
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karma Yeshi | + 2094 word(s) | 2094 | 2022-01-12 09:06:13 | | | |
| 2 | Vicky Zhou | Meta information modification | 2094 | 2022-01-21 05:08:20 | | |
Video Upload Options
Plant secondary metabolites (PSMs) are vital for human health and constitute the skeletal framework of many pharmaceutical drugs. Indeed, more than 25% of the existing drugs belong to PSMs. One of the continuing challenges for drug discovery and pharmaceutical industries is gaining access to natural products, including medicinal plants. This bottleneck is heightened for endangered species prohibited for large sample collection, even if they show biological hits. While cultivating the pharmaceutically interesting plant species may be a solution, it is not always possible to grow the organism outside its natural habitat. Plants affected by abiotic stress present a potential alternative source for drug discovery. In order to overcome abiotic environmental stressors, plants may mount a defense response by producing a diversity of PSMs to avoid cells and tissue damage. Plants either synthesize new chemicals or increase the concentration (in most instances) of existing chemicals, including the prominent bioactive lead compounds morphine, camptothecin, catharanthine, epicatechin-3-gallate (EGCG), quercetin, resveratrol, and kaempferol. Most PSMs produced under various abiotic stress conditions are plant defense chemicals and are functionally anti-inflammatory and antioxidative. The major PSM groups are terpenoids, followed by alkaloids and phenolic compounds.
1. Introduction
2. Reported Pharmacological Properties of PSMs Present in Plants Affected by Ex Situ Abiotic Stresses
| Stress Condition(s) | Plant Species (Family) | PSMs Produced | Effects on PSMs Concentration | Compound Class | Bioactive Compounds | Reported Pharmacological Properties |
|---|---|---|---|---|---|---|
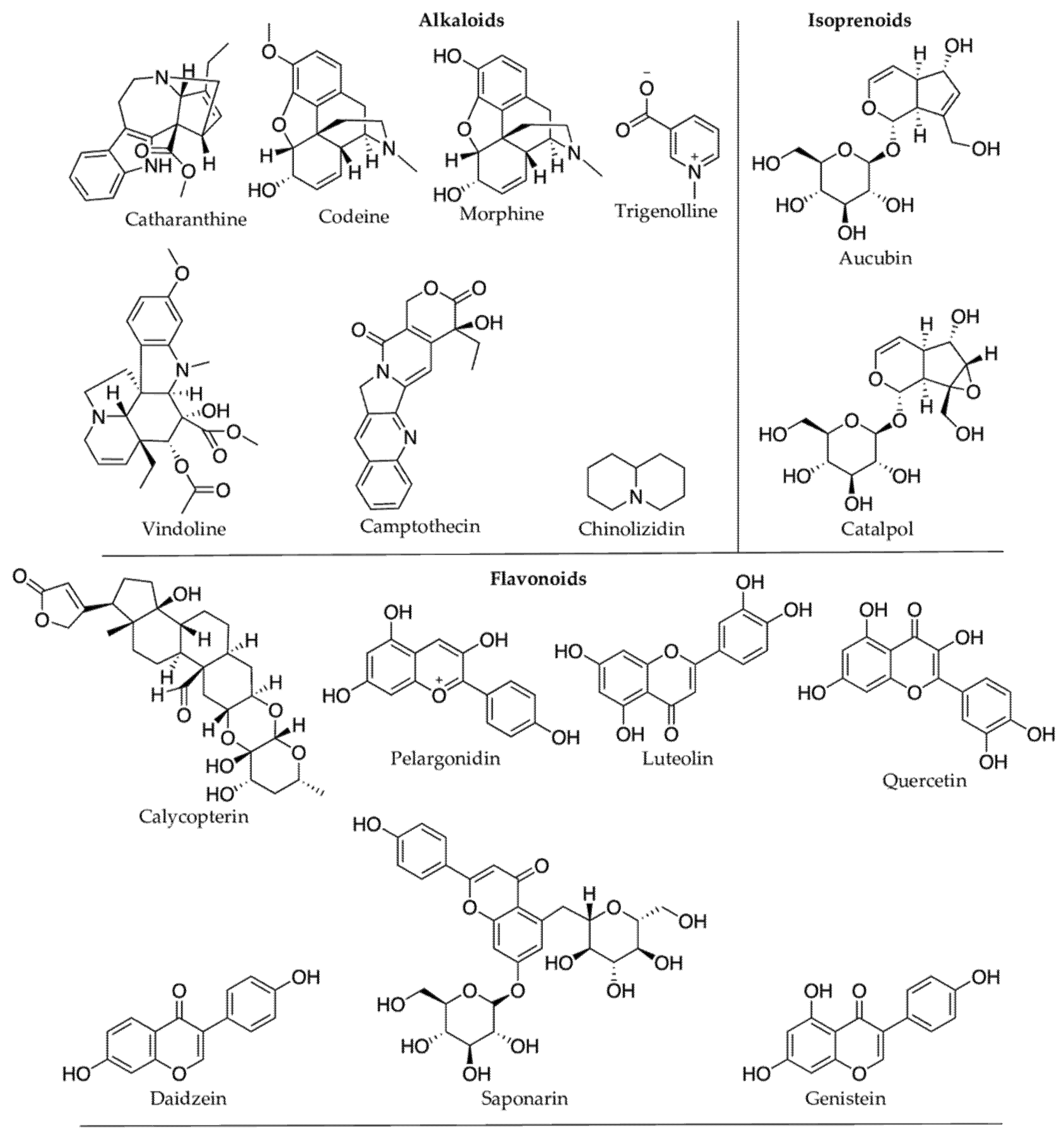
| Cold stress | Catharanthus roseus (Apocynaceae) [39] | vindoline | Decrease | Alkaloids | vindoline | Antidiabetic [35] |
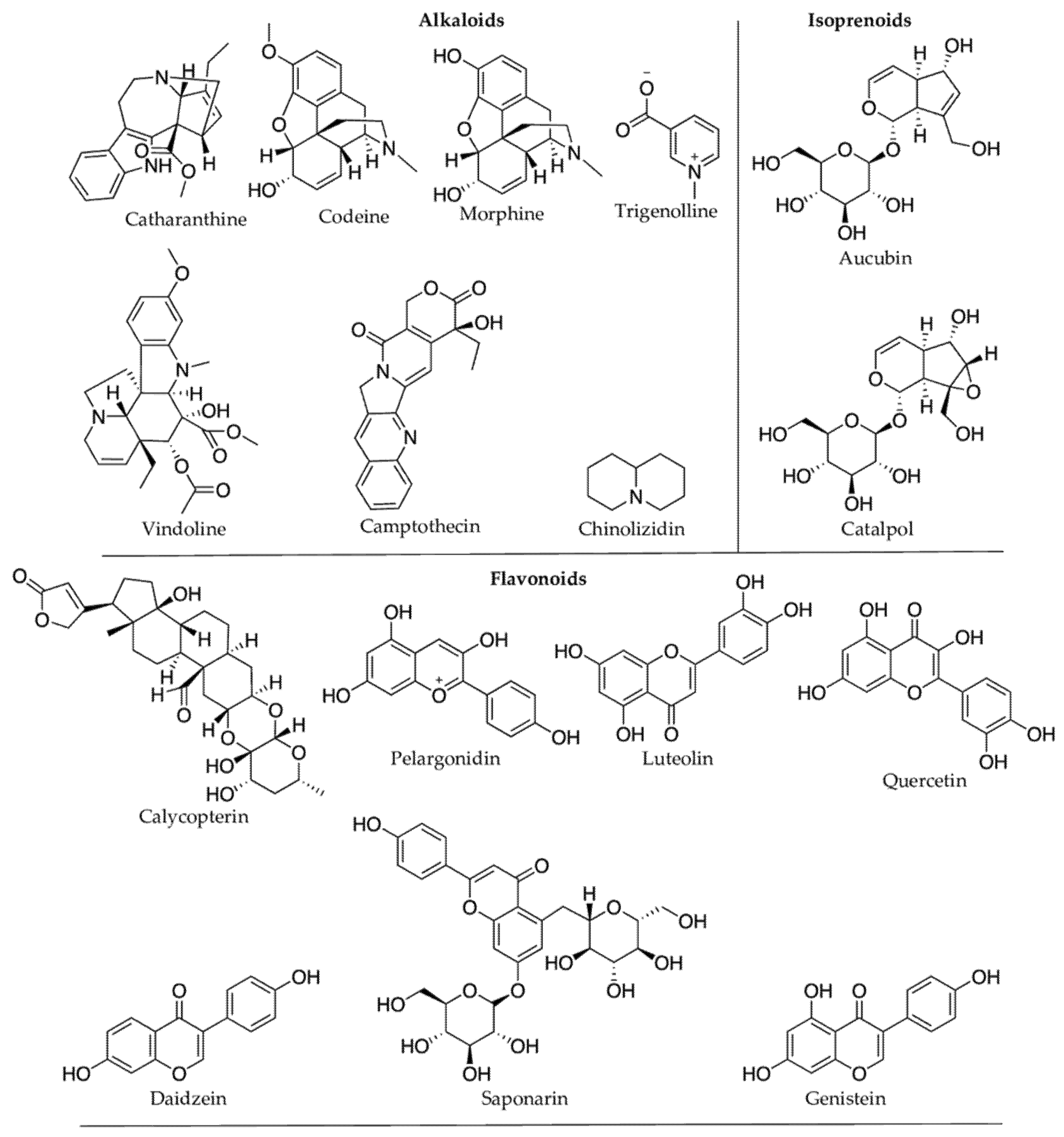
| Cold stress | Glycine max (Fabaceae) [40] | genistein, daidzein | Increase | Phenolics | genistein, daidzein | Antiproliferative [41][42] |
| Cold stress | Solanum lycopersicon (Solanaceae) [43][44] | (Z)-3-hexenol and (E)-2-hexenal (dominant); 1-hexanol and 1,4-hexadienal (smaller quantities) | Increase | Fatty Acyls | (E)-2-hexenal | Antibacterial [45] |
| Cold stress | β-phellandrene, (E)-β-ocimene | Increase | Terpenoids | NA | NA | |
| Cold stress | δ-elemene, α-humulene and β-caryophyllene (dominant); in severe cold: β-elemene is produced. | Increase | Terpenoids | δ-elemene, α-humulene and β-caryophyllene | Antiproliferative [46]; anticancer [47]; anti-inflammatory [48] | |
| Cold stress | Zea mays (Poaceae) [49] | pelargonidin | Increase | Phenolics | pelargonidin | Antithrombotic [50] |
| Cold stress | Fagopyrum tartaricum (Polygonaceae) [51] | anthocyanins (e.g.,3-O-galactosides) and anthocyanidins (e.g., malvidin) | Increase | Phenolics | anthocyanins | Antioxidant [52] |
| Cold stress | Withania somnifera (Solanaceae) [53] | withanolide A, withaferin A | Increase | Terpenoids | withanolide A; withferin A | Neuroprotective [54]; anticancer [55] |
| Cold stress | Camellia sinensis (Theaceae) [56] | nerolidol glucoside | Increase | Terpenoids | NA | NA |
| Drought | Amaranthus tricolor (Amaranthaceae) [57] | hydroxybenzoic acids (gallic acid, vanillic acid, syringic acid, p-hydroxybenzoic acid, salicylic acid, ellagic acid), hydroxycinnamic acids (caffeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, m-coumaric acid, sinapic acid, trans-cinnamic acid), flavonoids (iso-quercetin, hyperoside, rutin). | Increase | Phenolics (Flavonoids) | p-hydroxybenzoic acid | Antisickling activity [58] |
| Drought | Camellia sinensis (Theaceae) [59] | Epicatechins | Increase | Phenolics (Flavonoids) | epicatechins | Antioxidant [60] |
| Drought | Camptotheca acuminata (Nyssaceae) [61] | camptothecin | Increase | Alkaloids | camptothecin | Antitumour [62] |
| Drought (PEG-induced) | Catharanthus roseus (Apocyanaceae) [63] | vinblastine | Increase | Alkaloids | vinblastine | Anticancer [64] |
| Drought | Cistus clusii (Cistaceae) [65] | epigallocatechin gallate, epicatechin, epicatechin gallate, and ascorbic acid. | Increase | Phenolics (Flavonols) | epigallocatechin gallate | Anticancer [66]; antibacterial [67] |
| Drought | Crataegus laevigata, C. monogyna (Rosaceae) [68] | chlorogenic acid, catechin, (−)-epicatechin | Increase | Phenolics | chlorogenic acid, (−)-epicatechin | Antioxidant [69][70] |
| Drought | Glycine max (Fabaceae) [71] | trigonelline | Increase | Alkaloids | trigonelline | Antidiabetic [72] |
| Drought | Hypericum brasiliense (Hypericaceae) [73] | isouliginosin B, rutin, 1,5-dihydroxyxanthone | Increase | Phenolics | isouliginosin B, rutin, | Antinociceptive [74]; Anticancer [75] |
| betulinic acid | Terpenoids | betulinic acid | Anticancer [76] | |||
| Drought | Lupinus angustifolius (Fabaceae) [77] | chinolizidin | Increase | Alkaloids | NA | NA |
| Drought | Papaver somniferum (Papaveraceae) [78] | morphine, codeine | Increase | Alkaloids | morphine, codeine | Analgesic [79][80] |
| Drought | Pinus sylvestris (Pinaceae) [81] | abietic acid | Increase | Terpenoids | abietic acid | Antiallergic [82]; anti-inflammatory [83] |
| Drought | Salvia miltiorrhiza (Lamiaceae) [29] | tanshinones, cryptotanshinone | Increase | Terpenoids | cryptotanshinone | Anticancer [84]. |
| Drought | S. miltiorrhiza [29] | rosmarinic acid | Decrease | Phenolics | rosmarinic acid | Antioxidant [85] |
| salvianolic acid | Increase | salvianolic acids | Antioxidant [86] | |||
| Drought | Scrophularia ningpoensis (Scrophulariaceae) [87] | catalpol, harpagide, aucubin, harpagoside | Increase | Glycosides | catalpol, aucubin | Hepatoprotective [88]; neuroprotective [89] |
| Ozone (O3) stress | S. lycopersicon [43][44] | α-carotene, β-carotene, violoxanthin | Increase | Terpenoids | β-carotene | Antioxidants [90]; anti-inflammatory [91] |
| isoprene, α-pinene, β-pinene, myrcene, limonene, sabinene, (E)-β-ocimene, (Z)-β-ocimene, α-humulene, (E)-β-farnesene, (E,E)-α-farnesene, (E)-β-caryophyllene, δ-cadinene | Increase | Terpenoids | α-pinene; myrcene; limonene; α-humulene. | Anti-inflammatory [92]; anti-asthmatic [93]; antioxidant [94]; anti-inflammatory [95] | ||
| O3 | Gingko biloba (Ginkgoaceae) [96] | ginkgolide A | Increase | Terpenoids | ginkgolide A | Neuroprotective [97] |
| Ultraviolet radiation-B (UV-B) | Arabidopsis thaliana (Brassicaceae) [98] | kaempferol 3-gentiobioside-7-rhamnoside; kaempferol 3,7-dirhamnoside. | Increase | Phenolics (Flavonoids) | NA | NA |
| UV-B | Brassica napus (Brassicaceae) [99] | quercetin 3-sophoroide-7-glucoside; quercetin 3-sinapyl sophoroside-7-glucoside | Increase | Phenolics (Flavonoids) | NA | NA |
| UV-B | Brassica oleracea (Brassicaceae) [100] | cyanidine glycosides; sinapyl alcohol | Increase | Phenolics (Flavoboids) | NA | NA |
| UV-B | C. roseus (Apocynaceae) [101][102] | catharanthine, vindoline | Increase | Alkaloids | catharanthine | Anticancer [103] |
| Clarkia breweri (Onagraceae) [104] | eugenol, isoeugenol, methyleugenol, and isomethyleugenol | Increase | Phenolics | eugenol | Antifungal [105]; anti-inflammatory [106] | |
| UV-B | Fagopyrum esculentum (Polygonaceae) [107] | rutin, quercetin, catechin | Increase | Phenolics | quercetin; catechin | Antioxidant [108]; anticancer and antioxidant [109][110] |
| UV-B | Gnaphalium luteoalbum (Asteraceae) [28] | calycopterin; 3’-methoxycalycopterin | Increase | Phenolics (Flavonoids) | calycopterin | Anticancer [27] |
| UV-B | G. viravira [111] | 7-O-methyl araneol | Increase | Phenolics (Flavonoids) | NA | NA |
| UV-B | Hordeum vulgare (Poaceae) [112] | saponarin; luteolin | Increase | Phenolics (Flavonoids) | saponarin; luteolin | Antihypertensive [113]; antibacterial [114] |
| UV-B | Marchantia polymorpha (Marchantiaceae) [115] | luteolin 7-glucuronide; luteolin 3,4’-di-p-coumaryl-quercetin 3-glucoside. | Increase | Phenolics (Flavonoids) | NA | NA |
| UV-B | Quercus ilex (Fagaceae) [116] | acylated kaempferol glycosides | Increase | Phenolics (Flavonoids) | kaempferol | Anticancer [117]; anti-inflammatory [118] |
| Heat stress | C. acuminata [119] | 10-hydroxycamptothecin | Increase | Alkaloids | 10-hydroxycamptothecin | Anticancer [120] |
| Heat stress | Daucus carota (Apiaceae) [121][122][123] | α-terpinolene | Decrease | Terpenoids | α-terpinolene | Antioxidant and anticancer [124] |
| α-caryophyllene, β-farnesene | Increase | NA | NA | |||
| anthocyanins, coumaric and caffeic acid; | Increase | Phenolics | p-coumaric acid and caffeic acid | Antioxidant [125][126] | ||
| Heat stress | Q. rubra (Fagaceae) [127] | isoprene (2-methyl-1,3-butadiene) | Increase | Terpenoids | NA | NA |
| Heat stress | S. lycopersicon [43][44] | β-phellandrene (dominant), 2-carene, α-phellandrene, limonene; increased emission of (E)-β-ocimene after treatment above 46 °C; β-caryophyllene. | Increase | Terpenoids | α-phellandrene; β-caryophyllene | Antifungal [128]; anticancer and anti-inflammatory [47][48] |
| α-humulene | Decrease | α-humulene | Anticancer [129] | |||
| Heat stress (increased humidity) | Centella asiatica (Apiaceae) [130] | asiaticoside | Increase | Phenolics | asiaticoside | Anti-cellulite agent [131] |
3. Conclusions
References
- Kessler, A.; Kalske, A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138.
- Sallas, L.; Luomala, E.M.; Utriainen, J.; Kainulainen, P.; Holopainen, J.K. Contrasting effects of elevated carbon dioxide concentration and temperature on rubisco activity, chlorophyll fluorescence, needle ultrastructure and secondary metabolites in conifer seedlings. Tree Physiol. 2003, 23, 97–108.
- Julkunen-Tiitto, R.; Nenadis, N.; Neugart, S.; Robson, M.; Agati, G.; Vepsäläinen, J.; Zipoli, G.; Nybakken, L.; Winkler, B.; Jansen, M.A.K. Assessing the response of plant flavonoids to UV radiation: An overview of appropriate techniques. Phytochem. Rev. 2014, 14, 273–297.
- Kaling, M.; Kanawati, B.; Ghirardo, A.; Albert, A.; Winkler, J.B.; Heller, W.; Barta, C.; Loreto, F.; Schmitt-Kopplin, P.; Schnitzler, J.P. UV-B mediated metabolic rearrangements in poplar revealed by non-targeted metabolomics. Plant. Cell Environ. 2015, 38, 892–904.
- Hošek, J.; Šmejkal, K. Flavonoids as anti-inflammatory agents. In Encyclopedia of Inflammatory Diseases; Miller, R., Ed.; Hayle Medical: New York, NY, USA, 2015; pp. 1–17.
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93.
- Yeshi, K.; Yangdon, P.; Kashyap, S.; Wangchuk, P. Antioxidant activity and the polyphenolic and flavonoid contents of five high altitude medicinal plants used in Bhutanese sowa rigpa medicine. JBAPN 2017, 7, 18–26.
- Pinto, D.M.; Blande, J.D.; Souza, S.R.; Nerg, A.M.; Holopainen, J.K. Plant volatile organic compounds (VOCs) in ozone (O3) polluted atmospheres: The ecological effects. J. Chem. Ecol. 2010, 36, 22–34.
- Hamilton, A.C. Medicinal plants, conservation and livelihoods. Biodivers. Conserv. 2004, 13, 1477–1517.
- Applequist, W.L.; Brinckmann, J.A.; Cunningham, A.B.; Hart, R.E.; Heinrich, M.; Katerere, D.R.; van Andel, T. Scientists’ warning on climate change and medicinal plants. Planta Med. 2020, 86, 10–18.
- Roy, S.K.; Roy, D.K. Use of medicinal plant and its vulnerability due to climate change in northern part of Bangladesh. Am. J. Plant. Sci. 2016, 07, 1782–1793.
- Gupta, A.; Singh, P.P.; Singh, P.; Singh, K.; Singh, A.V.; Singh, S.K.; Kumar, A. Medicinal Plants Under Climate Change: Impacts on Pharmaceutical Properties of Plants. In Climate Change and Agricultural Ecosystems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 181–209.
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. BBA-GEN SUBJECTS 2013, 1830, 3670–3695.
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 12, 539–573.
- Melidou, M.; Riganakos, K.; Galaris, D. Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: The role of iron chelation. Free Radic. Biol. Med. 2005, 39, 1591–1600.
- Selway, J.T. Antiviral activity of flavones and flavans. Prog Clin. Biol. Res. 1986, 213, 521–536.
- Piao, M.J.; Kim, K.C.; Chae, S.; Keum, Y.S.; Kim, H.S.; Hyun, J.W. Protective effect of fisetin (3,7,3’,4’-tetrahydroxyflavone) against gamma-irradiation-induced oxidative stress and cell damage. Biomol. Ther. 2013, 21, 210–215.
- Maher, P. A comparison of the neurotrophic activities of the flavonoid fisetin and some of its derivatives. Free Radic. Res. 2006, 40, 1105–1111.
- Maher, P. The flavonoid fisetin promotes nerve cell survival from trophic factor withdrawal by enhancement of proteasome activity. Arch. Biochem. Biophy. 2008, 476, 139–144.
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434.
- Sung, B.; Pandey, M.K.; Aggarwal, B.B. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-κB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IκBα kinase activation. Mol. Pharmacol. 2007, 71, 1703–1714.
- Hussain, T.; Al-Attas, O.S.; Alamery, S.; Ahmed, M.; Odeibat, H.A.M.; Alrokayan, S. The plant flavonoid, fisetin alleviates cigarette smoke-induced oxidative stress, and inflammation in Wistar rat lungs. J. Food Biochem. 2019, 43, 1–11.
- Choi, I.S.; Choi, E.Y.; Jin, J.Y.; Park, H.R.; Choi, J.I.; Kim, S.J. Kaempferol inhibits P. intermedia lipopolysaccharide-induced production of nitric oxide through translational regulation in murine macrophages: Critical role of heme oxygenase-1-mediated ROS reduction. J. Periodontol. 2013, 84, 545–555.
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107.
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A review on the dietary flavonoid kaempferol. Mini. Rev. Med. Chem. 2011, 11, 298–344.
- Kalantari, H.; Das, D.K. Physiological effects of resveratrol. Biofactors 2010, 36, 401–406.
- Faham, N.; Javidnia, K.; Bahmani, M.; Amirghofran, Z. Calycopterin, an immunoinhibitory compound from the extract of Dracocephalum kotschyi. Phytother. Res. 2008, 22, 1154–1158.
- Cuadra, P.; Harborne, J.B.; Waterman, P.G. Increases in surface flavonols and photosynthetic pigments in Gnaphalium luteo-album in response to UV-B radiation. Phytochemistry 1997, 45, 1377–1383.
- Liu, H.; Wang, X.; Wang, D.; Zou, Z.; Liang, Z. Effect of drought stress on growth and ccumulation of active constituents in Salvia miltiorrhiza Bunge. Ind. Crops. Prod. 2011, 33, 84–88.
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, critical pharmacological components in Salvia miltiorhiza. Front. Pharmacol. 2019, 10, 1–14.
- Oliviera, A.B.; Dolabela, M.F.; Braga, F.C.; Jacome, R.L.R.P.; Varotti, F.P.; Povoa, M.M. Plant-derived antimalarial agents: New leads and efficient phytomedicines. Part I. Alkaloids. An. Acad. Bras. Cienc. 2009, 81, 715–740.
- Levin, D.A. Alkaloids bearing plants: An ecogeographic perspective. Am. Nat. 1976, 110, 261–284.
- Binder, B.Y.K.; Peebles, C.A.M.; Shanks, J.V.; San, K.-Y. The effects of UV-B stress on the production of terpenoid indole alkaloids in Catharanthus roseus hairy roots. Biotechnol. Prog. 2009, 25, 861–865.
- Wilson, L.; Creswell, K.M.; Chin, D. The mechanism of action of vinblastine. Binding of vinblastine to embryonic chick brain tubulin and tubulin from sea urchin sperm tail outer doublet microtubules. Biochemistry 1975, 14, 5586–5592.
- Goboza, M.; Aboua, Y.G.; Chegou, N.; Oguntibeju, O.O. Vindoline effectively ameliorated diabetes-induced hepatotoxicity by docking oxidative stress, inflammation and hypertriglyceridemia in type 2 diabetes-induced male Wistar rats. Biomed. Pharmacother 2019, 112, 1–11.
- Zi, J.; Mafu, S.; Peters, R.J. To gibberellins and beyond! surveying the evolution of (di) terpenoid metabolism. Annu. Rev. Plant Biol. 2014, 65, 259–286.
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural inhibitors of NF-kB signaling with anti-inflammatory and anticancer potential Cell Mol. Life Sci. 2008, 65, 2979–2999.
- Geu-Flores, F.; Sherden, N.H.; Courdavault, V.; Burlat, V.; Glenn, W.S.; Wu, C.; Nims, E.; Cui, Y.; O’Connor, S.E. An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature 2012, 492, 138–142.
- Dutta, A.; Sen, J.; Deswal, R. Downregulation of terpenoid indole alkaloid biosynthetic pathway by low temperature and cloning of a AP2 type C-repeat binding factor (CBF) from Catharanthus roseus (L). G. Don. Plant Cell Rep. 2007, 26, 1869–1878.
- Janas, K.M.; Cvikrová, M.; Pałagiewicz, A.; Szafranska, K.; Posmyk, M.M. Constitutive elevated accumulation of phenylpropanoids in soybean roots at low temperature. Plant Sci. 2002, 163, 369–373.
- Ono, M.; Takeshima, M.; Nishi, A.; Higuchi, T.; Nakano, S. Genistein suppresses v-Src-driven proliferative activity by arresting the cell-cycle at G2/M through increasing p21 level in Src-activated human gallbladder carcinoma cells. Nutr. Cancer 2020, 1–9.
- Choi, E.J.; Kim, G.H. Antiproliferative activity of daidzein and genistein may be related to ERalpha/c-erbB-2 expression in human breast cancer cells. Mol. Med. Rep. 2013, 7, 781–784.
- Vickers, C.E.; Gershenzon, J.; Lerdau, M.T.; Loreto, F. A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 2009, 5, 283–291.
- Copolovici, L.; Kannaste, A.; Pazouki, L.; Niinemets, U. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. J. Plant. Physiol. 2012, 169, 664–672.
- Kubo, A.; Lunde, C.S.; Kubo, I. Indole and (E)-2-hexenal, phytochemical potentiators of polymyxins against Pseudomonas aeruginosa and Escherichia coli. Antimicrob. Agents Chemother. 1996, 40, 1438–1441.
- Wang, X.-S.; Yang, W.; Tao, S.-J.; Li, K.; Li, M.; Dong, J.-H.; Wang, M.-W. The effect of delta-elemene on hela cell lines by apoptosis induction. Yakugaku Zasshi 2006, 126, 979–990.
- Legault, J.; Pichette, A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647.
- Dahham, S.S.; Tabana, Y.M.; Khadeer Ahamed, M.B.; Abdul Majid, A.M.S. In vivo anti-inflammatory activity of β-caryophyllene, evaluated by molecular imaging. Molecules Med. Chem. 2016, 162, 108–117.
- Christie, P.J.; Alfenito, M.R.; Walbot, V. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: Enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 1994, 194, 541–549.
- Ku, S.K.; Yoon, E.K.; Lee, W.; Kwon, S.; Lee, T.; Bae, J.S. Antithrombotic and antiplatelet activities of pelargonidin in vivo and in vitro. Arch. Pharm. Res. 2016, 39, 398–408.
- Jeon, J.; Kim, J.K.; Wu, Q.; Park, S.U. Effects of cold stress on transcripts and metabolites in tartary buckwheat (Fagopyrum tataricum). Environ. Exp. Bot. 2018, 155, 488–496.
- Khoo, H.E.; Azlan, A.; Nurulhuda, M.H.; Ismail, A.; Abas, F.; Hamid, M.; Roowi, S. Antioxidative and cardioprotective properties of anthocyanins from defatted dabai extracts. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–13.
- Mir, B.A.; Mir, S.A.; Khazir, J.; Tonfack, L.B.; Cowan, D.A.; Vyas, D.; Koul, S. Cold stress affects antioxidative response and accumulation of medicinally important withanolides in Withania somnifera (L.) Dunal. Ind. Crops. Prod. 2015, 74, 1008–1016.
- Akhoon, B.A.; Pandey, S.; Tiwari, S.; Pandey, R. Withanolide A offers neuroprotection, ameliorates stress resistance and prolongs the life expectancy of Caenorhabditis elegans. Exp. Gerontol. 2016, 78, 47–56.
- Hahm, E.R.; Moura, M.B.; Kelley, E.E.; Van Houten, B.; Shiva, S.; Singh, S.V. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS ONE 2011, 6, 1–12.
- Zhao, M.; Zhang, N.; Gao, T.; Jin, J.; Jing, T.; Wang, J.; Wu, Y.; Wan, X.; Schwab, W.; Song, C. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytol. 2020, 226, 362–372.
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant. Biol. 2018, 18, 1–15.
- Akojie, F.O.; Fung, L.W. Antisickling activity of hydroxybenzoic acids in Cajanus cajan. Planta Med. 1992, 58, 317–320.
- Hernandez, I.; Alegre, L.; Munne-Bosch, S. Enhanced oxidation of flavan-3-ols and proanthocyanidin accumulation in water-stressed tea plants. Phytochemistry 2006, 67, 1120–1126.
- Anitha, S.; Krishnan, S.; Senthilkumar, K.; Sasirekha, V. Theoretical investigation on the structure and antioxidant activity of (+) catechin and (−) epicatechin—A comparative study. Mol. Phys. 2020, 118, 1–12.
- Liu, Z. Drought-induced in vivo synthesis of camptothecin in Camptotheca acuminata seedlings. Physiol. Plant 2000, 110, 483–488.
- Adams, D.J.; Dewhirst, M.W.; Flowers, J.L.; Gamcsik, M.P.; Colvin, O.M.; Manikumar, G.; Wani, M.C.; Wall, M.E. Camptothecin analogues with enhanced antitumor activity at acidic pH. Cancer Chemother. Pharmacol. 2000, 46, 263–271.
- Liu, Y.; Meng, Q.; Duan, X.; Zhang, Z.; Li, D. Effects of PEG-induced drought stress on regulation of indole alkaloid biosynthesis in Catharanthus roseus. J. Plant Interact. 2017, 12, 87–91.
- Zhou, X.; Xu, Z.; Li, A.; Zhang, Z.; Xu, S. Double-sides sticking mechanism of vinblastine interacting with alpha, beta-tubulin to get activity against cancer cells. J. Biomol. Struct. Dyn. 2019, 37, 4080–4091.
- Hernandez, I.; Alegre, L.; Munne-Bosch, S. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions. Tree Physiol. 2004, 24, 1303–1311.
- Rao, S.D.; Pagidas, K. Epigallocatechin-3-gallate, a natural polyphenol, inhibits cell proliferation and induces apoptosis in human ovarian cancer Cclls. Anticancer Res. 2010, 30, 2519–2524.
- Falcinelli, S.D.; Shi, M.C.; Friedlander, A.M.; Chua, J. Green tea and epigallocatechin-3-gallate are bactericidal against Bacillus anthracis. FEMS Microbiol. Lett. 2017, 364, 1–8.
- Kirakosyan, A.; Kaufman, P.; Warber, S.; Zick, S.; Aaronson, K.; Bolling, S.; Chul Chang, S. Applied environmental stresses to enhance the levels of polyphenolics in leaves of hawthorn plants. Physiol. Plant 2004, 121, 182–186.
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138.
- Xu, J.Z.; Yeung, S.Y.; Chang, Q.; Huang, Y.; Chen, Z.Y. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr. 2004, 91, 873–881.
- Cho, Y.; Njiti, V.N.; Lightfoot, D.A.; Wood, A.J. Trigonelline concentration in field-grown soybean in response to irrigation. Biol. Plant 2003, 46, 405–410.
- Yoshinari, O.; Sato, H.; Igarashi, K. Anti-diabetic effects of pumpkin and its components, trigonelline and nicotinic acid, on Goto-Kakizaki rats. Biosci. Biotechnol. Biochem. 2009, 73, 1033–1041.
- Nacif de Abreu, I.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248.
- Bridi, H.; Dischkaln Stolz, E.; Maikon Corrêa de Barros, F.; Elingson da Silva Costa, B.; Guerini, L.; Maris Kuze Rates, S.; Lino von Poser, G. Antinociceptive activity of phloroglucinol derivatives isolated from southern Brazilian Hypericum species. Chem. Biodivers 2018, 15, 66–87.
- Lin, J.P.; Yang, J.S.; Lin, J.J.; Lai, K.C.; Lu, H.F.; Ma, C.Y.; Sai-Chuen Wu, R.; Wu, K.C.; Chueh, F.S.; Gibson Wood, W.; et al. Rutin inhibits human leukemia tumor growth in a murine xenograft model in vivo. Environ. Toxicol. 2012, 27, 480–484.
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines but not on normal cells. Cancer Lett. 2002, 175, 17–25.
- Christiansen, J.L.; Jornsgard, B.; Buskov, S.; Oslen, C.E. Effect of drought stress on content and composition of seed alkaloids in narrow-leafed lupin, Lupinus angustifolius L. Eur. J. Agron. 1997, 7, 307–314.
- Szabó, B.; Tyihák, E.; Szabó, G.; Botz, L. Mycotoxin and drought stress induced change of alkaloid content of Papaver somniferum plantlets. Acta. Bot. Hung. 2003, 45, 409–417.
- Stein, C.; Comisel, K.; Haimerl, E.; Yassouridis, A.; Lehrberger, K.; Herz, A.; Peter, K. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N. Engl. J. Med. 1991, 325, 1123–1126.
- Clark, E.; Plint, A.C.; Correll, R.; Gaboury, I.; Passi, B. A randomized, controlled trial of acetaminophen, ibuprofen, and codeine for acute pain relief in children with musculoskeletal trauma. Pediatrics 2007, 119, 460–467.
- Turtola, S.; Manninen, A.M.; Rikala, R.; Kainulainen, P. Drought stress alters the concentration of wood terpenoids in Scots pine and Norway spruce seedlings. J. Chem. Ecol. 2003, 29, 1981–1995.
- Ulusu, N.N.; Ercil, D.; Sakar, M.K.; Tezcan, E.F. Abietic acid inhibits lipoxygenase activity. Phytother Res. 2002, 16, 88–90.
- Fernandez, M.A.; Tornos, M.P.; GarcIa, M.D.; de las Heras, B.; Villar, A.M.; Saenz, M.T. Anti-inflammatory activity of abietic acid, a diterpene isolated from Pimenta racemosa var. grissea. J. Pharm. Pharmacol. 2001, 53, 867–872.
- Chen, W.; Lu, Y.; Chen, G.; Huang, S. Molecular evidence of cryptotanshinone for treatment and prevention of human cancer. Anticancer Agents Med. Chem. 2013, 13, 979–987.
- Adomako-Bonsu, A.G.; Chan, S.L.F.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. In Vitro 2017, 40, 248–255.
- Sun, Y.; Zhu, H.; Wang, J.; Liu, Z.; Bi, J. Isolation and purification of salvianolic acid A and salvianolic acid B from Salvia miltiorrhiza by high-speed counter-current chromatography and comparison of their antioxidant activity. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 733–737.
- Wang, D.H.; Du, F.; Liu, H.Y.; Liang, Z.S. Drought stress increases iridoid glycosides biosynthesis in the roots of Scrophularia ningpoensis seedlings. J. Med. Plant. Res. 2010, 4, 2691–2699.
- Liu, L.; Cao, X.; Li, T.; Li, X. Effects of catalpol on the activity of human liver cytochrome P450 enzymes. Xenobiotica 2019, 49, 1289–1295.
- Song, M.; Han, M.; Kim Kwon, Y. Effect of aucubin on neural precursor cell survival during neuronal differentiation. Int. J. Neurosci. 2018, 128, 899–905.
- Marquardt, D.; Williams, J.A.; Kucerka, N.; Atkinson, J.; Wassall, S.R.; Katsaras, J.; Harroun, T.A. Tocopherol activity correlates with its location in a membrane: A new perspective on the antioxidant vitamin E. J. Am. Chem Soc. 2013, 135, 7523–7533.
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory activity of beta-carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo 2018, 32, 255–264.
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-alpha-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269.
- Du, Y.; Luan, J.; Jiang, R.P.; Liu, J.; Ma, Y. Myrcene exerts anti-asthmatic activity in neonatal rats via modulating the matrix remodeling. Int. J. Immunopathol. Pharmacol. 2020, 34, 1–10.
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic. Clin. Pharmacol. Toxicol. 2010, 106, 38–44.
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236.
- He, X.; Huang, W.; Chen, W.; Dong, T.; Liu, C.; Chen, Z.; Xu, S.; Ruan, Y. Changes of main secondary metabolites in leaves of Ginkgo biloba in response to ozone fumigation. J. Environ. Sci. 2009, 21, 199–203.
- Feng, Z.; Sun, Q.; Chen, W.; Bai, Y.; Hu, D.; Xie, X. The neuroprotective mechanisms of ginkgolides and bilobalide in cerebral ischemic injury: A literature review. Mol. Med. 2019, 25, 1–8.
- Ormrod, D.P.; Landry, L.G.; Coclin, P.L. Short-term UV-B radiation and ozone exposure effects on aromatic secondary metabolite accumulation and shoot growth of flavonoid-deficient Arabidopsis mutants. Physiol. Plant 1995, 93, 602–610.
- Olsson, L.C.; Veit, M.; Weissenbock, G.; Bornman, J.F. Flavonoid response to UV-B radiation in Brassica napus. Phytochemistry 1998, 49, 1021–1028.
- Gitz, D.C.; Liu, L.; McClure, J.W. Phenolic metabolism, growth and UV-B tolerance in phenylalanine ammonia lyase inhibited red cabbage seedlings. Phytochemistry 1998, 49, 377–386.
- Ramani, S.; Jayabaskaran, C. Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. J. Mol. Signal. 2008, 3, 1–6.
- Ramani, S.; Chelliah, J. UV-B-induced signaling events leading to enhanced-production of catharanthine in Catharanthus roseus cell suspension cultures. BMC Plant. Biol. 2007, 7, 1–17.
- Siddiqui, M.J.; Ismail, Z.; Aisha, A.F.A.; Abdul Majid, A.M.S. Cytotoxic activity of Catharanthus roseus (Apocynaceae) crude extracts and pure compounds against human colorectal carcinoma cell line. Int. J. Pharmacol. 2010, 6, 43–47.
- Wang, J.; Dudareva, N.; Bhakta, S.; Raguso, R.A.; Pichersky, E. Floral scent production in Clarkia breweri (Onagraceae). II. Localization and developmental modulation of the enzyme SAM:(Iso)eugenol O-methyltransferase and phenylpropanoid emission. Plant. Physiol. 1997, 114, 213–221.
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M. Antifungal activity of eugenol against Penicillium, Aspergillus, and Fusarium species. J. Food Prot. 2010, 73, 1124–1128.
- Lee, Y.Y.; Hung, S.L.; Pai, S.F.; Lee, Y.H.; Yang, S.F. Eugenol suppressed the expression of lipopolysaccharide-induced proinflammatory mediators in human macrophages. J. Endod. 2007, 33, 698–702.
- Regvar, M.; Bukovnik, U.; Likar, M.; Kreft, I. UV-B radiation affects flavonoids and fungal colonisation in Fagopyrum esculentum and F. tataricum. Open Life Sci. 2012, 7, 275–283.
- Zheng, Y.Z.; Deng, G.; Liang, Q.; Chen, D.F.; Guo, R.; Lai, R.C. Antioxidant activity of quercetin and its glucosides from Propolis: A theoretical study. Sci. Rep. 2017, 7, 1–11.
- Sun, H.; Yin, M.; Hao, D.; Shen, Y. Anti-Cancer Activity of Catechin against A549 Lung Carcinoma Cells by Induction of Cyclin Kinase Inhibitor p21 and Suppression of Cyclin E1 and P–AKT. Appl. Sci. 2020, 10, 2065.
- Simos, Y.V.; Verginadis, I.I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox. Rep. 2012, 17, 181–186.
- Cuadra, P.; Harborne, J.B. Changes in epicuticular flavonoids and photosynthetic pigments as a plant response to UV-B radiation. Z. Naturforsch. 1996, 51c, 671–680.
- Reuber, S.; Bornman, J.F.; Weissenbock, G. A flavonoid mutant of barley exhibits increased sensitivity to UV-B radiation in primary leaves. Plant Cell Environ. 1996, 19, 593–599.
- Ra, J.-E.; Woo, S.-Y.; Jin, H.; Lee, M.J.; Kim, H.Y.; Ham, H.; Chung, I.-M.; Seo, W.D. Evaluation of antihypertensive polyphenols of barley (Hordeum vulgare L.) seedlings via their effects on angiotensin-converting enzyme (ACE) inhibition. Appl. Biol. Chem. 2020, 63, 1–9.
- Guo, Y.; Liu, Y.; Zhang, Z.; Chen, M.; Zhang, D.; Tian, C.; Liu, M.; Jiang, G. The antibacterial activity and mechanism of action of luteolin against Trueperella pyogenes. Infect. Drug Resist. 2020, 13, 1697–1711.
- Markham, K.R.; Ryan, K.G.; Bloor, S.J.; Mitchell, K.A. An increase in the luteolin-apigenin ratio in Marchantia polymorpha on UV-B enhancement. Phytochemistry 1998, 48, 791–794.
- Skaltsa, H.; Verykokidou, E.; Harvala, C.; Karabourniotis, G.; Manetas, Y. UV-B protective potential and flavonoid content of leaf hairs in Quercus ilex. Phytochemistry 1994, 37, 987–990.
- Lee, K.M.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Heo, Y.S.; Bode, A.M.; Lubet, R.A.; Lee, H.J.; Dong, Z. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem. Pharmacol. 2010, 80, 2042–2049.
- Kadioglu, O.; Nass, J.; Saeed, M.E.M.; Schuler, B.; Efferth, T. Kaempferol is an anti-inflammatory compound with activity towards NF-κB pathway proteins. Anticancer Res. 2015, 35, 2645–2650.
- Zu, Y.G.; Tang, Z.H.; Yu, J.H.; Liu, S.G.; Wang, W.; Guo, X.R. Different responses of camptothecin and 10-hydroxycamptothecin to heat shock in Camptotheca acuminata seedlings. Acta. Bot. Sin. 2003, 45, 809–814.
- Ping, Y.-H.; Lee, H.-C.; Lee, J.-Y.; Wu, P.-H.; Ho, L.-K.; Chi, C.-W.; Lu, M.-F.; Wang, J.-J. Anticancer effects of low-dose 10-hydroxycamptothecin in human colon cancer. Oncol. Rep. 2006, 15, 1273–1279.
- Rosenfeld, H.J.; Aaby, K.; Lea, P. Influence of temperature and plant density on sensory quality and volatile terpenoids of carrot (Daucus carota L.) root. J. Sci. Food Agric. 2002, 82, 1384–1390.
- Helmig, D.; Ortega, J.; Duhl, T.; Tanner, D.; Guenther, A.; Harley, P.; Wiedinmyer, C.; Milford, J.; Sakulyanontvittaya, T. Sesquiterpene emissions from pine trees-identifications, emission rates and flux estimates for the contiguous United States. Environ. Sci. Technol. 2007, 41, 1545–1553.
- Commisso, M.; Toffali, K.; Strazzer, P.; Stocchero, M.; Ceoldo, S.; Baldan, B.; Levi, M.; Guzzo, F. Impact of phenylpropanoid compounds on heat stress tolerance in carrot cell cultures. Front. Plant Sci. 2016, 7, 1–16.
- Aydin, E.; Turkez, H.; Tasdemir, S. Anticancer and antioxidant properties of terpinolene in rat brain cells. Arh. Hig. Rada. Toksikol. 2013, 64, 415–424.
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587.
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Correa, M.A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim Acta A Mol. Biomol. Spectrosc. 2019, 219, 358–366.
- Hanson, D.T.; Sharkey, T.D. Effect of growth conditions on isoprene emission and other thermotolerance-enhancing compounds. Plant Cell Environ. 2001, 24, 929–936.
- Zhang, J.H.; Sun, H.L.; Chen, S.Y.; Zeng, L.; Wang, T.T. Anti-fungal activity, mechanism studies on alpha-phellandrene and nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 1–9.
- Hadri, A.; Gomez del Rio, M.A.; Sanz, J.; Coloma, A.G.; Idaomar, M.; Ozonas, B.R.; Gonzalez, J.B.; Reus, M.I.S. Cytotoxic activity of α-humulene and trans-caryophyllene from Salvia officinalis in animal and human tumor cells. An. R. Acad. Nac. Farm. 2010, 76, 343–356.
- Randriamampionona, D.; Diallo, B.; Rakotoniriana, F.; Rabemanantsoa, C.; Cheuk, K.; Corbisier, A.M.; Jaziri, M. Comparative analysis of active constituents in Centella asiatica samples from Madagascar: Application for ex situ conservation and clonal propagation. Fitoterapia 2007, 78, 482–489.
- Sondari, D.; Harmami, S.B.; Ghozali, M.; Randy, A.; Amanda, A.; Irawan, Y. Determination of the active asiaticoside content in Centella asiatica as anti-cellulite agent. Indones J. Cancer Chemoprev. 2011, 2, 222–227.




