Plant secondary metabolites (PSMs) are vital for human health and constitute the skeletal framework of many pharmaceutical drugs. Indeed, more than 25% of the existing drugs belong to PSMs. One of the continuing challenges for drug discovery and pharmaceutical industries is gaining access to natural products, including medicinal plants. This bottleneck is heightened for endangered species prohibited for large sample collection, even if they show biological hits. While cultivating the pharmaceutically interesting plant species may be a solution, it is not always possible to grow the organism outside its natural habitat. Plants affected by abiotic stress present a potential alternative source for drug discovery. In order to overcome abiotic environmental stressors, plants may mount a defense response by producing a diversity of PSMs to avoid cells and tissue damage. Plants either synthesize new chemicals or increase the concentration (in most instances) of existing chemicals, including the prominent bioactive lead compounds morphine, camptothecin, catharanthine, epicatechin-3-gallate (EGCG), quercetin, resveratrol, and kaempferol. Most PSMs produced under various abiotic stress conditions are plant defense chemicals and are functionally anti-inflammatory and antioxidative. The major PSM groups are terpenoids, followed by alkaloids and phenolic compounds.
- secondary metabolites
- climate change
- drug discovery
- abiotic stress
1. Introduction
2. Reported Pharmacological Properties of PSMs Present in Plants Affected by Ex Situ Abiotic Stresses
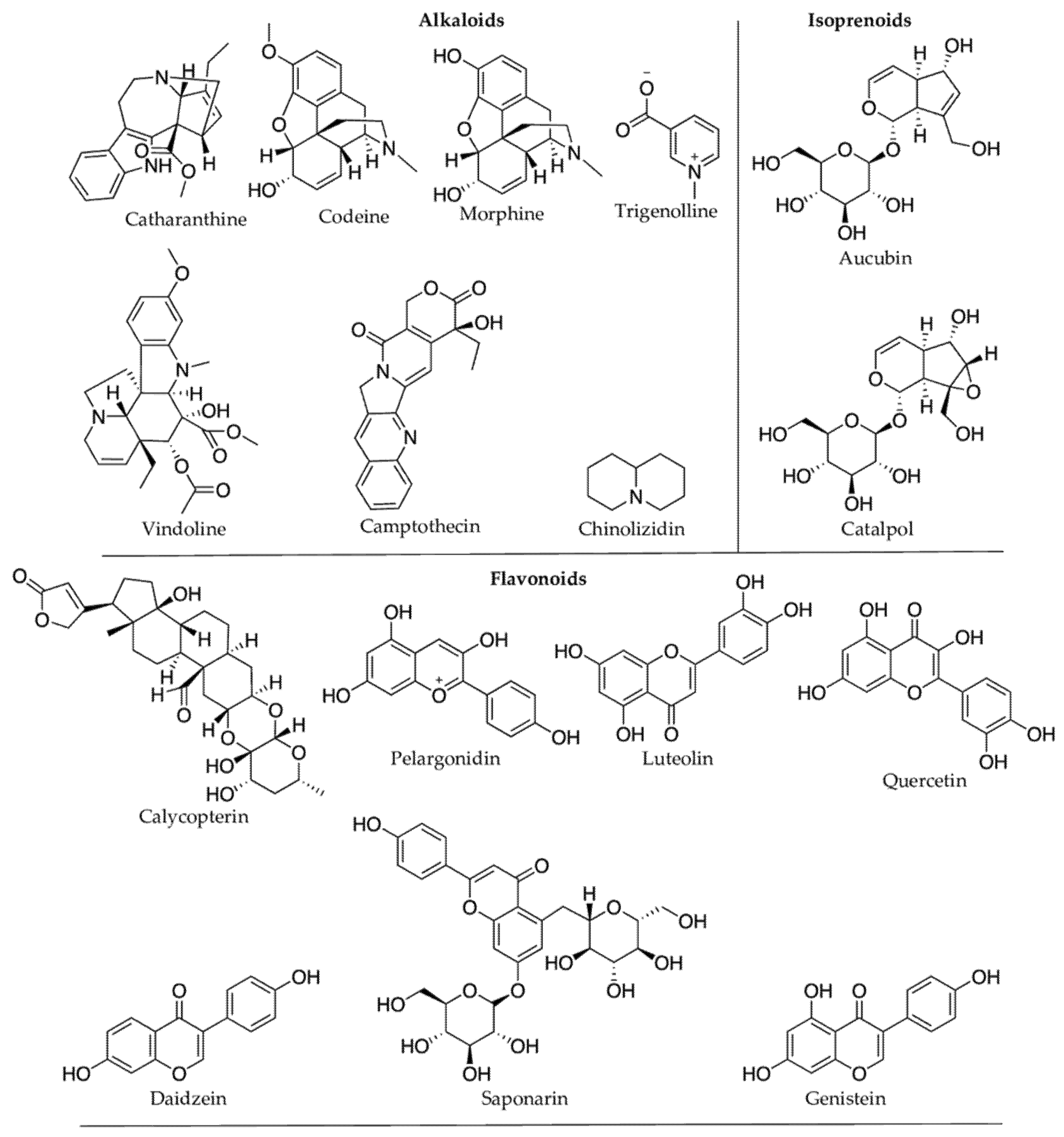
Plant protective secondary metabolites are diverse in structure and biological properties, and they have been continuously exploited for pharmaceutical, nutraceutical, and cosmetic uses [13][251] (Figure 17). Flavonoids and other phenolic compounds are predominant among secondary metabolites produced in response to climatic/or abiotic stress (Table 1). Flavonoids confer protection against inflammation, allergy, and bacterial infections [14][252]. Flavonols (or 3-hydroxy flavones), one of the main subclass of flavonoids, are apparent antioxidants in stressed plants, and they are known to prevent nuclear DNA damage by free radicals like H2O2 [15][253]. Flavonols are polyaromatic secondary metabolites with three rings, and many of them are bioactive. Many flavonoids possess antiviral properties. For instance, the hydroxy (OH) group in the ring-C of flavonols makes them more effective against herpes simplex virus type I than flavones [16][254]. Fisetin is another example of an active flavonoid produced by plants under oxidative stress, preventing membrane lipid peroxidation, DNA damage, and protein carbonylation [17][247]. Fisetin showed numerous biological activities such as protection against cell death from oxidative stress, growth, and maintenance of nerve cells (primary cortical neurons from a rat) [18][19][248,255]. Fisetin suppresses many inflammatory pathways, including Nuclear Factor-kappa B (NF-kB) pathway, helping prevent cancerous growth [20][21][256,257]. Similarly, Hussain et al. [22][258] also observed the protective effect of fisetin against smoke-induced oxidative stress and inflammation in rat lungs. Plant UV filters, kaempferol, and quercetin are a few other examples of bioactive flavonoids. Kaempferol is an anti-inflammatory [23][259], chemo-protective [24][260], and cardio-protective [25][261]. Polyphenolic resveratrol is one of the essential stilbene phytoalexin produced by a plant’s defense mechanism, and it possesses antioxidant, anticancer, and anti-estrogenic properties [26][262]. The immunoinhibitory compound, calycopterin isolated from the medicinal plant Dracocephalum kotschyi [27][168], was elevated upon UV irradiation in Gnaphalium luteo-album [28][167]. Tanshinones are other examples of bioactive phenols. In response to severe drought stress, their concentration in the Salvia miltiorrhiza increases, including tanshinone I and tanshinone IIA by 182% and 322%, respectively, compared to 148% under the moderate drought stress [29][139]. Tanshinones are known for their anti-inflammatory, antioxidant, and anticancer properties [30][263].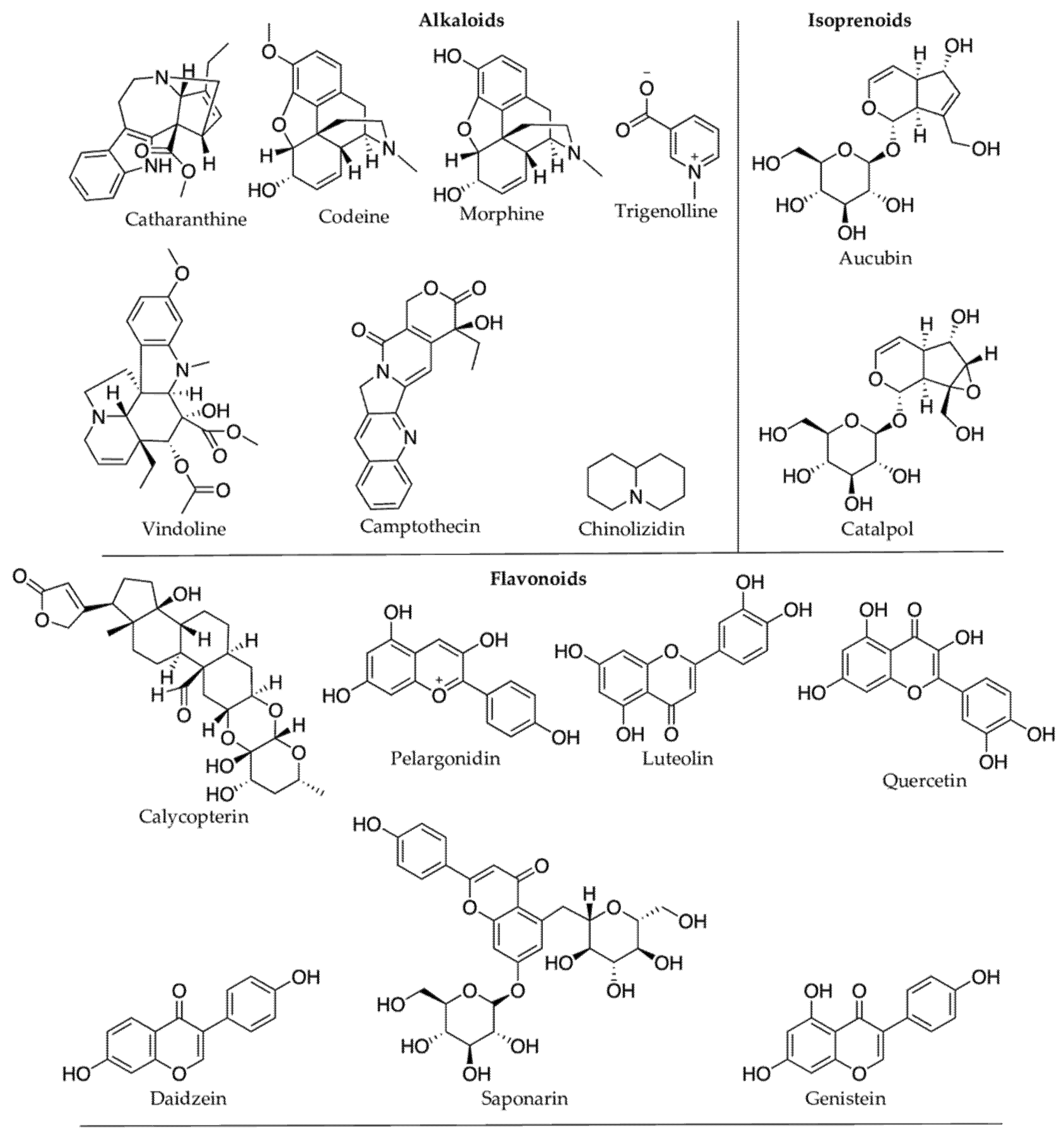
| Stress Condition(s) | Plant Species (Family) | PSMs Produced | Effects on PSMs Concentration | Compound Class | Bioactive Compounds | Reported Pharmacological Properties | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cold stress | Catharanthus roseus (Apocynaceae) [39] | (Apocynaceae) [98] | vindoline | Decrease | Alkaloids | vindoline | Antidiabetic [35] | Antidiabetic [99] | |||||||||
| Cold stress | Glycine max (Fabaceae) [40] | (Fabaceae) [94] | genistein, daidzein | Increase | Phenolics | genistein, daidzein | Antiproliferative [41][42] | Antiproliferative [95,96] | |||||||||
| Cold stress | Solanum lycopersicon (Solanaceae) [43][44] | (Solanaceae) [87,97] | ( | Z | )-3-hexenol and ( | E | )-2-hexenal (dominant); 1-hexanol and 1,4-hexadienal (smaller quantities) | Increase | Fatty Acyls | ( | E | )-2-hexenal | Antibacterial [45] | Antibacterial [100] | |||
| Cold stress | β-phellandrene, ( | E | )-β-ocimene | Increase | Terpenoids | NA | NA | ||||||||||
| Cold stress | δ-elemene, α-humulene and β-caryophyllene (dominant); in severe cold: β-elemene is produced. | Increase | Terpenoids | δ-elemene, α-humulene and β-caryophyllene | Antiproliferative [46]; anticancer [47]; anti-inflammatory [48] | Antiproliferative [101]; anticancer [102]; anti-inflammatory [103] | |||||||||||
| Cold stress | Zea mays (Poaceae) [49] | (Poaceae) [104] | pelargonidin | Increase | Phenolics | pelargonidin | Antithrombotic [50] | Antithrombotic [105] | |||||||||
| Cold stress | Fagopyrum tartaricum (Polygonaceae) [51] | (Polygonaceae) [106] | anthocyanins (e.g.,3- | O | -galactosides) and anthocyanidins (e.g., malvidin) | Increase | Phenolics | anthocyanins | Antioxidant [52] | Antioxidant [107] | |||||||
| Cold stress | Withania somnifera (Solanaceae) [53] | (Solanaceae) [108] | withanolide A, withaferin A | Increase | Terpenoids | withanolide A; withferin A | Neuroprotective [54]; anticancer [55] | Neuroprotective [109]; anticancer [110] | |||||||||
| Cold stress | Camellia sinensis (Theaceae) [56] | (Theaceae) [111] | nerolidol glucoside | Increase | Terpenoids | NA | NA | ||||||||||
| Drought | Amaranthus tricolor (Amaranthaceae) [57] | (Amaranthaceae) [112] | hydroxybenzoic acids (gallic acid, vanillic acid, syringic acid, | p | -hydroxybenzoic acid, salicylic acid, ellagic acid), hydroxycinnamic acids (caffeic acid, chlorogenic acid, | p | -coumaric acid, ferulic acid, | m | -coumaric acid, sinapic acid, | trans | -cinnamic acid), flavonoids (iso-quercetin, hyperoside, rutin). | Increase | Phenolics (Flavonoids) | p | -hydroxybenzoic acid | Antisickling activity [58] | Antisickling activity [113] |
| Drought | Camellia sinensis (Theaceae) [59] | (Theaceae) [114] | Epicatechins | Increase | Phenolics (Flavonoids) | epicatechins | Antioxidant [60] | Antioxidant [115] | |||||||||
| Drought | Camptotheca acuminata (Nyssaceae) [61] | (Nyssaceae) [116] | camptothecin | Increase | Alkaloids | camptothecin | Antitumour [62] | Antitumour [117] | |||||||||
| Drought (PEG-induced) | Catharanthus roseus (Apocyanaceae) [63] | (Apocyanaceae) [118] | vinblastine | Increase | Alkaloids | vinblastine | Anticancer [64] | Anticancer [119] | |||||||||
| Drought | Cistus clusii (Cistaceae) [65] | (Cistaceae) [120] | epigallocatechin gallate, epicatechin, epicatechin gallate, and ascorbic acid. | Increase | Phenolics (Flavonols) | epigallocatechin gallate | Anticancer [66]; antibacterial [67] | Anticancer [121]; antibacterial [122] | |||||||||
| Drought | Crataegus laevigata | , | C. monogyna (Rosaceae) [68] | (Rosaceae) [123] | chlorogenic acid, catechin, (−)-epicatechin | Increase | Phenolics | chlorogenic acid, (−)-epicatechin | Antioxidant [69][70] | Antioxidant [124,125] | |||||||
| Drought | Glycine max (Fabaceae) [71] | (Fabaceae) [126] | trigonelline | Increase | Alkaloids | trigonelline | Antidiabetic [72] | Antidiabetic [127] | |||||||||
| Drought | Hypericum brasiliense (Hypericaceae) [73] | (Hypericaceae) [128] | isouliginosin B, rutin, 1,5-dihydroxyxanthone | Increase | Phenolics | isouliginosin B, rutin, | Antinociceptive [74]; Anticancer [75] | Antinociceptive [129]; Anticancer [130] | |||||||||
| betulinic acid | Terpenoids | betulinic acid | Anticancer [76] | Anticancer [131] | |||||||||||||
| Drought | Lupinus angustifolius (Fabaceae) [77] | (Fabaceae) [132] | chinolizidin | Increase | Alkaloids | NA | NA | ||||||||||
| Drought | Papaver somniferum (Papaveraceae) [78] | (Papaveraceae) [133] | morphine, codeine | Increase | Alkaloids | morphine, codeine | Analgesic [79][80] | Analgesic [134,135] | |||||||||
| Drought | Pinus sylvestris (Pinaceae) [81] | (Pinaceae) [136] | abietic acid | Increase | Terpenoids | abietic acid | Antiallergic [82]; anti-inflammatory [83] | Antiallergic [137]; anti-inflammatory [138] | |||||||||
| Drought | Salvia miltiorrhiza (Lamiaceae) [29] | (Lamiaceae) [139] | tanshinones, cryptotanshinone | Increase | Terpenoids | cryptotanshinone | Anticancer [84]. | Anticancer [140]. | |||||||||
| Drought | S. miltiorrhiza [29] | [139] | rosmarinic acid | Decrease | Phenolics | rosmarinic acid | Antioxidant [85] | Antioxidant [141] | |||||||||
| salvianolic acid | Increase | salvianolic acids | Antioxidant [86] | Antioxidant [142] | |||||||||||||
| Drought | Scrophularia ningpoensis (Scrophulariaceae) [87] | (Scrophulariaceae) [143] | catalpol, harpagide, aucubin, harpagoside | Increase | Glycosides | catalpol, aucubin | Hepatoprotective [88]; neuroprotective [89] | Hepatoprotective [144]; neuroprotective [145] | |||||||||
| Ozone (O | 3 | ) stress | S. lycopersicon [43][44] | [87,97] | α-carotene, β-carotene, violoxanthin | Increase | Terpenoids | β-carotene | Antioxidants [90]; anti-inflammatory [91] | Antioxidants [146]; anti-inflammatory [147] | |||||||
| isoprene, α-pinene, β-pinene, myrcene, limonene, sabinene, ( | E | )-β-ocimene, ( | Z | )-β-ocimene, α-humulene, ( | E | )-β-farnesene, ( | E,E | )-α-farnesene, ( | E | )-β-caryophyllene, δ-cadinene | Increase | Terpenoids | α-pinene; myrcene; limonene; α-humulene. | Anti-inflammatory [92]; anti-asthmatic [93]; antioxidant [94]; anti-inflammatory [95] | Anti-inflammatory [148]; anti-asthmatic [149]; antioxidant [150]; anti-inflammatory [151] | ||
| O | 3 | Gingko biloba (Ginkgoaceae) [96] | (Ginkgoaceae) [152] | ginkgolide A | Increase | Terpenoids | ginkgolide A | Neuroprotective [97] | Neuroprotective [153] | ||||||||
| Ultraviolet radiation-B (UV-B) | Arabidopsis thaliana (Brassicaceae) [98] | (Brassicaceae) [154] | kaempferol 3-gentiobioside-7-rhamnoside; kaempferol 3,7-dirhamnoside. | Increase | Phenolics (Flavonoids) | NA | NA | ||||||||||
| UV-B | Brassica napus (Brassicaceae) [99] | (Brassicaceae) [155] | quercetin 3-sophoroide-7-glucoside; quercetin 3-sinapyl sophoroside-7-glucoside | Increase | Phenolics (Flavonoids) | NA | NA | ||||||||||
| UV-B | Brassica oleracea (Brassicaceae) [100] | (Brassicaceae) [156] | cyanidine glycosides; sinapyl alcohol | Increase | Phenolics (Flavoboids) | NA | NA | ||||||||||
| UV-B | C. roseus (Apocynaceae) [101][102] | (Apocynaceae) [157,158] | catharanthine, vindoline | Increase | Alkaloids | catharanthine | Anticancer [103] | Anticancer [159] | |||||||||
| Clarkia breweri (Onagraceae) [104] | (Onagraceae) [160] | eugenol, isoeugenol, methyleugenol, and isomethyleugenol | Increase | Phenolics | eugenol | Antifungal [105]; anti-inflammatory [106] | Antifungal [161]; anti-inflammatory [162] | ||||||||||
| UV-B | Fagopyrum esculentum (Polygonaceae) [107] | (Polygonaceae) [163] | rutin, quercetin, catechin | Increase | Phenolics | quercetin; catechin | Antioxidant [108]; anticancer and antioxidant [109][110] | Antioxidant [164]; anticancer and antioxidant [165,166] | |||||||||
| UV-B | Gnaphalium luteoalbum (Asteraceae) [28] | (Asteraceae) [167] | calycopterin; 3’-methoxycalycopterin | Increase | Phenolics (Flavonoids) | calycopterin | Anticancer [27] | Anticancer [168] | |||||||||
| UV-B | G. viravira [111] | [169] | 7-O-methyl araneol | Increase | Phenolics (Flavonoids) | NA | NA | ||||||||||
| UV-B | Hordeum vulgare (Poaceae) [112] | (Poaceae) [170] | saponarin; luteolin | Increase | Phenolics (Flavonoids) | saponarin; luteolin | Antihypertensive [113]; antibacterial [114] | Antihypertensive [171]; antibacterial [172] | |||||||||
| UV-B | Marchantia polymorpha (Marchantiaceae) [115] | (Marchantiaceae) [173] | luteolin 7-glucuronide; luteolin 3,4’-di- | p | -coumaryl-quercetin 3-glucoside. | Increase | Phenolics (Flavonoids) | NA | NA | ||||||||
| UV-B | Quercus ilex (Fagaceae) [116] | (Fagaceae) [174] | acylated kaempferol glycosides | Increase | Phenolics (Flavonoids) | kaempferol | Anticancer [117]; anti-inflammatory [118] | Anticancer [175]; anti-inflammatory [176] | |||||||||
| Heat stress | C. acuminata [119] | [177] | 10-hydroxycamptothecin | Increase | Alkaloids | 10-hydroxycamptothecin | Anticancer [120] | Anticancer [178] | |||||||||
| Heat stress | Daucus carota (Apiaceae) [121][122][123] | (Apiaceae) [179,180,181] | α-terpinolene | Decrease | Terpenoids | α-terpinolene | Antioxidant and anticancer [124] | Antioxidant and anticancer [182] | |||||||||
| α-caryophyllene, β-farnesene | Increase | NA | NA | ||||||||||||||
| anthocyanins, coumaric and caffeic acid; | Increase | Phenolics | p | -coumaric acid and caffeic acid | Antioxidant [125][126] | Antioxidant [183,184] | |||||||||||
| Heat stress | Q. rubra (Fagaceae) [127] | (Fagaceae) [185] | isoprene (2-methyl-1,3-butadiene) | Increase | Terpenoids | NA | NA | ||||||||||
| Heat stress | S. lycopersicon [43][44] | [87,97] | β-phellandrene (dominant), 2-carene, α-phellandrene, limonene; increased emission of ( | E | )-β-ocimene after treatment above 46 °C; β-caryophyllene. | Increase | Terpenoids | α-phellandrene; β-caryophyllene | Antifungal [128]; anticancer and anti-inflammatory [47][48] | Antifungal [186]; anticancer and anti-inflammatory [102,103] | |||||||
| α-humulene | Decrease | α-humulene | Anticancer [129] | Anticancer [187] | |||||||||||||
| Heat stress (increased humidity) | Centella asiatica (Apiaceae) [130] | (Apiaceae) [188] | asiaticoside | Increase | Phenolics | asiaticoside | Anti-cellulite agent [131] | Anti-cellulite agent [189] |
