Globally, lignocellulosic biomass has great potential for industrial production of materials and products, but this resource must be used in an environmentally friendly, socially acceptable and sustainable manner. Wood and agricultural residues such as walnut shells as lignocellulosic biomass are one of the most affordable and important renewable resources in the world, which can partially replace fossil resources. The entry revealed that walnut shell biomass can be effectively liquefied into glycerol using H2SO4 as the catalyst, with liquefaction efficiency ranging from 89.21 to 90.98%.
1. Introduction
A large part of agricultural waste consists of lignocellulosic material, and the main properties of lignocellulosic biomass are very good strength, flammability, biodegradability and reactivity [4]. Agricultural biomass can be used as a raw material for the production of natural fibers and is particularly interesting from the point of view of environmental protection, since it is available, renewable and acceptable as a raw material source independent of petroleum products [5,6,7].
With the growing trends of production based on the biological basis of products, the concept of biorefinery is continuously gaining in importance [17]. Although the definition of the term biorefinery itself is subject to debate, the ultimate goal of biorefinery production is to create diverse products from different biomass as raw materials [16,18]. Processes and technologies from different fields, including polymer chemistry, bioengineering and agriculture [19,20,21], used in biorefineries can result in different products such as fuel, energy, chemicals and different matter [22,23,24].
After walnut kernels are used for food purposes, the shell remains as a by-product or waste, although walnut shell biomass is also a renewable source of raw material [25]. The structural polymers of lignocellulosic materials are cellulose, hemicellulose, and lignin, and their proportions depend on the plant species [27].
Development of new technologies for the effective utilization of biomass will enable the production of environmentally friendly bio-based products such as biofuels, bioenergy, biochemicals and biomaterials [30]. Common methods used in the production of bio-based products from biomass are biochemical and thermochemical conversion methods [31]. Of the thermochemical conversion methods, the most attention was drawn to the wood liquefaction in the presence of some organic reagents [32,33].
After the discovery of the wood liquefaction phenomena, research of different liquefaction parameters was conducted in terms of increasing of biomass concentration in liquefaction mixture, achieving the real liquefaction degree with respect to solubility properties of liquefied biomass in organic solvents, comprehension and understanding of the wood liquefaction mechanism, and further application [33,34].
2. Chemical Composition of Walnut Shell
The analysis of non-combustible properties determined: nitrogen (N), water (H2O), ash, coke, fixed carbon (Cfix), and the obtained values are shown in Table 1.
Table 1. Non-combustible matter content in the walnut shell.
| Walnut shell |
Moisture |
Ash |
Coke |
Fixed Carbon |
Nitrogen |
| (%, mass) |
| 12.23 ± 0.10 |
1.26 ± 0.08 |
17.15 ± 0.30 |
15.89 ± 0.47 |
0.56 ± 0.14 |
3. Liquefaction Properties of Liquefied Biomass
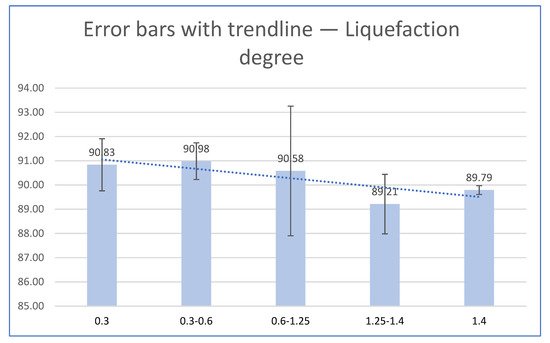
Among the liquefied different biomass particle sizes of walnut shell, the lowest undissolved residue content was achieved with granulation 0.3–0.6 mm, so the highest liquefaction degree was achieved with the same particle size (Figure 3).
Figure 3. Liquefaction degree as a function of particle size.
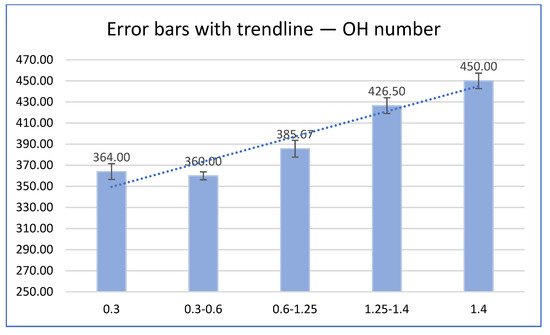
Obtained results have shown that liquefaction degree depends on particle size and with a decrease in granulation there is an increase in liquefaction degree. Among the liquefied different biomass particle sizes, the lowest OH-number was achieved with granulation 0.3-0.6 mm (Figure 4).
Figure 4. OH-number as a function of particle size.
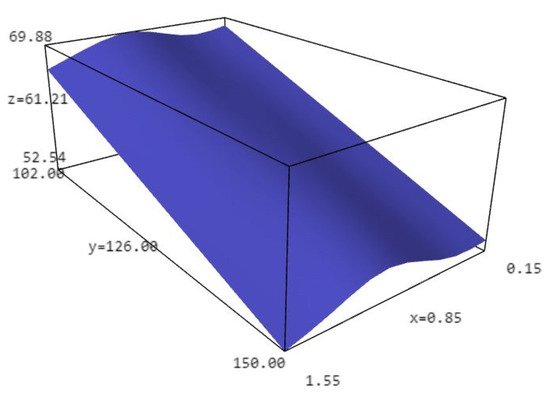
Obtained results have shown that dry matter depends on temperature and particle size. The lowest content of dry matter was at the smallest and at the biggest particle size of walnut shell (Table 5 and Figure 5).
Figure 5. Dry matter dependence on temperature and particle size (x—particle size; y—temperature; z—dry matter).
Results have also shown that the relationship between dry matter and particle size remains the same regardless of temperature, i.e., given the particle size interval, the change of dry matter is the same no matter the temperature (Figure 5).
4. Findings
Moisture content depends on environmental air temperature during the winter season, and it is one of the most important parameters when it comes to fuel properties. The moisture content was 12.23%. Ash content, which is also one of the main factors of biomass quality, since higher amounts of ash diminishes the quality of fuels, especially solid ones, was 1.26%. Ash originates simultaneously from natural and technogenic inorganic, organic and fluid matter during biomass combustion [
35]. This result is in the range reported for nut shells, although a large variation of values may be found for a specific nut shell. Queirós [
36] also reported a low content of ash for walnut shell (0.7%). Coke content and fixed carbon content are considered positive properties of biomass because they represent the quantity of energy released by the combustion of a specific amount of biomass [
38].
In the chemical composition of fuel, carbon (C) is one of the basic elements and makes up to 95%. The amount of carbon also determines the quality of fuel; i.e. by increasing the carbon content the quality of fuel improves. The carbon content in the walnut shell was 52.11% while the hydrogen content was 5.86%. The obtained results are slightly higher than those obtained by Matin et al., [
40] who found the carbon content in walnut shell to be from 57.55 to 58.01%, depending on the variety, while Demirbaş [
39] found that walnut shell contains 53.50% carbon (C). Hydrogen is the second most important ingredient in fuel, which with its high energy increases the thermal value of the fuel, and by burning it creates a visible flame.
Liquefaction comprises a complex set of reactions taking place on the polymeric components of biomass. They include derivatization such as esterification or etherification of free hydroxyl groups in cellulose or lignin as well as reactions that break the polymer chain of cellulose. In addition, liquefaction is affected by physical constraints on biomass reactivity such as the high crystallinity of cellulose. The tight packing of cellulose in the crystalline domains makes the reaction kinetics of otherwise reactive functional groups dependent on the diffusion of reagents into the tightly packed system. To overcome this limitation and speed up the liquefaction, increasingly harsh catalysts and reaction conditions, mainly mineral acids and high temperatures, have been employed. In short, macromolecule compounds in biomass are degraded into micro molecules and the obtained small molecules are unstable, reactive and can re-polymerize into oily products with a wide range of molecular weight distribution [
33,
34].
Results have shown that OH-number depends on particle size and with an increase in granulation there is an increase in OH-number. Results also shown that the relationship between dry matter and particle size remains the same regardless of temperature, i.e., given the particle size interval, the change of dry matter is the same no matter the temperature.
5. Conclusions
The study revealed that walnut shell biomass can effectively be liquefied in glycerol using H2SO4 as a catalyst with liquefaction efficiency ranging from 89.21–90.98% under the same liquefaction conditions, which is similar to the results obtained for liquified wood (88.47–94.94%).
Hydroxyl number as the most important liquefaction property in further application for various bioproducts have priority over undissolved residue and liquefaction degree, and according to that bigger particle size of walnut shell biomass show better properties in further application compared to smaller. Results have shown that OH-number depends on particle size and with the increase in granulation there is an increase in OH-number, so the biggest particle size (>1.4) had the biggest OH number (450). The lowest content of dry matter was at the smallest and at the biggest particle size of walnut shell so the obtained results have shown that the relationship between dry matter and particle size remains the same regardless of temperature, i.e., given the particle size interval, the change of dry matter is the same no matter the temperature.
The use of a catalytic liquefaction method with polyhydric alcohol glycerol was found to be suitable for liquefying walnut shells. Finally, the present study revealed unimaginable opportunities for scientific research and development aimed at novel bioproducts from liquefied biomass and opened new challenges in exploring natural, ecologically sound products with unlimited raw materials.
This entry is adapted from the peer-reviewed paper 10.3390/en15020495