
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nives Jovicic | + 1322 word(s) | 1322 | 2022-01-12 07:01:55 | | | |
| 2 | Yvaine Wei | + 59 word(s) | 1381 | 2022-01-24 01:49:55 | | |
Video Upload Options
Globally, lignocellulosic biomass has great potential for industrial production of materials and products, but this resource must be used in an environmentally friendly, socially acceptable and sustainable manner. Wood and agricultural residues such as walnut shells as lignocellulosic biomass are one of the most affordable and important renewable resources in the world, which can partially replace fossil resources. The entry revealed that walnut shell biomass can be effectively liquefied into glycerol using H2SO4 as the catalyst, with liquefaction efficiency ranging from 89.21 to 90.98%.
1. Introduction
A large part of agricultural waste consists of lignocellulosic material, and the main properties of lignocellulosic biomass are very good strength, flammability, biodegradability and reactivity [1]. Agricultural biomass can be used as a raw material for the production of natural fibers and is particularly interesting from the point of view of environmental protection, since it is available, renewable and acceptable as a raw material source independent of petroleum products [2][3][4].
With the growing trends of production based on the biological basis of products, the concept of biorefinery is continuously gaining in importance [5]. Although the definition of the term biorefinery itself is subject to debate, the ultimate goal of biorefinery production is to create diverse products from different biomass as raw materials [6][7]. Processes and technologies from different fields, including polymer chemistry, bioengineering and agriculture [8][9][10], used in biorefineries can result in different products such as fuel, energy, chemicals and different matter [11][12][13].
After walnut kernels are used for food purposes, the shell remains as a by-product or waste, although walnut shell biomass is also a renewable source of raw material [14]. The structural polymers of lignocellulosic materials are cellulose, hemicellulose, and lignin, and their proportions depend on the plant species [15].
Development of new technologies for the effective utilization of biomass will enable the production of environmentally friendly bio-based products such as biofuels, bioenergy, biochemicals and biomaterials [16]. Common methods used in the production of bio-based products from biomass are biochemical and thermochemical conversion methods [17]. Of the thermochemical conversion methods, the most attention was drawn to the wood liquefaction in the presence of some organic reagents [18][19].
After the discovery of the wood liquefaction phenomena, research of different liquefaction parameters was conducted in terms of increasing of biomass concentration in liquefaction mixture, achieving the real liquefaction degree with respect to solubility properties of liquefied biomass in organic solvents, comprehension and understanding of the wood liquefaction mechanism, and further application [19][20].
2. Chemical Composition of Walnut Shell
| Walnut shell | Moisture | Ash | Coke | Fixed Carbon | Nitrogen |
| (%, mass) | |||||
| 12.23 ± 0.10 | 1.26 ± 0.08 | 17.15 ± 0.30 | 15.89 ± 0.47 | 0.56 ± 0.14 | |
3. Liquefaction Properties of Liquefied Biomass
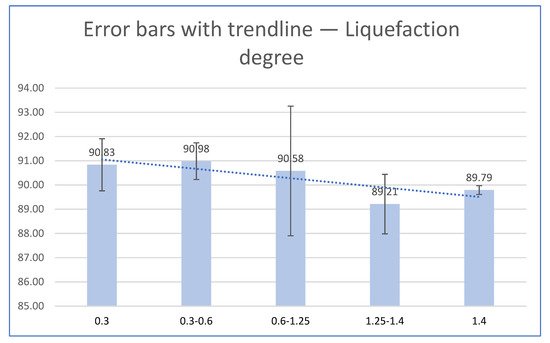
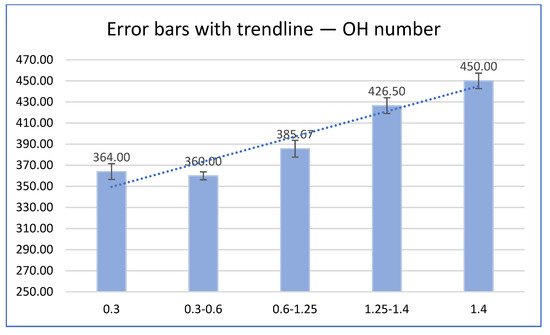
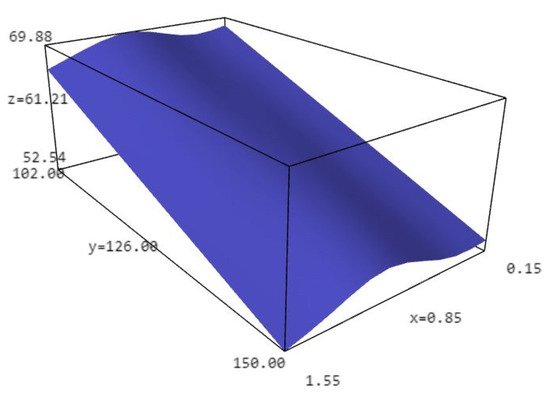
| Particle Size | Dry Matter (%) | |||
|---|---|---|---|---|
| 102 °C | 150 °C | |||
| Walnut shell PS < 0.3 | 68.71 ± 0.79 a | 53.82 ± 0.85 a | ||
| Walnut shell PS 0.30–0.60 | 68.10 ± 4.13 a | 53.82 ± 1.19 a | ||
| Walnut shell PS 0.60–1.25 | 69.89 ± 6.81 a | 56.10 ± 1.79 a | ||
| Walnut shell PS 1.25–1.40 | 68.24 ± 3.03 a | 53.85 ± 2.16 a | ||
| Walnut shell PS > 1.40 | 66.66 ± 5.12 a | 52.54 ± 1.17 a | ||
| F | P | F | P | |
| 0.205 | 0.93 | 2.193 | 0.143 | |
4. Findings
5. Conclusions
References
- Putro, J.; Soetaredjo, F.; Lin, S.-Y.; Ju, Y.-H.; Ismadji, S. Pretreatment and Conversion of Lignocellulose Biomass into Valuable Chemicals. R. Soc. Chem. 2016, 6, 46834–46852.
- Krička, T.; Jurišić, V.; Matin, A.; Bilandžija, N.; Antonović, A. Mogućnosti pretvorbe i iskorištenja ostataka poljoprivredne biomase nakon procesa pirolize. In Proceedings of the 51st Croatian and 11th International Symposium on Agriculture, Opatija, Croatia, 15–18 February 2016; pp. 485–489.
- Bhat Subrahmanya, K.; Yashas Gowda, T.G.; Sanjay, M.R.; Yogesha, B. Polymer Matrix-Natural Fiber Composites: An Overview. Cogent Eng. 2018, 5, 1.
- Maj, G. Emission Factors and Energy Properties of Agro and Forest Biomass in Aspect of Sustainability of Energy Sector. Energies 2018, 11, 1516.
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235.
- Lynd, L.R.; Larson, E.; Greene, N.; Laser, M.; Sheehan, J.; Dale, B.E.; McLaughlin, S.; Wang, M. The role of biomass in America’s energy future: Framing the analysis. Biofuels Bioprod. Biorefining 2009, 3, 113–123.
- Kaparaju, P.; Serrano, M.; Thomsen, A.B.; Kongjan, P.; Angelidaki, I. Bioethanol, biohydrogen and biogas production from wheat straw in a biorefinery concept. Bioresour. Technol. 2009, 100, 2562–2568.
- Fernando, S.; Adhikari, S.; Chandrapal, C.; Murali, N. Biorefineries: Current status, challenges, and future direction. Energy Fuels 2006, 20, 1727–1737.
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective. Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922.
- Clark, J.H.; Budarin, V.; Deswarte, F.E.I.; Hardy, J.J.E.; Kerton, F.M.; Hunt, A.J.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; Rodriguez, A.; et al. Green chemistry and the biorefinery: A partnership for a sustainable future. Green Chem. 2006, 8, 853–860.
- Dodds, D.R.; Gross, R.A. CHEMISTRY: Chemicals from biomass. Science 2007, 318, 1250–1251.
- Kumar, D.; Kumar, S. Mechanical Behaviour of Hybrid Bio-composite Reinforced with Walnut (Juglans regia L.) Shell Particle and Coconut Fibre. Int. J. Emerg. Technol. 2017, 8, 604–608.
- Ohara, H. Biorefinery. Appl. Microbiol. Biotechnol. 2003, 62, 474–477.
- Rahul, K.; Gourav, G.; Ripudaman, S.N. Walnut Shell Reinforced Composite: A Review. Int. J. Sci. Eng. Res. 2016, 7, 179–189.
- Sluiter, J.B.; Ruiz, O.R.; Scarlata, C.J.; Sluiter, A. Compositional Analysis of Lignocellulosic Feedstocks. Review and Description of Methods. J. Agric. Food Chem. 2010, 58, 9043–9053.
- Misra, M.; Mohanty, A.K.; Pandey, J. Biocomposites: Design and Mechanical Performance; Woodhead Publishing, Elsevier: London, UK, 2015.
- Antonović, A.; Jambreković, V.; Pervan, S.; Ištvanić, J.; Moro, M.; Zule, J. Utjecaj lokaliteta uzorkovanja na grupni kemijski sastav bijeli bukovine (Fagus sylvatica L.). Drv. Ind. 2007, 58, 119–125.
- Antonović, A.; Jambreković, V.; Pervan, S.; Ištvanić, J.; Greger, K.; Bublić, A. A supplement to the research of native lignin of beech sapwood (Fagus sylvatica L.). Wood Res. 2008, 53, 55–68.
- Antonović, A.; Ištvanić, J.; Medved, S.; Antolović, S.; Stanešić, J.; Kukuruzović, J.; Đurović, A.; Španić, N. Influence of Different Wood Species Chemical Composition on the Liquefaction Properties. In Implementation of Wood Science to Woodworking Sector; Sveučilište u Zagrebu Šumarski fakultet: Zagreb, Croatia, 2019; pp. 25–34.
- Antonović, A.; Barčić, D.; Kljak, J.; Ištvanić, J.; Podvorec, T.; Stanešić, J. The quality of fired Aleppo pine wood (Pinus halepensis Mill) biomass for biorefinery products. Croat. J. For. Eng. J. Theory Appl. For. Eng. 2018, 39, 3013–3024.
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885.
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefinery 2020, 10, 175–188.
- Matin, A.; Krička, T.; Jurišić, V.; Bilandžija, N.; Voća, N.; Mrkšić, J. Energetska Iskoristivost Ljuske Oraha i Lješnjaka. Zbornik Radova 48. Hrvatskog i 8. Međunarodnog Simpozija Agronoma; Publisher: Poljoprivredni fakultet. Sveučilište Josipa Jurja Strossmayera u Osijeku. Dubrovnik, Croatia, 2013; pp. 836–840.
- Pirayesh, H.; Khazaeian, A.; Tabarsa, T. The potential for using walnut (Juglans regia L.) shell as a raw material for wood-based particleboard manufacturing. Compos. Part B-Eng. 2012, 43, 3276–3280.
- Demirbas, A. Fuel characteristics of olive husk and walnut, hazelnut, sunflower, and almond shells. Energy Source 2002, 24, 215–221.




