Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Krzysztof Lukasz Fijalkowski | + 3980 word(s) | 3980 | 2022-01-11 04:57:22 | | | |
| 2 | Amina Yu | Meta information modification | 3980 | 2022-01-25 01:51:59 | | | | |
| 3 | Amina Yu | -1 word(s) | 3979 | 2022-01-25 01:52:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fijalkowski, K. Biodegradable Waste and Degraded Soil. Encyclopedia. Available online: https://encyclopedia.pub/entry/18700 (accessed on 07 February 2026).
Fijalkowski K. Biodegradable Waste and Degraded Soil. Encyclopedia. Available at: https://encyclopedia.pub/entry/18700. Accessed February 07, 2026.
Fijalkowski, Krzysztof. "Biodegradable Waste and Degraded Soil" Encyclopedia, https://encyclopedia.pub/entry/18700 (accessed February 07, 2026).
Fijalkowski, K. (2022, January 24). Biodegradable Waste and Degraded Soil. In Encyclopedia. https://encyclopedia.pub/entry/18700
Fijalkowski, Krzysztof. "Biodegradable Waste and Degraded Soil." Encyclopedia. Web. 24 January, 2022.
Copy Citation
Soil degradation is the modification of its physical, chemical and biological properties that worsen the biological activity of the environment, with particular emphasis on food production, water quality, ecosystem services, flooding, eutrophication, biodiversity and carbon stock shrinkage. Soil degradation has many forms and genesis: (i) geotechnical soil degradation caused by deformation of the relief resulting from the activities of opencast and underground mining as well as construction (including road, rail and water). This form of soil degradation covers the entire territory, but the greatest damage should be noted in the areas of high concentration of the mining industry and in large urban agglomerations.
soil degradation
biodegradable waste
compost
biochar
remediation
revegetation
soil organic matter
plant ecosystem restoration contamination immobilization/degradation
1. Bio-Based Waste Substrates
Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Towards a circular economy: a zero-waste program for Europe published in 2014 clearly stressed the need to reuse waste in the closed-loop cycle [1]. In 2019 European Commission published a new EU fertiliser regulation [2]. In order to protect primary raw materials, it recommends the introduction of secondary raw materials for fertilizer production in the EU. This is to allow access to the EU internal market for organic fertilizers and soil improvers from recycling (compost and digestate) [3]. However, the list of biodegradable waste could be used as substrate to products for degraded soil fertilization is very wide (Table 1) and after decomposition introduce several valuable compounds such polysaccharides, proteins, polyphenols, lipids, macro and micronutrients and many others (Figure 1).
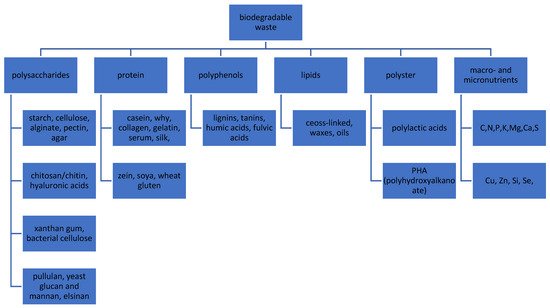
Figure 1. The samples of valuable compounds from biodegradable waste that can be used for degraded soil remediation/revitalization.
Table 1. The most frequently used biodegradable waste and products for degraded soils improvement.
| Product | Biodegradable Waste |
|---|---|
| Compost Vermicompost Digestate Organic-mineral fertilizers Soil substitutes Plant growth promoting substrates Biochar Ash Struvite |
Food, agricultural, forest waste Pig, cow, poultry manure Sewage sludge Organic fraction of municipal solid waste (ofmsw) Waste from the food (such slaughterhouses, bakeries, dairies, breweries) Pulp and paper mill by-products Wastewater |
1.1. Compost
Biological methods are most often used to transform and utilize biodegradable waste: composting and/or fermentation. It is estimated that out of the total mass of bio-waste, about 30 ÷ 50% is suitable for composting. The composting process is a very effective way of transforming and neutralizing various bio-waste, and then reusing the final product. It is an aerobic process during which microorganisms (aerobic bacteria, nematodes, fungi, etc.) break down biodegradable organic compounds, leading to the formation of compost—an organic fertilizer used to improve soil fertility and the quantity and quality of crops [4]. Composting is carried out both on an industrial scale (in reactors, tunnels, containers or prisms) and, on a much smaller scale, in domestic composters.
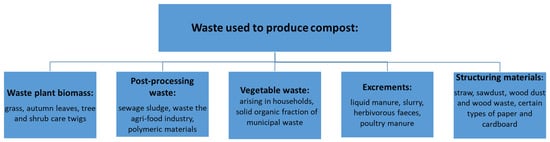
Many different substrates are used in the process of composting (Figure 2), mainly waste materials, which are produced both in industry, agriculture and municipal management.
Several factors influence the correct operation of the composting process. The input material must have an appropriate carbon/nitrogen (C/N) ratio, moisture content and structure, while maintaining the correct process conditions (aeration, moisture content, obtaining and maintaining the right temperature) allows the production of high-quality compost [11]. The basic parameter which determines the correct progress of the process and the quality of the resulting product is C/N—mass ratio of organic carbon to total nitrogen. Carbon is a source of energy for microorganisms while nitrogen influences the growth rate of their populations (Figure 3).
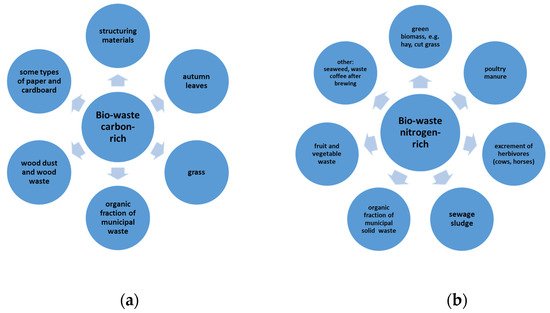
If the nitrogen content is too high, its excess is released into the atmosphere during composting and can cause odor problems. In the opposite situation, the process slows down rapidly because of the insufficient amount of nitrogen needed for the microorganisms to grow properly. After mixing all the selected waste to be composted, the C:N ratio should be between 25 ÷ 35:1. In addition, it is important that the batch mix has a moisture content of approximately 55% and a minimum organic matter content of 30%. An incorrect C/N ratio during the process can degrade the quality of the compost and decrease its fertilizer values. Any biodegradable material can be used for composting as long as there is sufficient time for decomposition [17]. However, not every raw material is suitable for the production of compost of a good quality, e.g., because of its above-standard heavy metal content (a common problem with sewage sludge). In addition, the compost mixture, in which the temperature during the thermophilic phase (according to various authors 60 ÷ 65 °C) necessary for the hygienization of the final product is not achieved, may cause the occurrence of harmful microorganisms in the finished compost [18]. Therefore, animal manure, meat residues and milk products should only be composted under an appropriate technological control. The base for obtaining good quality material for the composting process is the separate collection of bio-waste [19]. Good quality compost was produced using selectively collected green waste [20][21], vegetable waste [22][23][24][25] or agricultural waste [7][26][27]. In comparison, composts made from sewage sludge or the biodegradable solid fraction of municipal waste require more attention in both production and application [28][29][30][31].
1.2. Organic–Mineral Fertilizers
Processing of biodegradable waste into organic–mineral fertilizers or plant growth promoter products are technological solutions exist on commercial market as an alternative way for composts. The production of such fertilizers requires the addition of a significant amount of inorganic substrates. These can be calcium compounds, sulfuric acid, magnesium and potassium compounds or fly ashes from the combustion of hard coal or brown coal. Their role is primarily [32]:
-
Elimination of pathogens, as the product for natural use should be safe in terms of sanitation;
-
Correction and harmonization of the chemical composition and physical properties, as sewage sludge is a variable substrate;
-
Giving the fertilizer mixture a practical, usable and storable form.
Usually the process is based on the adding significant amount of quicklime (containing active calcium oxide CaO):
CaO + H2O → Ca(OH)2 + heat
The amount of heat generated by a strong exothermic reaction is directly proportional to the amount of water needed for evaporation and the amount of added quicklime CaO (Equation (1)). According to the fertilizer manufacturers, the resulting slaked lime can react at a temperature elevated to 135–140 °C with the presence of, for example, amorphous silicic acid or aluminum compounds present in the sediments. In addition, lime used as a fertilizing component also has an added value in the form of a positive effect on acidic soils, where it will provide not only lime as a fertilizing element, but also increase the pH of such soils, improving their fertility.
Another possibility is technology using roasted magnesite and sulfuric acid. In this method, calcined magnesite with a high MgO content and then sulfuric acid are added to the waste. As a result of an exothermic reaction (temperature above 100 °C), excess heat escapes in the form of water vapor (Equation (2)):
MgO + H2SO4 → MgSO4 + H2O + heat
The reaction also results in binding the water contained in the waste into the water of crystallization of magnesium sulphate (Equation (3)):
MgO + H2SO4 + nH2O → MgSO4 + (n + 1) H2O
In this technology, the process is carried out in such a way as not to fully saturate the water with magnesium sulphate, which protects the product against caking and is easily granulated. The fertilizer is an additional source of magnesium and sulphur for plants.
In turn, the fly ashes from biomass combustion is one of the oldest natural mineral fertilizers. Generally, ash from biomass has a much higher content of components such as CaO, MgO, Na2O, K2O, P2O5 and, at the same time, a lower content of SiO2, Al2O3 and TiO2 compared to ash from coal combustion. The pH of ashes from the combustion of various types of biomass ranges from 9.3 for oat grain ash to 13.9 for oak wood ashes. Due to its alkaline properties, these ashes can act as a hygienising substance of biodegradable waste (pH of aqueous extracts approx. 13, high content of reactive CaO). The condition for full hygienization of the waste is to maintain a high temperature of the mixture of 55–70 °C for 24 h and at the beginning of the process—a high pH of min. 12.5 As a result of homogenization of the mixture a number of exothermic reactions which lead to physicochemical changes [33]. Fertilizer contains significant amounts of calcium, magnesium and potassium as well as microelements, which are an important nutrient for plants. Moreover, due to high alkalinity it has de-acidifying properties and can be a substitute for calcium.
1.3. Biochar
Biochar is obtained in the pyrolysis process, i.e., thermal processing of biomass without oxygen at a temperature of 350–700 °C, which produces oil (mixture of hydrocarbons), synthetic gas (mixture of gaseous hydrocarbons) and biochar (solid residue). Generally, biochar can be obtained from all types of biodegradable waste. The most frequent waste used in the production of biochar include: forest waste, residues from agri-food processing (e.g., fermentation oats, rice husks, nut shells, coconut and bagasse), sewage sludge, organic fraction of municipal waste, poultry processing waste, chicken manure, cow manure and waste algae biomass. The selection of appropriate substrates depends on the physicochemical properties (including water and carbon content, particle size), potential applications of biochar and the parameters of the pyrolysis process.
The great advantage of processing waste into biochar is also the creation of a product with a uniform structure and stable properties. The obtained biochars are characterized, among others, by high content of organic carbon in the stable form, presence of mineral active chemical groups, developed porosity and large specific surface area (1 g of the material has a surface area of 400 to 800 m2), negative charge, alkaline reaction and resistance to degradation. It is assumed that the higher the pyrolysis temperature, the greater the specific surface area of the resulting biochar, although there is evidence that at very high temperatures some pores collapse and the surface area diminishes. The negative charge on the surface of biochar attracts positive charged metal ions and organic compounds from the soil solution, which significantly reduces their bioavailability to living organisms. The sorption capacity of metals increases with the pyrolysis temperature until it reaches a maximum at about 350–400 °C, then sorption decreases. The surface of biochar produced at such temperatures is rich in “oxygen-containing functional groups”, which enable the formation of complexes with cations, e.g., Cu2+, Ni2+, Cd2+, Pb2+ or Zn2+. Metal sorption is the effect of ion exchange with functional groups such as hydroxy-, carboxy- and phenols. In contrast, at high pyrolysis temperatures, the C/O ratio increases, and the surface becomes more electronegative. As a result, metal sorption is the effect of the electrostatic interaction between positively charged metal ions and the negative charge related to the delocalization of π-electrons on aromatic structures (Figure 5). The chemisorption of metals is generally much stronger than their physical sorption. However, it is believed that biochars produced at higher temperatures have a greater ability to absorb hydrocarbons.
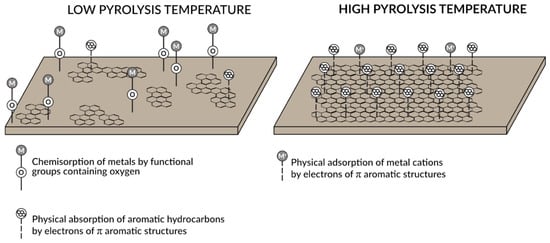
Figure 5. A schematic diagram showing the mechanisms of sorption of organic and inorganic pollutants on biocarbons produced at high and low temperatures [34], modified.
Generally, biochar has a high value of pH. This is due to the formation of metal oxides from basic cations (e.g., K, Ca, Si and Mg) during pyrolysis. Moreover, some biochars contain a lot of mineral ash (up to 50% for animal waste and up to 85% for bone meal materials). The minerals present in the ash, such as carbonates, phosphates and sulphates, can cause precipitation of some toxic elements e.g., Pb in the form of insoluble salts.
2. Advantages of Use the Biodegradable Waste on/to Degraded Soil
In the literature many studies have demonstrated that organic substrates added to degraded soil improve quality, resulting first at all in increase of organic matter content and stabilize soil structure. Garcia et al. [35] identify some main functions of organic amendments in soils: (1) promotion of soil aggregation; (2) provision of plant nutrients; and (3) a reduction in water content loss, in addition to other beneficial functions. The many other authors find several samples described in literature which confirm the positive effect on soil physical, chemical and microbiological properties. The study of Soria et al. [36] evaluates the effects of technosols made with different organic amendments (waste of gardening, greenhouse horticultural, stabilized sewage sludge and two mixtures of sludge with both vegetable composts) to restore degraded soils in a semiarid limestone quarry. Amended technosols after 6 and 18 months increased water retention capacity, electrical conductivity, total organic carbon and nitrogen, as compared to not amended and natural soils. In turn Arif et al. [37] carried out 5-year consecutive application of fresh industrial sludge (FIS) and composted industrial sludge (CIS) to restore soil functions at surface (0–15 cm) and subsurface (15–30 cm) of the degraded agricultural land. The authors found that sludge amendments increased such soil parameters like total organic carbon (TOC), soil available nitrogen (SAN), soil available phosphorus (SAP) and soil available potassium (SAK) at 0–15 cm depth. Taking into consideration of microbial activities they noted significant increase of value of dehydrogenase (DHA), β-glucosidase (BGA) and alkaline phosphatase (ALp) after FIS and CIS applications. However, other enzymes, such as urease activity (UA) and acid phosphatase (ACp), were significantly reduced compared to control soil. Moreover, sludge amendments significantly increased microbial biomass nitrogen (MBN) and microbial biomass phosphorus (MBP). Significant changes were noted in the increase population of soil culturable microflora (bacteria, fungi and actinomycetes) after sludge application into soil [37][38]. Composts produced from biodegradable waste not only help to improve soil fertility and plant yield, but also are able to control of soil erosion, biocontrol of diseases and bioremediation [39]. The optimal rates (not greater than 50 t ha−1) of different organic amendments can improve physical (soil structure, permeability, water holding capacity, etc.) and chemical (pH, cation exchangeable capacity, etc.) soil properties, favoring plant growth and microbial activity, without any risks for the environment (subsoil and groundwater contamination) [40]. Other studies have indicated the effectiveness of organic matter addition on increase surface roughness resulting in a large decline in soil erosion rates [41]. The use organic matter on salt-degraded soils caused following benefits: (i) aggregate formation, (ii) pores: soil aeration and plant root prolongation, (iii) water leaching [42]. Several advantages of using of biochar on degraded land identified IPCC special report [43]: (i) improved nutrient use efficiency due to reduced leaching of nitrate and ammonium and increased availability of phosphorus in soils with high phosphorus fixation capacity, (ii) management of heavy metals and organic pollutants: through reduced bioavailability of toxic elements, (iii) stimulation of beneficial soil organisms, (iv) improved porosity and water-holding capacity, (v) amelioration of soil acidification.
2.1. Soil Organic Matter (SOM)
Healthy soils are able to store significant quantities of carbon (C) in the form of soil organic carbon (SOC) or soil organic matter (SOM). Navarro-Pedreño et al. [44] noted that around 45% of the mineral soils in Europe have low or very low organic carbon content (0–2%) and 45% have a medium content (2–6%). SOC is included as a metrics for the regular assessment of land degradation in reporting for SDG target 15.3 [45]. The source of carbon in the soil is above and below ground plant biomass, animal residues, organic products of edaphone and biomass introduced in the form of fertilizers (manure, slurry, compost or green fertilizers). As a result of the mineralisation of organic compounds in the soil, under aerobic conditions, the available nutrients for plants are created, but at the same time the production of CO2 increases. However, in the process of humification, i.e., chemical, biological and biochemical transformations of various degrees of advancement, humus and other non-humus substances (fats, carbohydrates and lignins) are formed. One should strive for humification processes (accumulation of organic matter) to prevail over mineralization processes. Soil organic matter (SOM), and in it organic carbon, determines the physical, chemical and biological properties. It is one of the main components of forming soil fertility and influences the formation and durability of soil aggregates. Its content determines soil sorption capacity, water retention, biodiversity and soil density. Organic fertilisation is necessary to maintain and improve soil fertility, although affects yields more slowly and to a lesser extent [46]. Ngo et al. [47] compared several additives (mineral fertilizers, buffalo manure, compost, vermicompost and biochar) to check effect on degraded soils. They found the synergistic effects between plants and different organic amendments on carbon storage and soil organic matter composition. The biowaste compost (BWC) amended soils were assessed during 180 days under arid ambient conditions and in comparison with control soil [48]. It was shown a significant increase in SOM and SOC in dependence on used BWC quantities to 120 days, and then decrease in SOM and SOC levels.
2.2. Carbon Sequestration and Climate Mitigate
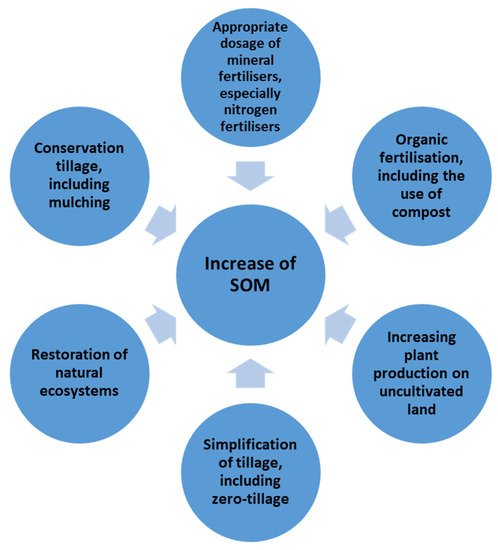
Increasing the organic matter content in soils can be fundamental in reducing CO2 concentration in the atmosphere. Two processes can be defined: carbon biosequestration in plants and carbon sequestration in soil. Photosynthesis reduces the amount of carbon dioxide in the atmosphere: thanks to chlorophyll, plants absorb CO2 from the atmosphere and as a result of biochemical transformations convert it into organic compounds necessary for their life processes. Increasing the yields of selected plants with appropriate agrotechnology helps to reduce CO2 emissions into the atmosphere (biosequestration). An effective reduction of the carbon dioxide content in the atmosphere can be achieved by sequestering CO2 in SOM [49]. An increase in soil organic matter content in Europe is estimated to have the potential to absorb about 0.8% of the current CO2 emissions from the burning of fossil fuels in the world, improving compliance with the international Kyoto Protocol [50]. Figure 4 shows the good agricultural practices described in the literature that influence the SOM content of soils.
The initiative to increase the global soil organic matter resource by 0.4% (four per mille) per year as compensation for global greenhouse gases (GHGs) emissions from anthropogenic sources was initiated during COP 21. Most carbon sequestration research only considers 30 cm of topsoil because it is considered that farming techniques have the greatest impact on this layer. It is estimated that agricultural land accumulates around 600 Gt C in a 1 m thick soil layer. Increasing SOC inventories by 4 per mille (around 2.5 Gt C per year) could offset about 30% of global greenhouse gas emissions [53]. It has been found that by using best land management practices, a C absorption rate of up to 10 per mille can be achieved in the first 20 years for soils with low initial SOC resources [49]. Earlier investigations on impact of biosolids on soil organic carbon buildup in calcareous strip-mined land in soil was done in Illinois [54]. Biosolids were applicated at a cumulative loading rate from 455 to 1654 Mg ha −1 (dry wt.) for 8 to 23 yr in rotation from 1972 to 2004. Over a 34 years reclamation period, mean net soil carbon accumulation rate was 1.73 (0.54 to 3.05) Mg carbon ha −1 yr −1 in biosolids amended fields compared with 0.07 to 0.17 Mg carbon ha−1 yr−1 in fertilizer controls. Soil carbon accumulation rate was significantly correlated with biosolids application rate, expressed as (Equation (4)):
where:
y = 0.064 x − 0.11
-
y is the annual net soil carbon sequestration (Mg C ha −1 yr −1),
-
x is annual biosolids application (Mg ha −1 yr −1, dry wt.).
Placek et al. [55] proposed several factors for calculating of carbon sequestration in degraded soil of zinc smelter and post-mining areas: (i) carbon management index (CMI), (ii) carbon lability (L), (iii) soil organic carbon (SOC) pool; (iv) carbon stock (C stock); (v) carbon sequestration rate (C sequestration); (vi) soil organic carbon build up rate (SOC build up rate). The authors used municipal compost, lacustrine chalk and coal slurry, the improvement of soil fertility and soil quality (increase value of total Kjeldahl nitrogen TN, total carbon TC, total organic carbon TOC). They found that CMI and SOC sequestration rate were the best methods to determine carbon sequestration in the soil during conducted pot experiment.
2.3. Biodiversity and Microbial Activity
Soil inhabiting microorganisms play an important role in organic matter mineralization and nutrient cycling. The activity and diversity of microorganisms are crucial for the stability and function of soil ecosystems. Usually the addition of organic matter restored the microbial activity over a long time period, and also increased the diversity of soil communities. The positive effect on the growth of both the biomass of microorganisms and their activity after the application of waste origin substrates with high organic matter content to soils is widely described in the literature, although the results are quite difficult to interpret. In the previous studies, a significant increase in the number of soil bacteria and fungi was observed as a result of the application of sewage sludge to soil [56][57]. The qualitative differences in the communities inhabiting the substrates enriched with additives resulted mainly from the presence of the so-called yeast-like fungi of the genus Saccharomyces, Candida, Rhodotorula, members of the Mucoraceae family (Mucor, Absidia) and the genus Trichoderma. However, this impact is highly dependent on climatic conditions and time. One of the causes of significant fluctuations in the number of individual groups of microorganisms is the rapid uptake/depletion of one or several essential nutrients, production of toxins antibiotics and “devouring” of bacteria and fungi by protozoa. On the other hand, the activity of soil dehydrogenases, related mainly to the catabolic processes of heterotrophic prokaryotic cells and fungi, generally reacts strongly to changes in soil oxygenation, although it is not the only factor modifying the activity of these oxidoreductases in such a biodiverse environment as soil. Similarly, significant effect of the introduction of N-rich sludge into the soil on the enzymatic activity was observed, although a significant decrease in activity 90 days after the application of the sewage sludge was noted [58].
2.4. Plant Ecosystem Restoration
Brownfield sites are usually devoid of vegetation. There are many publications in the literature confirming the fact that organic fertilization usually creates favorable conditions for plant growth and vitality [59]. Due to the fact that organic amendments provide both nutrients and water, the plants have better conditions to adopt to live in difficult degraded soil ecosystems. Usually many benefits are noted, such as an increase of total biomass weight, root and stem length and diameter, the number of leaves and foliar area. Some authors describe specific reactions of plants for organic amendment to degraded/contaminated soils. Khan et al. [60] used hard wood biochar (HWB), bagasse (BG), rice husk (RH) and maize comb waste (MCW) to chromite mine degraded soil containing Cd. The results indicated that the biochar added to soil, significantly increased chlorophyll contents (20–40%) and biomass (40–63%) of tomato and cucumber. Moreover, HWB was the most effective at reducing Cd bioavailability and significantly decrease Cd levels in vegetables. Good sample of such effect was induced phytoremediation carried out on degraded terrain around zinc mill (Miasteczko Slaskie, Silesia Region, Poland). The soil is characterized by extremally high concentration of heavy metals (mainly Zn, Pb, Cd) and totally degraded. Addition of sewage sludge in the dose 30 t ha−1 boosted the survival of trees such as Scots pine, birch, beech and oak (Figure 5). Similar results were obtained with use of municipal green waste (MGW) on degraded former opencast coal land on the margins of UNESCO’s Blaenavon Industrial Landscape World Heritage site in southeast Wales [61]. The application of MGW into soil significantly supported the growth of Silver Birch (Betula pendula, Roth) and European Larch (Larix decidua), but no Common Alder (Alnus glutinosa (L.) Gaertn.) [61]. There are many advantages of use the so called “aided phytostabilizaion technologies”. The technologies are widely proposed as a suitable strategy ecosystem plant revegetation. As a results of organic additives, metals bioavailability can be reduced. At the same time tolerant plant species find the suitable conditions to growth and further improves the soil characteristics boosted by the increase soil organic matter and biological activity [62].
. Mycorrhized Scots pine growing in a plot in the field growing on control soil (a) and on soil enriched with sewage sludge (b), (photo M. Kacprzak).
Application of organic fertilizers to degraded soil caused that the plants receive better conditions to develop and produce better defense responses, then are in general less susceptible to infection by phytopathogens such Pythium, Phytophthora and Fusarium spp. [63].
References
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions towards a Circular Economy: A Zero Waste Programme for Europe/* COM/2014/0398 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A52014DC0398 (accessed on 11 November 2021).
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union. 2019, 170, 1–114.
- 161024 ECN Biowaste Recycling in Europe. Available online: https://www.compostnetwork.info/download/ecn-status-report-2019-european-bio-waste-management-overview-of-bio-waste-collection-treatment-markets-across-europe-2/ (accessed on 11 November 2021).
- Razza, F.; D’Avino, L.; L’Abate, G.; Lazzeri, L. The role of compost in bio-waste management and circular economy. In Designing Sustainable Technologies, Products and Policies; Springer: Cham, Switzerland, 2018; pp. 133–143.
- Zhang, D.; Luo, W.; Li, Y.; Wang, G.; Li, G. Performance of co-composting sewage sludge and organic fraction of municipal solid waste at different proportions. Bioresour. Technol. 2018, 250, 853–859.
- Jalili, M.; Mokhtari, M.; Eslami, H.; Abbasi, F.; Ghanbari, R.; Ebrahimi, A.A. Toxicity evaluation and management of co-composting pistachio wastes combined with cattle manure and municipal sewage sludge. Ecotoxicol. Environ. Saf. 2019, 171, 798–804.
- Ajmal, M.; Aiping, S.; Awais, M.; Ullah, M.S.; Saeed, R.; Uddin, S.; Ahmad, I.; Zhou, B.; Zihao, X. Optimization of pilot-scale in-vessel composting process for various agricultural wastes on elevated temperature by using Taguchi technique and compost quality assessment. Process Saf. Environ. Prot. 2020, 140, 34–45.
- Cerda, A.; Artola, A.; Font, X.; Barrena, R.; Gea, T.; Sánchez, A. Composting of food wastes: Status and challenges. Bioresour. Technol. 2018, 248, 57–67.
- Onwosi, C.O.; Igbokwe, V.C.; Odimba, J.N.; Eke, I.E.; Nwankwoala, M.O.; Iroh, I.N.; Ezeogu, L.I. Composting technology in waste stabilization: On the methods, challenges and future prospects. J. Environ. Manag. 2017, 190, 140–157.
- Meng, L.; Li, W.; Zhang, S.; Wu, C.; Lv, L. Feasibility of co-composting of sewage sludge, spent mushroom substrate and wheat straw. Bioresour. Technol. 2017, 226, 39–45.
- Jędrczak, A. Biological Treatment of Waste; Polish Scientific Publishers PWN: Warsaw, Poland, 2007; ISBN 978-83-01-15166-9. (In Polish)
- De Mendonça Costa, M.S.S.; Bernardi, F.H.; de Mendonça Costa, L.A.; Pereira, D.C.; Lorin, H.E.F.; Rozatti, M.A.T.; Carneiro, L.J. Composting as a cleaner strategy to broiler agro-industrial wastes: Selecting carbon source to optimize the process and improve the quality of the final compost. J. Clean. Prod. 2017, 142, 2084–2092.
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137.
- Chen, M.; Huang, Y.; Liu, H.; Xie, S.; Abbas, F. Impact of different nitrogen source on the compost quality and greenhouse gas emissions during composting of garden waste. Process Saf. Environ. Prot. 2019, 124, 326–335.
- Koyama, M.; Nagao, N.; Syukri, F.; Abd Rahim, A.; Kamarudin, M.S.; Toda, T.; Mitsuhasi, T.; Nakasaki, K. Effect of temperature on thermophilic composting of aquaculture sludge: NH3 recovery, nitrogen mass balance, and microbial community dynamics. Bioresour. Technol. 2018, 265, 207–213.
- Li, M.X.; He, X.S.; Tang, J.; Li, X.; Zhao, R.; Tao, Y.Q.; Wang, C.; Qiu, Z.P. Influence of moisture content on chicken manure stabilization during microbial agent-enhanced composting. Chemosphere 2021, 264, 128549.
- Diaz, L.F.; Golueke, C.G.; Savage, G.M.; Eggerth, L.L. Composting and Recycling Municipal Solid Waste; CRC Press: Boca Raton, FL, USA, 2020.
- Barthod, J.; Rumpel, C.; Dignac, M.F. Composting with additives to improve organic amendments. A review. Agron. Sustain. Dev. 2018, 38, 17.
- Siebert, S. Bio-Waste Recycling in Europe against the Backdrop of the Circular Economy Package. Available online: https://www.compostnetwork.info/download/bio-waste-recycling-europe-backdrop-circular-economy-package/ (accessed on 21 November 2021).
- Longhurst, P.J.; Tompkins, D.; Pollard, S.J.; Hough, R.L.; Chambers, B.; Gale, P.; Tyrrel, S.; Villa, R.; Taylor, M.; Wu, S.; et al. Risk assessments for quality-assured, source-segregated composts and anaerobic digestates for a circular bioeconomy in the UK. Environ. Int. 2019, 127, 253–266.
- Massa, D.; Malorgio, F.; Lazzereschi, S.; Carmassi, G.; Prisa, D.; Burchi, G. Evaluation of two green composts for peat substitution in geranium (Pelargonium zonale L.) cultivation: Effect on plant growth, quality, nutrition, and photosynthesis. Sci. Hortic. 2018, 228, 213–221.
- Ali, M.; Griffiths, A.J.; Williams, K.P.; Jones, D.L. Evaluating the growth characteristics of lettuce in vermicompost and green waste compost. Eur. J. Soil Biol. 2007, 43, S316–S319.
- Chang, J.I.; Tsai, J.J.; Wu, K.H. Composting of vegetable waste. Waste Manag. Res. 2006, 24, 354–362.
- Kalamdhad, A.S.; Singh, Y.K.; Ali, M.; Khwairakpam, M.; Kazmi, A.A. Rotary drum composting of vegetable waste and tree leaves. Bioresour. Technol. 2009, 100, 6442–6450.
- Sarkar, S.; Pal, S.; Chanda, S. Optimization of a vegetable waste composting process with a significant thermophilic phase. Procedia Environ. Sci. 2016, 35, 435–440.
- Pergola, M.; Persiani, A.; Pastore, V.; Palese, A.M.; D’Adamo, C.; De Falco, E.; Celano, G. Sustainability assessment of the green compost production chain from agricultural waste: A case study in southern Italy. Agronomy 2020, 10, 230.
- Gupta, C.; Prakash, D.; Gupta, S.; Nazareno, M.A. Role of vermicomposting in agricultural waste management. In Sustainable Green Technologies for Environmental Management; Springer: Singapore, 2019; pp. 283–295.
- Picariello, E.; Pucci, L.; Carotenuto, M.; Libralato, G.; Lofrano, G.; Baldantoni, D. Compost and sewage sludge for the improvement of soil chemical and biological quality of Mediterranean agroecosystems. Sustainability 2021, 13, 26.
- Khaliq, S.J.A.; Al-Busaidi, A.; Ahmed, M.; Al-Wardy, M.; Agrama, H.; Choudri, B.S. The effect of municipal sewage sludge on the quality of soil and crops. Int. J. Recycl. Org. Waste Agric. 2017, 6, 289–299.
- Wei, Y.; Li, J.; Shi, D.; Liu, G.; Zhao, Y.; Shimaoka, T. Environmental challenges impeding the composting of biodegradable municipal solid waste: A critical review. Resour. Conserv. Recycl. 2017, 122, 51–65.
- Soobhany, N. Preliminary evaluation of pathogenic bacteria loading on organic Municipal Solid Waste compost and vermicompost. J. Environ. Manag. 2018, 206, 763–767.
- Grobelak, A.; Stępień, W.; Kacprzak, M. Sewage sludge as an ingredient in fertilizers and soil substitutes. Ecol. Eng. 2016, 48, 52–60.
- Poluszyńska, J. Possibilities of applications of fly ash from the biomass combustion in the sludge management. Sci. Work. Inst. Ceram. Build. Mater. 2013, 13, 48–59. (In Polish)
- Sizmur, T.; Quilliam, R.; Puga, A.P.; Moreno-Jiménez, E.; Beesley, L.; Gomez-Eyles, J.L. Application of Biochar for Soil Remediation. Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, M., Eds.; SSSA Special Publication (SSSA): Madison, WI, USA, 2015; Volume 63.
- Garcia, C.; Hernandez, T.; Coll, M.D.; Ondoño, S. Organic amendments for soil restoration in arid and semiarid areas: A review. AIMS Environ. Sci. 2017, 4, 640–676.
- Soria, R.; González-Pérez, J.A.; de la Rosa, J.M.; San Emeterio, L.M.; Domene, M.A.; Ortega, R.; Miralles, I. Effects of technosols based on organic amendments addition for the recovery of the functionality of degraded quarry soils under semiarid Mediterranean climate: A field study. Sci. Total Environ. 2021, 151572, in press.
- Arif, M.S.; Riaz, M.; Shahzad, S.M.; Yasmeen, T.; Ashraf, M.; Siddique, M.; Mubarik, M.S.; Bragazza, L.; Buttler, A. Fresh and composted industrial sludge restore soil functions in surface soil of degraded agricultural land. Sci. Total Environ. 2018, 619–620, 517–527.
- Kacprzak, M. Wspomaganie procesów remediacji terenów zdegradowanych. Wydawnictwo. Politechniki Czestochowskiej. 2007, 128. (In Polish)
- Ayilara, M.S.; Olanrewaju, O.S.; Babalola, O.O.; Odeyemi, O. Waste Management through Composting: Challenges and Potentials. Sustainability 2020, 12, 4456.
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: A review. Arid. Land Res. Manag. 2018, 33, 1–21.
- Hueso-González, P.; Muñoz-Rojas, M.; Martínez-Murillo, J.F. The role of organic amendments in drylands restoration. Curr. Opin. Environ. Sci. Health 2018, 5, 1–6.
- Meena, M.D.; Yadav, R.K.; Narjary, B.; Yadav, G.; Jat, H.S.; Sheoran, P.; Meena, M.K.; Antil, R.S.; Meena, B.L.; Singh, H.V.; et al. Municipal solid waste (MSW): Strategies to improve salt affected soil sustainability: A review. Waste Manag. 2019, 84, 38–53.
- Olsson, L.; Barbosa, H.; Bhadwal, S.; Cowie, A.; Delusca, K.; Flores-Renteria, D.; Hermans, K.; Jobbagy, E.; Kurz, W.; Li, D.; et al. Land Degradation. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendia, E.C., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., Eds.; IPPC: Rome, Italy, 2019.
- Navarro-Pedreño, J.; Almendro-Candel, M.B.; Zorpas, A.A. The Increase of Soil Organic Matter Reduces Global Warming, Myth or Reality? Science 2021, 3, 18.
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950.
- Rusco, E.; Jones, R.J.; Bidoglio, G. Organic matter in the soils of Europe: Present status and future trends. In JRC, Official Publications of the European Communities; European Communities: Brussels, Belgium, 2001.
- Ngo, P.-T.; Rumpel, C.; Thu, T.D.; Henry-des-Tureaux, T.; Dang, D.-K.; Jouquet, P. Use of organic substrates for increasing soil organic matter quality and carbon sequestration of tropical degraded soil: A 3-year mesocosms experiment. Carbon Manag. 2014, 5, 155–168.
- Mekki, A.; Aloui, F.; Sayadi, S. Influence of biowaste compost amendment on soil organic carbon storage under arid climate. J. Air Waste Manag. Assoc. 2019, 69, 867–877.
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86.
- Lal, R. Management of Carbon Sequestration in Soil; CRC Press: Boca Raton, FL, USA, 2019.
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196.
- Zang, H.; Blagodatskaya, E.; Wang, J.; Xu, X.; Kuzyakov, Y. Nitrogen fertilization increases rhizodeposit incorporation into microbial biomass and reduces soil organic matter losses. Biol. Fertil. Soils 2017, 53, 419–429.
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57.
- Tian, G.; Granato, T.C.; Cox, A.E.; Pietz, R.I.; Carlson, C.R.; Abedin, Z. Soil carbon sequestration resulting from long-term application of biosolids for land reclamation. J. Environ. Qual. 2009, 38, 6174.
- Placek, A.; Grobelak, A.; Włóka, D.; Kowalska, A.; Singh, B.R.; Almas, A.; Kacprzak, M. Methods for calculating carbon sequestration in degraded soil of zinc smelter and post-mining areas. Desalination Water Treat. 2018, 134, 233–243.
- Kacprzak, M.; Stańczyk–Mazanek, E. Changes in the structure of fungal communities of soil treated with sewage sludge. Biol. Fertil. Soils 2003, 38, 89–95.
- Kacprzak, M.; Woszczyk, K. The microfungal communities in sewage sludge treated soils. Environ. Prot. Eng. 2004, 30, 51–56.
- Kizilkaya, R.; Bayrakh, B. Effects of N-enriched sewage sludge on soil activities. Appl. Soil Ecol. 2005, 30, 192–202.
- Kowalska, A.; Grobelak, A.; Almås, Å.R.; Singh, B.R. Effect of Biowastes on Soil Remediation, Plant Productivity and Soil Organic Carbon Sequestration: A Review. Energies 2020, 13, 5813.
- Khan, M.A.; Ding, X.; Khan, S.; Brusseau, M.L.; Khan, A.; Nawab, J. The influence of various organic amendments on the bioavailability and plant uptake of cadmium present in mine-degraded soil. Sci. Total Environ. 2018, 636, 810–817.
- Sun, Y.; Xiong, X.; He, M.; Xu, Z.; Hou, D.; Zhang, W.; Ok, Y.S.; Rinklebe, J.; Wang, L.; Tsang, D.C.W. Roles of biochar-derived dissolved organic matter in soil amendment and environmental remediation: A critical review. Chem. Eng. J. 2021, 424, 130387.
- Barbosa, B.; Fernando, A.L. Chapter 9—Aided phytostabilization of mine waste. In Bio-Geotechnologies for Mine Site Rehabilitation; Prasad, M.N.V., de Favas, P.J., Maiti, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 147–157.
- Larkin, R.P. Soil Health Paradigms and Implications for Disease Management. Annu. Rev. Phytopathol. 2015, 53, 10.1–10.23.
More
Information
Subjects:
Soil Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Entry Collection:
Environmental Sciences
Revisions:
3 times
(View History)
Update Date:
25 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No