
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio J. Salinas | + 2796 word(s) | 2796 | 2022-01-17 03:49:00 | | | |
| 2 | Peter Tang | Meta information modification | 2796 | 2022-01-25 01:55:21 | | |
Video Upload Options
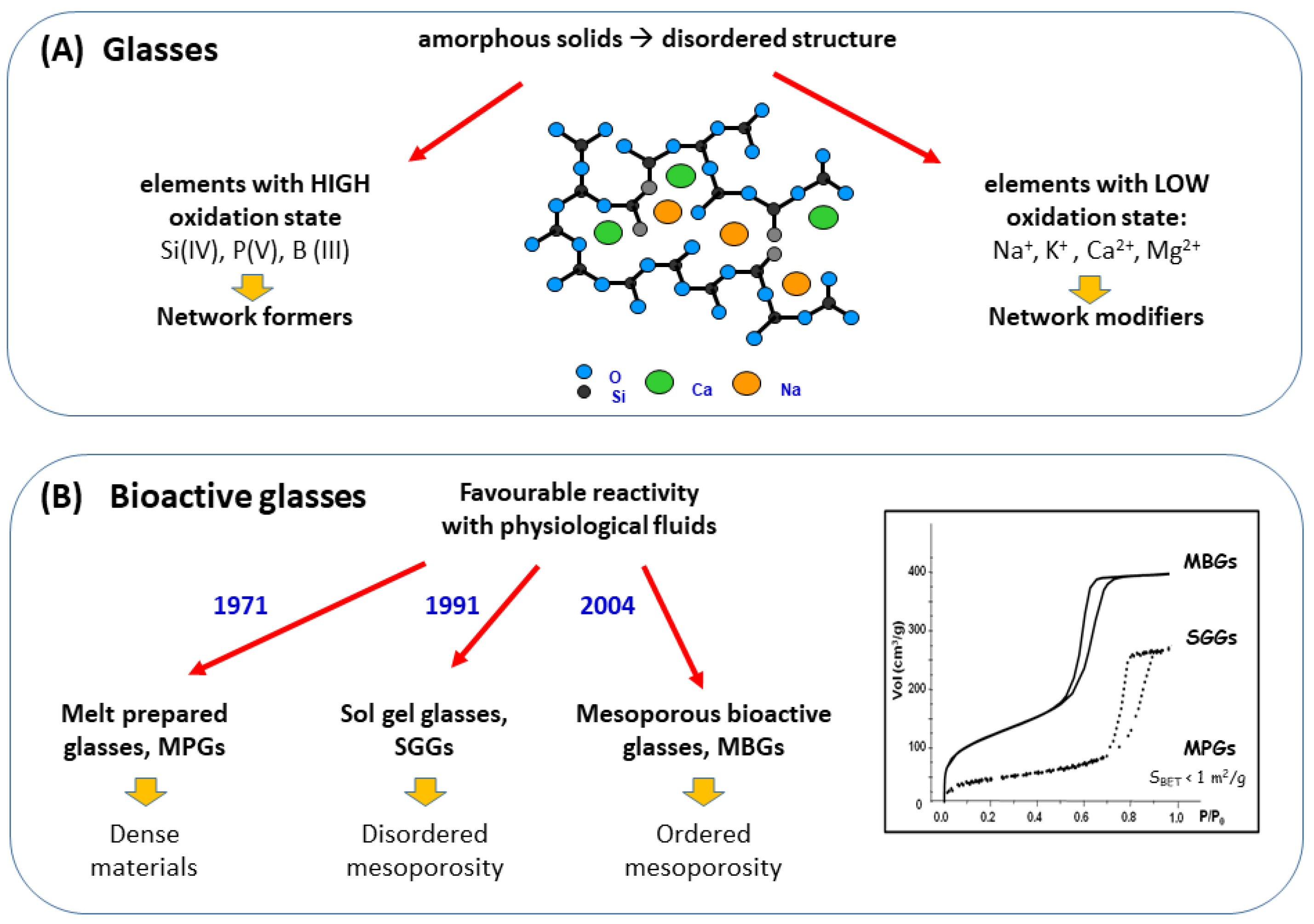
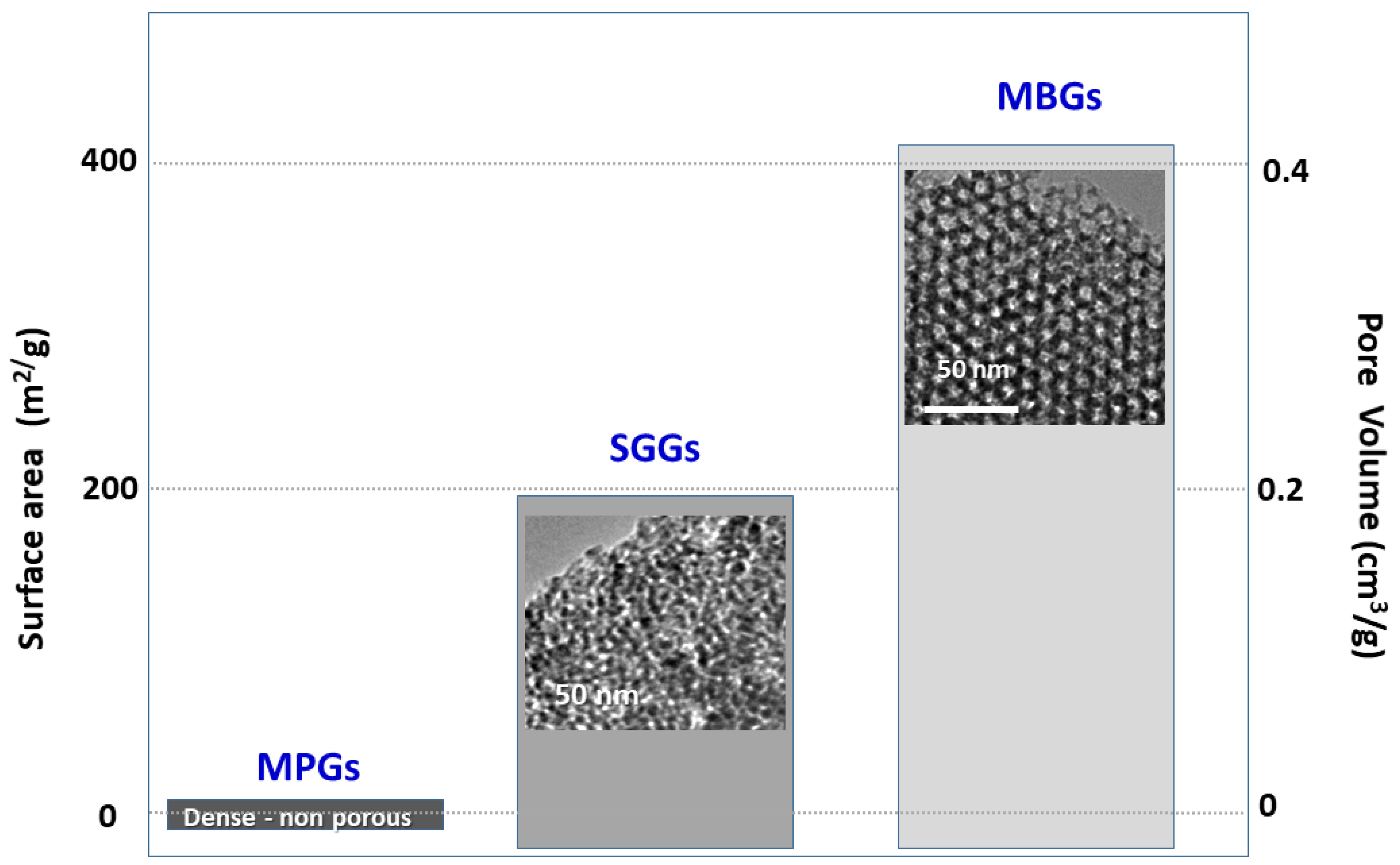
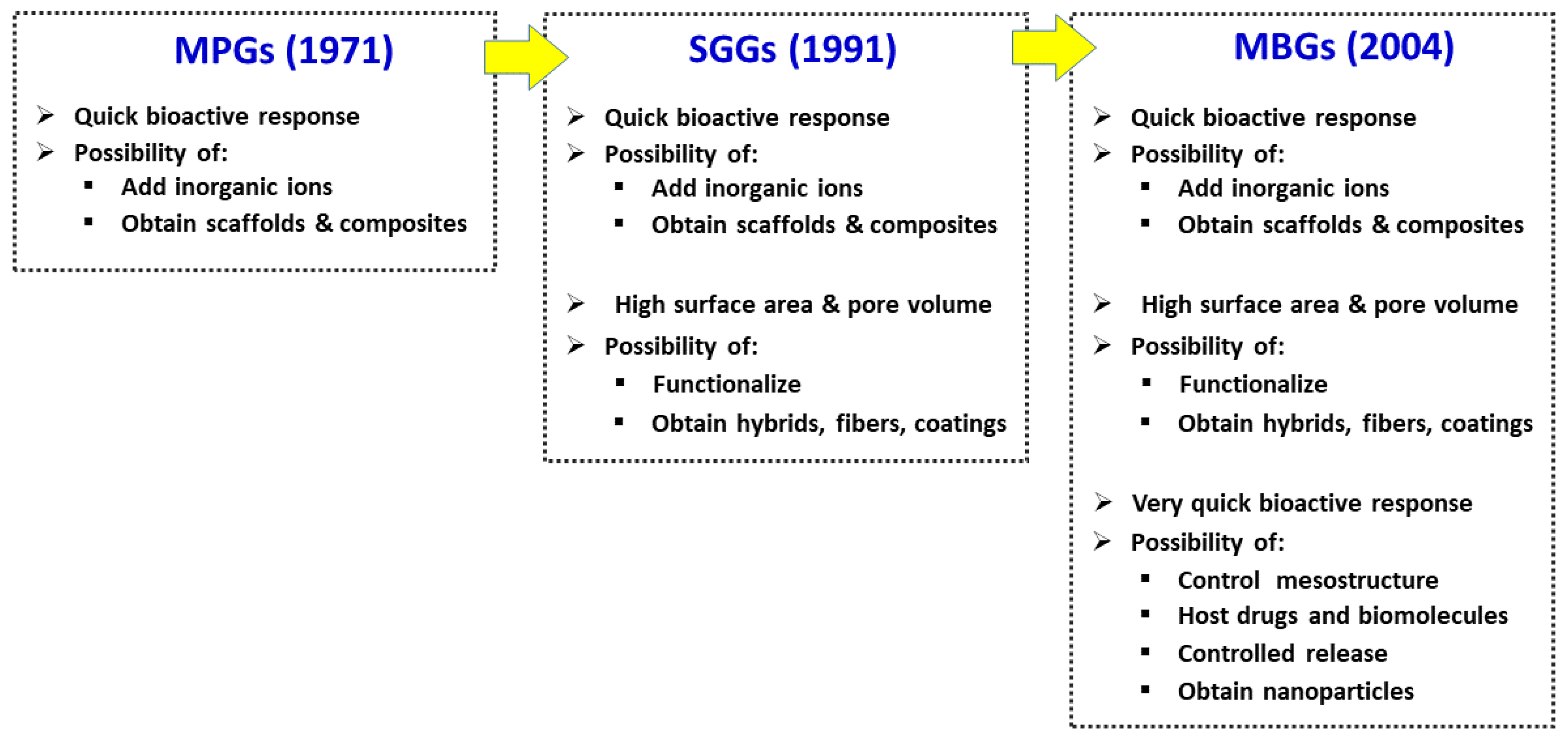
Mesoporous bioactive glasses (MBGs) is a category of bioceramics that, as will be explained, can be considered intermediate between traditional bioactive glasses—obtained by quenching of a melt or by the sol-gel method—and silica mesoporous materials. These bioactive glasses can be considered a spin-off of silica mesoporous materials because they are designed with a similar technical approach. Mesoporous glasses in addition to SiO2 contain significant amounts of other oxides, particularly CaO and P2O5 and therefore, they exhibit quite different properties and clinical applications than mesoporous silica compounds. Both materials exhibit ordered mesoporous structures with a very narrow pore size distribution that are achieved by using surfactants during their synthesis. The characteristics of mesoporous glasses made them suitable to be enriched with various osteogenic agents, namely inorganic ions and biopeptides as well as mesenchymal cells.
1. The Need of Synthetic Biomaterials in Bone Regeneration
2. The Three Families of Bioactive Glasses
3. Bioactive Glasses in Bone Regeneration
References
- Dimitriou, R.; Jones, E.; Mcgonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66.
- Fernández-Yagüe, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29.
- Nguyen, M.A.; Camci-Unal, G. Unconventional tissue engineering materials in disguise. Trends Biotechnol. 2020, 38, 178–190.
- Salinas, A.J.; Esbrit, P.; Vallet-Regí, M. A tissue engineering approach based on the use of bioceramics for bone repair. Biomater. Sci. 2013, 1, 40–51.
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459.
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698.
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824.
- Salinas, A.J.; Vallet-Regí, M. Glasses in bone regeneration: A multiscale issue. J. Non-Cryst. Solids 2016, 432, 9–14.
- Suárez, M.; Fernández-García, E.; Fernández, A.; López-Píriz, R.; Díaz, R.; Torrecillas, R. Novel antimicrobial phosphate-free glass–ceramic scaffolds for bone tissue regeneration. Sci. Rep. 2020, 10, 13171.
- Marie, P.J. The calcium-sensing receptor in bone cells: A potential therapeutic target in osteoporosis. Bone 2010, 46, 571–576.
- Julien, M.; Khoshniat, S.; Lacreusette, A.; Gatius, M.; Bozec, A.; Wagner, E.F.; Wittrant, Y.; Masson, M.; Weiss, P.; Beck, L.; et al. Phosphate-dependent regulation of MGP in osteoblasts: Role of ERK1/2 and Fra-1. J. Bone Miner. Res. 2009, 24, 1856–1868.
- Hoppe, A.; Boccaccini, A.R. Biological Impact of Bioactive Glasses and Their Dissolution Products. Front Oral Biol. 2015, 17, 22–32.
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramic. Biomaterials 2011, 32, 2757–2774.
- Ojansivu, M.; Vanhatupa, S.; Björkvik, L.; Häkkänen, H.; Kellomäki, M.; Autio, R.; Ihalainen, J.A.; Hupa, L.; Miettinen, S. Bioactive glass ions as strong enhancers of osteogenic differentiation in human adipose stem cells. Acta Biomater. 2015, 21, 190–203.
- Carlisle, E.M. Silicon: A possible factor in bone calcification. Science 1970, 167, 279–280.
- Li, H.; He, J.; Yu, H.; Green, C.R.; Chang, J. Glass-ceramic promotes wound healing by affecting gap junction connexin 43 mediated endothelial cell behavior. Biomaterials 2016, 84, 64–75.
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hamblin, M.R.; Mozafari, M. Nanotechnology for angiogenesis: Opportunities and challenges. Chem. Soc. Rev. 2020, 49, 5008–5057.
- Vallet-Regí, M.; Salinas, A.J. Mesoporous Bioactive Glasses in Tissue Engineering and Drug Delivery. In Bioactive Glasses: Fundamentals, Technology and Applications; Boccaccini, A.R., Brauer, D.S., Hupa, L., Eds.; The Royal Society of Chemistry: London, UK, 2017; pp. 393–419.
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 193, 282–295.
- Xie, J.; Peng, C.; Zhao, Q.; Wang, X.; Yuan, H.; Yang, L.; Li, K.; Lou, X.; Zhang, Y. Osteogenic differentiation and bone regeneration of iPSC-MSCs supported by a biomimetic nanofibrous scaffold. Acta Biomater. 2016, 29, 365–379.
- Lowe, B.; Ottensmeyer, M.P.; Xu, C.; He, Y.; Ye, Q.; Troulis, M.J. The regenerative applicability of bioactive glass and beta-tricalcium phosphate in bone tissue engineering: A transformation perspective. J. Funct. Biomater. 2019, 10, 16.
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141.
- Salinas, A.J.; Vallet-Regí, M.; Heikkila, J. Use of bioactive glasses as bone substitutes in orthopedics and traumatology. In Bioactive Glasses: Materials, Properties and Applications, 2nd ed.; Ylanen, H., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 337–364.
- Baino, F.; Novajra, G.; Miguez-Pacheco, V.; Boccaccini, A.R.; Vitale-Brovarone, C. Bioactive glasses: Special applications outside the skeletal system. J. Non-Cryst. Solids 2016, 432, 15–30.
- Li, R.; Clark, A.E.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991, 2, 231–239.
- Salinas, A.J. Mesoporous Bioactive Glasses: State of the Art and Future Prospects, In Bioactive Glasses: Properties, Composition and Recent Applications; Arcos, D., Vallet-Regí, M., Eds.; Nova Series; Materials Science and Technologies: Hauppauge, NY, USA, 2020; pp. 243–274.
- Kresge, C.T.; Leonowitz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712.
- Vallet-Regí, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–331.
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-Induced self-assembly: Nanostructures made easy. Adv. Mater. 1999, 11, 579–585.
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior V in vitro bone forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984.
- López-Noriega, A.; Arcos, D.; Izquierdo-Barballeta, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regí, M. Ordered mesoporous bioactive glasses for bone tissue regeneration. Chem. Mater. 2006, 18, 3137–3144.
- Vallet-Regí, M.; Salinas, A.J. Mesoporous bioactive glasses for regenerative medicine. Mater. Today Bio. 2021, 11, 100121.
- Lin, H.M.; Lin, Y.H.; Hsu, F.Y. Preparation and characterization of mesoporous bioactive glass/polycaprolactone nanofibrous matrix for bone tissues engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2619–2630.
- Yoon, J.Y.; Kim, J.J.; El-Fiqi, A.; Jang, J.H.; Kim, H.W. Ultrahigh protein adsorption capacity and sustained release of nanocomposite scaffolds: Implication for growth factor delivery systems. RSC Adv. 2017, 7, 16453–16459.
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431.
- Zhu, H.; Zheng, K.; Boccaccini, A.R. Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules. Acta Biomater. 2021, 129, 1–17.
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82.
- Greenspan, D.C. Bioglass and bioactivity: A brief look back. In Bioactive Glasses: Properties, Composition and Recent Applications; Arcos, D., Vallet-Regí, M., Eds.; Nova Series; Materials Science and Technologies: Hauppauge, NY, USA, 2020; pp. 1–26.
- Salinas, A.J.; Vallet Regí, M. Bioactive ceramics: From bone grafts to tissue engineering. RSC Adv. 2013, 3, 11116–11131.
- Han, P.; Wu, C.; Chang, J.; Xiao, Y. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/β-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials 2012, 33, 6370–6379.
- Fu, S.; Du, X.; Zhu, M.; Tian, Z.; Wei, D.; Zhu, Y. 3D printing of layered mesoporous bioactive glass/sodium alginate-sodium alginate scaffolds with controllable dual drug release behaviors. Biomed. Mater. 2019, 14, 065011.
- Zambon, A.; Malavasi, G.; Pallini, A.; Fraulini, F.; Lusvardi, G. Cerium Containing Bioactive Glasses: A Review. ACS Biomater. Sci. Eng. 2021, 7, 4388–4401.
- Saltman, P.D.; Strause, L.G. The role of trace minerals in osteoporosis. J. Am. Coll. Nutr. 1993, 12, 384–389.
- Beattie, J.H.; Avenell, A. Trace element nutrition and bone metabolism. Nutr. Res. Rev. 1992, 5, 167–188.
- Matter, M.T.; Probst, S.; Läuchli, S.; Herrmann, I.K. Uniting drug and delivery: Metal oxide hybrid nanotherapeutics for skin wound care. Pharmaceutics 2020, 12, 780.
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251.
- Marie, P.J.; Hott, M.; Modrowski, D.; de Pollak, C.; Guillemain, J.; Deloffre, P.; Tsouderos, Y. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen deficient rats. J. Bone Miner. Res. 2005, 20, 1065–1074.
- Arlot, M.E.; Jiang, Y.; Genant, H.K.; Zhao, J.; Burt-Pichat, B.; Roux, J.P.; Delmas, P.D.; Meunier, P.J. Histomorphometric and μCT analysis of bone biopsies from postmenopausal osteoporotic women treated with strontium ranelate. J. Bone Miner. Res. 2008, 23, 215–222.
- Fromigué, O.; Haÿ, E.; Barbara, A.; Petrel, C.; Traiffort, E.; Ruat, M.; Marie, P.J. Calcium sensing receptor-dependent and receptor-independent activation of osteoblast replication and survival by strontium ranelate. J. Cell Mol. Med. 2009, 13, 2189–2199.
- Iafisco, M.; Ruffini, A.; Adamiano, A.; Sprio, S.; Tampieri, A. Biomimetic magnesium-carbonate-apatite nanocrystals endowed with strontium ions as anti-osteoporotic trigger. Mat. Sci. Eng. C 2014, 35, 212–219.
- Lin, K.; Xia, L.; Li, H.; Jiang, X.; Pan, H.; Xu, Y.; Lu, W.W.; Zhang, Z.; Chang, J. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials 2013, 34, 10028–10042.
- Yang, G.L.; Song, L.N.; Jiang, Q.H.; Wang, X.X.; Zhao, S.F.; He, F.M. Effect of strontium substituted nanohydroxyapatite coating of porous implant surfaces on implant osseointegration in a rabbit model. Int. J. Oral Maxillofac. Implant. 2012, 27, 1332–1339.
- Ren, J.; Blackwood, K.A.; Doustgani, A. Melt-electrospun polycaprolactone strontium-substituted bioactive glass scaffolds for bone regeneration. J. Biomed. Mat. Res. A 2013, 102, 3140–3153.
- Zhang, J.; Zhao, S.; Zhu, Y. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014, 10, 2269–2281.
- Pan, H.B.; Zhao, X.L.; Zhang, X.; Zhang, K.B.; Li, L.C.; Li, Z.Y.; Lam, W.M.; Lu, W.W.; Wang, D.P.; Huang, W.H.; et al. Strontium borate glass: Potential biomaterial for bone regeneration. J. Royal Soc. Interface 2010, 7, 1025–1031.
- Sabareeswaran, A.; Basu, B.; Shenoy, S.J.; Jaffer, Z.; Saha, N.; Stamboulis, A. Early osseointegration of a strontium containing glass ceramic in a rabbit model. Biomaterials 2013, 34, 9278–9286.
- Jonas, J.; Burns, J.; Abel, E.W.; Cresswell, M.J.; Strain, J.J.; Paterson, C.R. Impaired mechanical strength of bone in experimental copper deficiency. Ann. Nutr. Metab. 1993, 37, 245–252.
- Smith, B.J.; King, J.B.; Lucas, E.A.; Akhter, M.P.; Arjmandi, B.H.; Stoecker, B.J. Nutrient metabolism skeletal unloading and dietary copper depletion are detrimental to bone quality of mature rats. J. Nutr. 2002, 132, 190–196.
- Nojiri, H.; Saita, Y.; Morikawa, D.; Kobayashi, K.; Tsuda, C.; Miyazaki, T.; Saito, M.; Marumo, K.; Yonezawa, I.; Kaneko, K.; et al. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J. Bone Miner. Res. 2011, 26, 2682–2694.
- Ye, J.; He, J.; Wang, C.; Yao, K.; Gou, Z. Copper-containing mesoporous bioactive glass coatings on orbital implants for improving drug delivery capacity and antibacterial activity. Biotechnol. Lett. 2014, 36, 961–968.
- Chitra, S.; Bargavi, P.; Balasubramaniam, M.; Chandran, R.R.; Balakumar, S. Impact of copper on in vitro biomineralization, drug release efficacy and antimicrobial properties of bioactive glasses. Mater. Sci. Eng. C 2020, 109, 110598.
- Jiménez-Holguín, J.; Sánchez-Salcedo, S.; Vallet-Regí, M.; Salinas, A.J. Development and evaluation of copper-containing mesoporous bioactive glasses for bone defects therapy. Micropor. Mesopor. Mat. 2020, 308, 1100454.
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915.
- Heras, C.; Jiménez-Holguín, J.; Doadrio, A.L.; Vallet-Regí, M.; Sánchez-Salcedo, S.; Salinas, A.J. Multifunctional antibiotic- and zinc-containing mesoporous bioactive glass scaffolds to fight bone infection. Acta Biomater. 2020, 114, 395–406.
- Guduric, V.; Belton, N.; Richter, R.F.; Bernhardt, A.; Spangenberg, J.; Wu, C.; Lode, A.; Gelinsky, M. Tailorable zinc-substituted mesoporous bioactive glass/alginate-methylcellulose composite bioinks. Materials 2021, 14, 1225.
- Westhauser, F.; Wilkesmann, S.; Nawaz, Q.; Hohenbild, F.; Rehder, F.; Saur, M.; Fellenberg, J.; Moghaddam, A.; Ali, M.S.; Peukert, W.; et al. Effect of manganese, zinc, and copper on the biological and osteogenic properties of mesoporous bioactive glass nanoparticles. J. Biomed. Mater. Res. 2021, 109, 1457–1467.
- Pérez, R.; Sánchez-Salcedo, S.; Lozano, D.; Heras, C.; Esbrit, P.; Vallet-Regí, M.; Salinas, A.J. Osteogenic effect of ZnO-mesoporous glasses loaded with osteostatin. Nanomaterials 2018, 8, 592.
- Heras, C.; Sánchez-Salcedo, S.; Lozano, D.; Peña, J.; Esbrit, P.; Vallet-Regí, M.; Salinas, A.J. Osteostatin potentiates the bioactivity of mesoporous glass scaffolds containing Zn2+ ions in human mesenchymal stem cells. Acta Biomater. 2019, 89, 359–371.
- Lozano, D.; Gil-Albarova, J.; Heras, C.; Sánchez-Salcedo, S.; Gómez-Palacio, V.E.; Gómez-Blasco, A.; Doadrio, J.C.; Vallet-Regí, M.; Salinas, A.J. ZnO-mesoporous glass scaffolds loaded with osteostatin and mesenchymal cells improve bone healing in a rabbit bone defect. J. Mater. Sci. Mater. Med. 2020, 31, 100.





