance, impaired feed efficiency, renal disease, insufficient egg quality, and decreased egg production are other issues associated with IBV infection [76]. Although IBV mainly replicates in the epithelial surface of chicken respiratory tracts and causes respiratory illness, it can also replicate in the enteric tract, oviducts, and the kidneys [77][78]. However, the replication of IBV in the chicken intestine does not cause any pathological changes [79]. The susceptibility of birds to the IBV strains is triggered by various factors, including environmental stress, age, and genetics [80]. The domestic chickens and pheasants (Phasianus spp.) are primarily the susceptible hosts for IBV [81].
Avian reovirus (ARV) is a nonenveloped, double-stranded RNA virus belonging to the genus Orthoreovirus in the Reoviridae family [82]. ARV is ubiquitous among commercial poultry and has been reported to be responsible for a variety of disease conditions in poultry, including malabsorption syndrome (MAS), runting-stunting syndrome (RSS), tenosynovitis, gastroenteritis, and immune suppression [83][84][85]. The most common mode of transmission of ARV is the fecal–oral route; following which, the initial replication occurs in the mucosa of the intestinal and respiratory tracts. However, infection via egg transmission has also been reported [86][87].
Adenoviruses are nonenveloped, icosahedral viruses that belong to the family Adenoviridae. Adenoviruses can be subdivided into a mammalian adenovirus (mastadenovirus) and avian adenovirus (aviadenoviruses) [88]. Further, avian adenoviruses can be subdivided into groups I, II, and III. Group I avian adenoviruses are usually found in excreta or in the tissues of birds with GI diseases. Various diseases, including runting/MAS [89], proventriculitis [89], ventriculitis [90], enteritis [91], and mortality syndrome [92], are associated with group I.
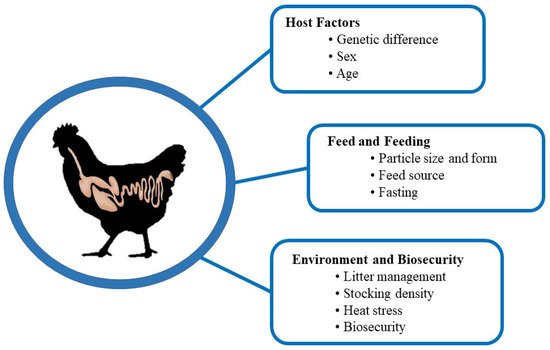
6. Factors Affecting Intestinal Health
References
- Thoo, L.; Noti, M.; Krebs, P. Keep calm: The intestinal barrier at the interface of peace and war. Cell Death Dis. 2019, 10, 849.
- Staudacher, H.M.; Loughman, A. Gut health: Definitions and determinants. Lancet Gastroenterol. Hepatol. 2021, 6, 269.
- Bischoff, S.C. Gut health: A new objective in medicine? BMC Med. 2011, 9, 24.
- Kogut, M.H.; Arsenault, R.J. Editorial: Gut health: The New Paradigm in Food Animal Production. Front. Vet. Sci. 2016, 3, 71.
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steiner, R.E.; Kluenter, A.-M.; Verlhac, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal nutrition. Anim. Feed Sci. Technol. 2017, 234, 88–100.
- Jha, R.; Fouhse, J.M.; Tivari, U.P.; Li, L.; Willing, B.P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 2019, 6, 48.
- Conway, P.L. Function and regulation of the gastrointestinal microbiota of the pig. In Proceedings of the VIth International Symposium on Digestive Physiology in Pigs; Souffrant, W., Hagemeister, H., Eds.; EAAP Publication: Ban Doberan, Germany, 1994; pp. 231–240.
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119.
- Susi, F.R. Keratinization in the mucosa of the ventral surface of the chicken tongue. J. Anat. 1969, 105, 477.
- Scanes, C.G.; Pierzchala-Koziec, K. Biology of the gastrointestinal tract in poultry. Avian Biol. Res. 2014, 7, 193–222.
- Alshamy, Z.; Richardson, K.C.; Hünigen, H.; Hafez, H.M.; Plendl, J.; Al Masri, S. Comparison of the gastrointestinal tract of a dual-purpose to a broiler chicken line: A qualitative and quantitative macroscopic and microscopic study. PLoS ONE 2018, 13, e0204921.
- Tabata, H.; Yasugi, S. Tissue interaction regulates expression of a spasmolytic polypeptide gene in chicken stomach epithelium. Dev. Growth Differ. 1998, 40, 519–526.
- van Dijk, A.; Veldhuizen, E.J.; Kalkhove, S.I.; Tjeerdsma-van Bokhoven, J.L.; Romijn, R.A.; Haagsman, H.P. The beta-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against foodborne pathogens. Antimicrob. Agents Chemother. 2007, 51, 912–922.
- Svihus, B. The gizzard: Function, influence of diet structure and effects on nutrient availability. World’s Poult. Sci. J. 2011, 67, 207–224.
- Aguzey, H.A.; Gao, Z.; Haohao, W.; Guilan, C. Influence of Feed Form and Particle Size on Gizzard, Intestinal Morphology and Microbiota Composition of Broiler Chicken. Poult. Fish. Wildl. Sci. 2018, 6, 2.
- Lillehoj, H.S.; Lillehoj, E.P. Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Dis. 2000, 44, 408–425.
- Hooper, L.V. Do symbiotic bacteria subvert host immunity? Nat. Rev. Microbiol. 2009, 7, 367–374.
- Coleman, O.I.; Haller, D. Bacterial signaling at the intestinal epithelial interface in inflammation and cancer. Front. Immunol. 2018, 8, 1927.
- Svihus, B.; Choct, M.; Classen, H.L. Function and nutritional roles of the avian caeca: A review. World’s Poult. Sci. J. 2013, 69, 249–264.
- Dunkley, K.D.; Callaway, T.R.; Chalova, V.I.; McReynolds, J.L.; Hume, M.E.; Dunkley, C.S.; Kubena, L.F.; Nisbet, D.J.; Ricke, S.C. Foodborne Salmonella ecology in the avian gastrointestinal tract. Anaerobe 2009, 15, 26–35.
- Majeed, M.F.; Al-Asadi, F.S.; Nassir, A.A.; Rahi, E.H. The morphological and histological study of the caecum in broiler chicken. Basra J. Vet. Res. 2009, 8, 19–25.
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain–gut–microbe communication in health and disease. Front. Physiol. 2011, 2, 94.
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203.
- Villageliũ, D.N.; Lyte, M. Microbial endocrinology: Why the intersection of microbiology and neurobiology matters to poultry health. Poult. Sci. 2017, 96, 2501–2508.
- Kabiersch, A.; del Rey, A.; Honegger, C.G.; Besedovsky, H.O. Interleukin-1 induces changes in norepinephrine metabolism in the rat brain. Brain Behav. Immun. 1988, 2, 267–274.
- Broom, L.J.; Kogut, M.H. Inflammation: Friend and foe for animal production? Poult. Sci. 2018, 94, 510–514.
- Kannaki, T.R.; Reddy, M.R.; Shanmugam, M.; Verma, P.C.; Sharma, R.P. Chicken toll-like receptors and their role in immunity. Worlds Poult. Sci. J. 2010, 66, 727–738.
- Zhang, J.; Li, C.; Tang, X.; Lu, Q.; Sa, R.; Zhang, H. Proteome changes in the small intestinal mucosa of broilers (Gallus gallus) induced by high concentrations of atmospheric ammonia. Proteome Sci. 2015, 13, 9.
- Deplancke, B.; Gaskins, H.R. Microbial modulation ofinnate defence: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131–1141.
- Wlodarska, M.; Willing, B.; Keeney, K.M.; Menendez, A.; Bergstrom, K.S.; Gill, N.; Russell, S.L.; Vallance, B.A.; Finlay, B.B. Antibiotic treatment alters the colonic mucus layer and pre-disposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 2011, 79, 1536–1545.
- Robinson, K.; Deng, Z.; Hou, Y.; Zhang, G. Regulation of the intestinal barrier function by host defense peptides. Front. Vet. Sci. 2015, 2, 57.
- Hong, Y.H.; Lillehoj, H.S.; Siragusa, G.R.; Bannerman, D.D.; Lillehoj, E.P. Antimicrobial activity of chicken NK-lysin against Eimeria sporozoites. Avian Dis. 2008, 52, 302–305.
- Kim, W.H.; Lillehoj, H.S.; Min, W. Evaluation of the Immunomodulatory Activity of the Chicken NK-Lysin-Derived Peptide cNK-2. Sci. Rep. 2017, 7, 45099.
- Wickramasuriya, S.S.; Park, I.; Lee, Y.; Kim, W.H.; Przybyszewski, C.; Gay, C.G.; van Oosterwijk, J.G.; Lillehoj, H.S. Oral Delivery of Bacillus subtilis Expressing Chicken NK-2 Peptide Protects Against Eimeria acervulina Infection in Broiler Chickens. Front. Vet. Sci. 2021, 8, 684818.
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594.
- Mantis, N.J.; Rol, N.; Corthesy, B. Secretory IgA’s com-plex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611.
- Honda, K.; Takeda, K. Regulatory mechanisms of immuneresponses to intestinal bacteria. Mucosal Immunol. 2009, 2, 187–196.
- Smirnov, A.; Perez, E.; Amit-Romach, E.; Sklan, D.; Uni, Z. Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. J. Nutr. 2005, 135, 187–192.
- Brisbin, J.T.; Gong, J.; Sharif, S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 2008, 9, 101–110.
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56.
- Rehman, H.U.; Vahjen, W.; Awad, W.A.; Zentek, J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch. Anim. Nutrit. 2007, 61, 319–335.
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254.
- Waite, D.W.; Taylor, M. Exploring the avian gut microbiota: Current trends and future directions. Front. Microbiol. 2015, 6, 673.
- Maki, J.J.; Klima, C.L.; Sylte, M.J.; Looft, T. The Microbial pecking order: Utilization of intestinal microbiota for poultry health. Microorganisms 2019, 7, 376.
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 1–11.
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112.
- Xi, Y.; Shuling, N.; Kunyuan, T.; Qiuyang, Z.; Hewen, D.; ChenCheng, G.; Tianhe, Y.; Liancheng, L.; Xin, F. Characteristics of the intestinal flora of specific pathogen free chickens with age. Microb. Pathog. 2019, 132, 325–334.
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24.
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063.
- Lee, W.J.; Hase, K. Gut microbiota-generated metabolites in animal health and disease. Nat. Chem. Biol. 2014, 10, 416–424.
- Nicholson, J.K.; Wilson, I.D. Opinion: Understanding ‘global’ systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. Drug Discov. 2003, 2, 668–676.
- Cully, M. Microbiome therapeutics go small molecule. Nat. Rev. Drug. Discov. 2019, 18, 569–572.
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481.
- Kogut, M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 2019, 250, 32–40.
- Tan, J.; Applegate, T.J.; Liu, S.; Guo, Y.; Eicher, S.D. Supplemental dietary L-Arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens. Br. J. Nutr. 2014, 112, 1098–1109.
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116.
- Sommer, F.; Backhed, F. The gut microbiota—masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238.
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115.
- Dalloul, R.A.; Lillehoj, H.S. Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Rev. Vaccines. 2006, 5, 143–163.
- Lillehoj, H.S.; Min, W.; Dalloul, R.A. Recent progress on the cytokine regulation of intestinal immune responses to Eimeria. Poult. Sci. 2004, 83, 611–623.
- Sharman, P.A.; Smith, N.C.; Wallach, M.G.; Katrib, M. Chasing the golden egg: Vaccination against poultry coccidiosis. Parasite Immunol. 2010, 32, 590–598.
- Kim, W.H.; Lillehoj, H.S.; Min, W. Indole treatment alleviates intestinal tissue damage induced by chicken coccidiosis through activation of the aryl hydrocarbon receptor. Front. Immunol. 2019, 10, 560.
- Mitra, T.; Kidane, F.A.; Hess, M.; Liebhart, D. Unravelling the immunity of poultry against the extracellular protozoan parasite Histomonas meleagridis is a cornerstone for vaccine development: A review. Front. Immunol. 2018, 9, 2518.
- Regmi, P.R.; Shaw, A.L.; Hungerford, L.L.; Messenheimer, J.R.; Zhou, T.; Pillai, P.; Omer, A.; Gilbert, J.M. Regulatory Considerations for the Approval of Drugs Against Histomoniasis (Blackhead Disease) in Turkeys, Chickens, and Game Birds in the United States. Avian Dis. 2016, 60, 725–730.
- Clarke, L.L.; Beckstead, R.B.; Hayes, J.R.; Rissi, D.R. Pathologic and molecular characterization of histomoniasis in peafowl (Pavo cristatus). J. Vet. Diagn. 2017, 29, 237–241.
- Sentíes-Cué, G.; Chin, R.P.; Shivaprasad, H.L. Systemic histomoniasis associated with high mortality and unusual lesions in the bursa of Fabricius, kidneys, and lungs in commercial turkeys. Avian Dis. 2009, 53, 231–238.
- Sigmon, C.S.; Malheiros, R.D.; Anderson, K.E.; Payne, J.A.; Beckstead, R.B. Blackhead Disease: Recovery of Layer Flock after Disease Challenge. J. Appl. Poult. Res. 2019, 28, 755–760.
- Keyburn, A.L.; Bannam, T.L.; Moore, R.J.; Rood, J.I. NetB, a Pore-forming toxin from necrotic enteritis strains of Clostridium perfringens. Toxins 2010, 2, 1913–1927.
- Lee, K.W.; Lillehoj, H.S.; Park, M.S.; Jang, S.I.; Ritter, G.D.; Hong, Y.H.; Jeong, W.; Jeoung, H.Y.; An, D.J.; Lillehoj, E.P. Clostridium perfringens α-toxin and NetB toxin antibodies and their possible role in protection against necrotic enteritis and gangrenous dermatitis in broiler chickens. Avian Dis. 2012, 56, 230–233.
- Lee, Y.; Kim, W.H.; jin Lee, S.; Lillehoj, H.S. Detection of chicken interleukin-10 production in intestinal epithelial cells and necrotic enteritis induced by Clostridium perfringens using capture ELISA. Vet. Immunol. Immunopathol. 2018, 204, 52–58.
- Kim, D.K.; Lillehoj, H.S.; Jang, S.I.; Lee, S.H.; Hong, Y.H.; Cheng, H.H. Transcriptional profiles of host-pathogen responses to necrotic enteritis and differential regulation of immune genes in two inbreed chicken lines showing disparate disease susceptibility. PLoS ONE 2014, 9, 114960.
- Bano, L.; Drigo, I.; Macklin, K.S.; Martin, S.W.; Miller, R.S.; Norton, R.A.; Oyarzabal, O.A.; Bilgili, S.F. Development of a polymerase chain reaction assay for specific identification of Clostridium colinum. Avian Pathol. 2008, 37, 179–181.
- Radi, Z.A. An epizootic of combined Clostridium perfringens, Eimeria spp. and Capillaria spp. enteritis and Histomonas spp. hepatitis with Escherichia coli septicemia in bobwhite quails (Colinus virginianus). Int. J. Poult. Sci. 2004, 3, 438–441.
- Porter, R.E. Bacterial Enteritides of Poultry. Poult. Sci. 1998, 77, 1159–1165.
- Barrow, P.A.; Freitas Neto, O.C. Pullorum disease and fowl typhoid-new thoughts on old diseases: A review. Avian Pathol. 2011, 40, 1–13.
- Bande, F.; Arshad, S.S.; Omar, A.R.; Hair-Bejo, M.; Mahmuda, A.; Nair, V. Global distributions and strain diversity of avian infectious bronchitis virus: A review. Anim. Health Res. Rev. 2017, 18, 70–83.
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297.
- Britton, P.; Armesto, M.; Cavanagh, D.; Keep, S. Modification of the avian coronavirus infectious bronchitis virus for vaccine development. Bioeng. Bugs. 2012, 3, 114–119.
- Raj, G.D.; Jones, R.C. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997, 26, 677–706.
- Liu, S.; Zhang, X.; Wang, Y.; Li, C.; Han, Z.; Shao, Y.; Li, H.; Kong, X. Molecular characterization and pathogenicity of infectious bronchitis coronaviruses: Complicated evolution and epidemiology in China caused by cocirculation of multiple types of infectious bronchitis coronaviruses. Intervirology 2009, 52, 223–234.
- Cavanagh, D.; Davis, P.J.; Mockett, A.P.A. Amino acids within hypervariable region 1 of avian coronavirus IBV (Massachusetts serotype) spike glycoprotein are associated with neutralization epitopes. Virus Res. 1988, 11, 141–150.
- Schat, K.A.; Skinner, M.A. Avian Immunosuppressive Diseases and Immuneevasion. Avian Immunol. 2008, 299–322.
- Montgomery, R.D.; Villegas, P.; Dawe, D.L.; Brown, J. Effect of Avian Reoviruses on Lymphoid Organ Weights and Antibody Response in Chickens. Avian Dis. 1985, 29, 552.
- Guy, J.S. Virus Infections of the Gastrointestinal Tract of Poultry. Poult. Sci. 1998, 77, 1166–1175.
- Shivaprasad, H.L.; Franca, M.; Woolcock, P.R.; Nordhausen, R.; Day, J.M.; Pantin-Jackwood, M. Myocarditis Associated with Reovirus in Turkey Poults. Avian Dis. 2009, 53, 523–532.
- Jones, R.C. Avian reovirus infections. OIE Rev. Sci. Technol. 2000, 19, 614–625.
- van der Heide, L. The History of Avian Reovirus. Avian Dis. 2000, 44, 638.
- Wigand, R.; Bartlia, A.; Dreizin, R.S.; Esche, H.; Ginsberg, H.S.; Green, M.; Hierholzer, J.C.; Kalter, S.S.; McFerran, J.B.; Pettersson, U.; et al. Adenoviridae: Second report. Intervirology 1982, 18, 169–176.
- Kouwenhoven, B.; Davelaar, F.G.; Van Walsum, J. Infectious proventriculitis causing runting in broilers. Avian Pathol. 1978, 7, 183–187.
- Goodwin, M.A. Adenovirus inclusion body ventriculitis in chickens and captive bobwhite quail (Colinus virginianus). Avian Dis. 1993, 37, 568.
- Goodwin, M.A.; Hill, D.L.; Dekich, M.A.; Putnam, M.R. Multisystemic Adenovirus infection in broiler chicks with hypoglycemia and spiking mortality. Avian Dis. 1993, 37, 625.
- Barnes, H.J.; Guy, J.S. Poult enteritis—Mortality syndrome. In Diseases of Poultry, 11th ed.; Saif, Y.M., Barnes, H.J., Glisson, J.R., Fadly, A.M., McDougald, L.R., Eds.; Iowa State University Press: Ames, IA, USA, 2003; pp. 320–326.
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Microbiol. 2018, 9, 235.





