
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rebecca Julianne Duncan | + 3022 word(s) | 3022 | 2022-01-19 07:11:40 | | | |
| 2 | Lindsay Dong | Meta information modification | 3022 | 2022-01-21 02:52:00 | | |
Video Upload Options
Microalgae growing on the underside of sea ice are key primary producers in polar marine environments. Their nutritional status, determined by their macromolecular composition, contributes to the region’s biochemistry and the unique temporal and spatial characteristics of their growth makes them essential for sustaining polar marine food webs. The importance of sea ice microalgae as primary producers in polar marine ecosystems means that ongoing research into climate-change driven macromolecular phenotyping is critical to understanding the implications for the regions biochemical cycling and carbon transfer.
1. Introduction
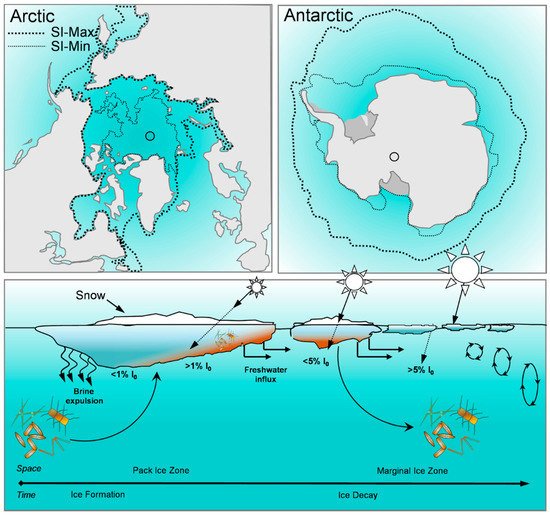
2. Biomolecular Composition of Sea Ice Algae from Polar Regions
| Study | Taxa | Location | Latitude, Longitude | Sampling Date | Biomolecules Investigated | |
|---|---|---|---|---|---|---|
| Antarctica | An et al., 2013 | Chlamydomonas sp. ICE-L | Zhongshan Research Station | 69° S, 77° E | N/A | Fatty acids |
| Cade-Menun & Paytan 2010 | Fragilariopsis curta, Fragilariopsis cylindrus, Nitzschia subcurvata, Phaeocystis Antarctica, Thalassiosira weissflogii, Dunaliella tertiolecta, Synechoccus sp. | Culture | N/A | N/A | Lipid, protein, carbohydrate |
|
| Gleitz & Kirst 1991 | Diatom-dominated mixed community, primarily Nitzschia sp., Chaetoceros sp., Navicula sp., Corethron sp., Rhizosolenia sp., Amphiprora sp., Dactyliosolen sp., Synedropsis sp., Tropidoneis and Phaeocystis pouchetii | Weddell Sea | 58–63° S, 55–45° W |
1988/1989 | Lipid, amino acid, carbohydrate | |
| Mock & Kroon 2002a | Fragilariopsis curta, Navicula gelida var.-antarctica, Nitzschia medioconstricta | Weddell Sea | 70°02′ S, 06°00′ W |
March–May 1999 | Lipid, protein | |
| Mock & Kroon 2002b | Fragilariopsis curta, Navicula gelida var.-antarctica, Nitzschia medioconstricta | Weddell Sea | 70°02′ S, 06°00′ W |
March–May 1999 | Lipid, protein | |
| Palmisano & Sullivan 1985 | Diatom-dominated mixed community, primarily Pleurosigma sp., Nitzschia stellata, Berkeleya sp., Amphiprora kuferathii, Phaeocystis sp. and small centrics. | McMurdo Sound | 77° S, 166° W |
November–December 1983 |
Lipid, protein, polysaccharide | |
| Teoh et al., 2004 | Chlamydomonas sp. and Navicula sp. | Windmill Islands | 66°17′ S, 110°29′ E |
N/A | Lipid, protein, carbohydrate, fatty acids | |
| Sackett et al., 2013 | Fragilariopsis cylindrus, Chaetoceros simplex and Pseudo-nitzschia subcurvata | Southern Ocean and Prydz Bay | 66° S, 147° E, 68° S, 73° E |
N/A | Lipid, protein, carbohydrate, fatty acids, amino acids | |
| Xu et al., 2014 | Chlamydomonas sp. ICE-L | Zhongshan Research Station | 69° S, 77° E | N/A | Lipid, fatty acids | |
| Arctic | Lee et al., 2008a | Mixed community dominated by large chain-forming diatoms | Barrow, Alaska | 71°20′ N, 156°39′ W |
April–June 2003 | Lipid, protein, polysaccharide |
| Lee et al., 2008b | Mixed community dominated by large chain-forming diatoms | Barrow, Alaska | 71°20′ N, 156°39′ W |
February–June 2003 |
Lipid, protein, polysaccharide | |
| Leu et al., 2006b | Thalassiosira antarctica var. borealis | Ny-Ålesund, Svalbard | 78°55′ N, 11°56′ E |
May–June 2004 | Fatty acids | |
| Leu et al., 2010 | Diatom-dominated mixed community, primarily Nitzschia frigida, Navicula septentrionalis and Fragilariopsis cylindrus. | Ripfjorden, Svalbard | 80° N, 22° E |
March–July 2007 | Fatty acids | |
| Lund-Hansen et al., 2020 | Mixed diatom-dominated community. Primarily Nitzschia frigida, Nitzschia longissima and Thalassiosira sp. | Kangerlussuaq West Greenland | 66°57′ N, 50°57′ W | March 2016 | Fatty acids | |
| Mock & Gradinger 2000 | Mixed community dominated by Nitzschia sp., Fragilariopsis sp. and Chaetoceros sp. | Barents Sea | 77°10′ N, 34°04′ E |
May–June 1997 | Lipid, protein, polysaccharides | |
| Pogorzelec et al., 2017 | Nitzschia frigida, pennate ribbon colonies and Attheya sp. | Dease Strait, Nunavut, Canada | 69°1′ N, 105°19′ W |
March–May 2014 | Lipid, protein | |
| Smith et al., 1987 | Mixed community | Resolute Passage, Canada | 74°41′ N, 95°50′ W |
April–June 1985 | Lipid, protein, polysaccharides | |
| Smith et al., 1989 | Diatom-dominated mixed community, primarily Nitzschia frigida and Nitzschia grunowii | Central Canadian Arctic | 74°40′ N, 94°54′ W |
April–May 1985; 1986 | Lipid, protein, amino acid, polysaccharide | |
| Smith et al., 1993 | Diatom-dominated mixed community | Resolute Passage, Canada | 74°41′ N, 95°50′ W |
March–June 1989 | Lipid | |
| Smith & Herman 1992 | Diatom-dominated mixed community | Resolute Passage, Canada | 74°41′ N, 95°50′ W |
May 1987, May–June 1988 |
Lipid, protein, polysaccharide | |
| Søreide et al., 2010 | Diatom-dominated mixed community | Ripfjorden, Svalbard | 80°27′ N, 22°29′ E |
March–July 2007 | Fatty acids | |
| Torstensson et al., 2013 | Nitzschia lecointei | Amundsen Sea | N/A | January 2011 | Fatty acids | |
| Torstensson et al., 2019 | Nitzschia lecointei | Amundsen Sea | N/A | N/A | Lipid, protein carbohydrate, fatty acids |
One of the strongest determinants of biomolecular composition is taxonomic composition. Phylogenetically distinct microalgal groups have been shown to vary in their proportional allocation of biomolecules. Diatoms (Orcophyta: Bacillariophyceae), for example, generally have higher lipid and lower carbohydrate content than other microalgal phyla, such as the Chlorophytes and Haptophytes [48]. More specifically, pennate diatoms within the sea ice have been shown to have higher lipid, fatty acid, and carbohydrate content than the centric diatoms from the same community [47]. Similarly, diatoms with a smaller cell volume (such as pennate diatoms) have been shown to have higher carbohydrate content than larger volume diatoms [68]. At the taxonomic level of species, differentiation is more subtle, but nevertheless has been shown [44][47][69][70]. However, by far most knowledge on species-specific biomolecular profiles is derived from single-species culture studies, providing a poor representation of what may be true for natural mixed communities. It is therefore important that studies on natural communities start to discriminate biomolecular profiles of individual taxa within a community if we are to improve our understanding of taxonomic biomolecular diversity.
3. Environmental Factors That Influence Bioomolecular Composition
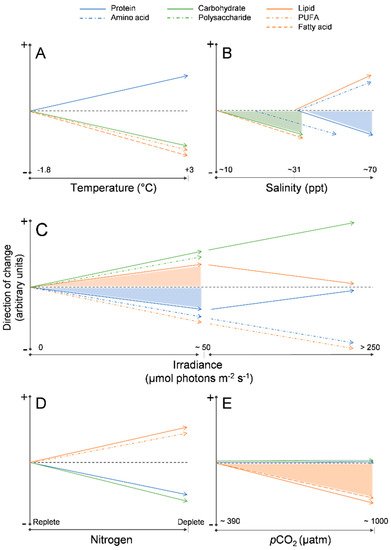
Temperature: At the ice–water interface, temperature generally remains around −1.8 °C throughout spring, however as sea surface temperatures rise with the onset of ocean warming [37], it is possible that warmer temperatures will alter the biochemical composition of the algae, drive an earlier ice melt, and ultimately inhibit sea ice microalgal growth completely [11][30]. Limited work has been completed on the effects of temperature on the biochemical composition of sea ice algae, but from these few studies some patterns have emerged. Extreme subzero temperatures (−20 °C) have been associated with a decline in fatty acids, especially PUFA content [71]. A decline in fatty acids has been observed also under moderate temperature increases (from −1.8 °C to 3 °C), as well as in temperature increases well beyond the natural range (~15 °C) [72][71][73][74] (Figure 2A). In several Antarctic sea-ice diatoms, an increase in relative protein content and decrease in carbohydrate content have been observed with exposure to warmer (up to 3 °C) temperatures [74], including temperatures well above (~20 °C) the natural range for sea ice microalgae [72].
References
- Parkinson, C.L. Global Sea Ice Coverage from Satellite Data: Annual Cycle and 35-Yr Trends. J. Clim. 2014, 27, 9377–9382.
- Arrigo, K.R. Sea ice as a habitat for primary producers. In Sea Ice, 3rd ed.; Thomas, D., Ed.; Wiley-Blackwell: Oxford, UK, 2017; pp. 352–369.
- Deming, J.W.; Eric Collins, R. Sea ice as a habitat for bacteria, archaea and viruses. In Sea Ice, 3rd ed.; Thomas, D., Ed.; Wiley-Blackwell: Oxford, UK, 2017; pp. 326–351.
- Belt, S.T. Source-specific biomarkers as proxies for Arctic and Antarctic sea ice. Org. Geochem. 2018, 125, 277–298.
- Kohlbach, D.; Hop, H.; Wold, A.; Schmidt, K.; Smik, L.; Belt, S.T.; Al-Habahbeh, A.K.; Woll, M.; Graeve, M.; Dąbrowska, A.M.; et al. Multiple trophic markers trace dietary carbon sources in barents sea zooplankton during late summer. Front. Mar. Sci. 2021, 7, 1216.
- Rontani, J.-F.; Belt, S.T.; Brown, T.; Amiraux, R.; Gosselin, M.; Vaultier, F.; Mundy, C.J. Monitoring abiotic degradation in sinking versus suspended Arctic sea ice algae during a spring ice melt using specific lipid oxidation tracers. Org. Geochem. 2016, 98, 82–97.
- Rontani, J.-F.; Amiraux, R.; Smik, L.; Wakeham, S.G.; Paulmier, A.; Vaultier, F.; Sun-Yong, H.; Jun-Oh, M.; Belt, S.T. Type II photosensitized oxidation in senescent microalgal cells at different latitudes: Does low under-ice irradiance in polar regions enhance efficiency? Sci. Total Environ. 2021, 779, 146363.
- Bélanger, S.; Xie, H.; Krotkov, N.; Larouche, P.; Vincent, W.F.; Babin, M. Photomineralization of terrigenous dissolved organic matter in Arctic coastal waters from 1979 to 2003: Interannual variability and implications of climate change. Glob. Biogeochem. Cycles 2006, 20, 1–13.
- Massicotte, P.; Amon, R.M.W.; Antoine, D.; Archambault, P.; Balzano, S.; Bélanger, S.; Benner, R.; Boeuf, D.; Bricaud, A.; Bruyant, F.; et al. The MALINA oceanographic expedition: How do changes in ice cover, permafrost and UV radiation impact biodiversity and biogeochemical fluxes in the Arctic Ocean? Earth Syst. Sci. Data 2021, 13, 1561–1592.
- Johnsen, G. Photoadaptation of sea-ice microalgae in the Barents Sea. Polar Biol. 1991, 11, 179–184.
- Leu, E.; Mundy, C.; Assmy, P.; Campbell, K.; Gabrielsen, T.; Gosselin, M.; Juul-Pedersen, T.; Gradinger, R. Arctic spring awakening—Steering principles behind the phenology of vernal ice algal blooms. Prog. Oceanogr. 2015, 139, 151–170.
- Cota, G.; Legendre, L.; Gosselin, M.; Ingram, R. Ecology of bottom ice algae: I. Environmental controls and variability. J. Mar. Syst. 1991, 2, 257–277.
- Selz, V.; Laney, S.; Arnsten, A.E.; Lewis, K.M.; Lowry, K.E.; Joy-Warren, H.L.; Mills, M.M.; van Dijken, G.L.; Arrigo, K.R. Ice algal communities in the Chukchi and Beaufort Seas in spring and early summer: Composition, distribution, and coupling with phytoplankton assemblages. Limnol. Oceanogr. 2018, 63, 1109–1133.
- Gosselin, M.; Levasseur, M.; Wheeler, P.A.; Horner, R.A.; Booth, B.C. New measurements of phytoplankton and ice algal production in the Arctic Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 1623–1644.
- Legendre, L.; Ackley, S.F.; Dieckmann, G.S.; Horner, R.; Hoshiai, T.; Melnikov, I.A.; Reeburgh, W.S.; Spindler, M.; Sullivan, C.W. Ecology of sea ice biota. Polar Biol. 1992, 12, 429–444.
- Gradinger, R. Sea-ice algae: Major contributors to primary production and algal biomass in the Chukchi and Beaufort Seas during May/June 2002. Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 1201–1212.
- Fernández-Méndez, M.; Katlein, C.; Rabe, B.; Nicolaus, M.; Peeken, I.; Bakker, K.; Flores, H.; Boetius, A. Photosynthetic production in the central Arctic Ocean during the record sea-ice minimum in 2012. Biogeosciences 2015, 12, 3525–3549.
- Rysgaard, S.; Kühl, M.; Glud, R.N.; Hansen, J.W. Biomass, production and horizontal patchiness of sea ice algae in a high-Arctic fjord (Young Sound, NE Greenland). Mar. Ecol. Prog. Ser. 2001, 223, 15–26.
- Lee, S.H.; Whitledge, T.E.; Kang, S.-H. Spring time production of bottom ice algae in the landfast sea ice zone at Barrow, Alaska. J. Exp. Mar. Biol. Ecol. 2008, 367, 204–212.
- Lizotte, M.P. The contributions of sea ice algae to Antarctic marine primary production1. Am. Zool. 2001, 41, 57–73.
- Bhavya, P.S.; Kim, B.K.; Jo, N.; Kim, K.; Kang, J.J.; Lee, J.H.; Lee, D.; Lee, J.H.; Joo, H.; Ahn, S.H.; et al. A Review on the Macromolecular compositions of phytoplankton and the implications for aquatic biogeochemistry. Ocean Sci. J. 2018, 54, 1–14.
- Bernard, K.S.; Gunther, L.A.; Mahaffey, S.H.; Qualls, K.M.; Sugla, M.; Saenz, B.T.; Cossio, A.M.; Walsh, J.; Reiss, C.S. The contribution of ice algae to the winter energy budget of juvenile Antarctic krill in years with contrasting sea ice conditions. ICES J. Mar. Sci. 2019, 76, 206–216.
- Graeve, M.; Kattner, G.; Hagen, W. Diet-induced changes in the fatty acid composition of Arctic herbivorous copepods: Experimental evidence of trophic markers. J. Exp. Mar. Biol. Ecol. 1994, 182, 97–110.
- Lee, R.; Hagen, W.; Kattner, G. Lipid storage in marine zooplankton. Mar. Ecol. Prog. Ser. 2006, 307, 273–306.
- Hagen, W.; Auel, H. Seasonal adaptations and the role of lipids in oceanic zooplankton. Zoology 2001, 104, 313–326.
- Falk-Petersen, S.; Mayzaud, P.; Kattner, G.; Sargent, J.R. Lipids and life strategy of Arctic Calanus. Mar. Biol. Res. 2009, 5, 18–39.
- Kohlbach, D.; Graeve, M.; Lange, B.A.; David, C.; Schaafsma, F.L.; Van Franeker, J.A.; Vortkamp, M.; Brandt, A.; Flores, H. Dependency of Antarctic zooplankton species on ice algae-produced carbon suggests a sea ice-driven pelagic ecosystem during winter. Glob. Chang. Biol. 2018, 24, 4667–4681.
- Meredith, M.; Sommerkorn, M.; Cassotta, S.; Derksen, C.; Ekaykin, A.; Hollowed, A.; Kofinas, G.; Macintosh, A.; Melbourne-Thomas, J.; Muelbert, M.M.C.; et al. Polar Regions. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019.
- Post, E.; Bhatt, U.S.; Bitz, C.M.; Brodie, J.F.; Fulton, T.L.; Hebblewhite, M.; Kerby, J.; Kutz, S.J.; Stirling, I.; Walker, D.A. Ecological consequences of sea-ice decline. Science 2013, 341, 519–524.
- Mallett, R.D.C.; Stroeve, J.C.; Tsamados, M.; Landy, J.C.; Willatt, R.; Nandan, V.; Liston, G.E. Faster decline and higher variability in the sea ice thickness of the marginal Arctic seas when accounting for dynamic snow cover. Cryosphere 2021, 15, 2429–2450.
- Post, E.; Alley, R.B.; Christensen, T.R.; Macias-Fauria, M.; Forbes, B.C.; Gooseff, M.N.; Iler, A.; Kerby, J.T.; Laidre, K.L.; Mann, M.E.; et al. The polar regions in a 2 °C warmer world. Sci. Adv. 2019, 5, eaaw9883.
- National Snow & Ice Data Center (NSIDC) Sea Ice Index. Available online: https://nsidc.org/data/seaice_index/ (accessed on 14 September 2021).
- Overland, J.E.; Wang, M. When will the summer Arctic be nearly sea ice free? Geophys. Res. Lett. 2013, 40, 2097–2101.
- Haine, T.W.N.; Martin, T. The Arctic-Subarctic sea ice system is entering a seasonal regime: Implications for future Arctic amplification. Sci. Rep. 2017, 7, 4618.
- Parkinson, C.L. A 40-y record reveals gradual Antarctic sea ice increases followed by decreases at rates far exceeding the rates seen in the Arctic. Proc. Natl. Acad. Sci. USA 2019, 116, 14414–14423.
- Stroeve, J.; Notz, D. Changing state of Arctic sea ice across all seasons. Environ. Res. Lett. 2018, 13, 103001.
- Timmermans, M.; Marshall, J. Understanding Arctic Ocean circulation: A review of ocean dynamics in a changing climate. J. Geophys. Res. Oceans 2020, 125, 1–35.
- Terhaar, J.; Kwiatkowski, L.; Bopp, L. Emergent constraint on Arctic Ocean acidification in the twenty-first century. Nature 2020, 582, 379–383.
- Terhaar, J.; Orr, J.C.; Ethé, C.; Regnier, P.; Bopp, L. Simulated Arctic Ocean response to doubling of riverine carbon and nutrient delivery. Glob. Biogeochem. Cycles 2019, 33, 1048–1070.
- Archer, S.; Leakey, R.; Burkill, P.; Sleigh, M.; Appleby, C. Microbial ecology of sea ice at a coastal Antarctic site: Community composition, biomass and temporal change. Mar. Ecol. Prog. Ser. 1996, 135, 179–195.
- Becquevort, S.; Dumont, I.; Tison, J.-L.; Lannuzel, D.; Sauvée, M.-L.; Chou, L.; Schoemann, V. Biogeochemistry and microbial community composition in sea ice and underlying seawater off East Antarctica during early spring. Polar Biol. 2009, 32, 879–895.
- Mock, T.; Kroon, B.M. Photosynthetic energy conversion under extreme conditions—II: The significance of lipids under light limited growth in Antarctic sea ice diatoms. Phytochemistry 2002, 61, 53–60.
- Leu, E.; Wiktor, J.; Søreide, J.E.; Berge, J.; Falk-Petersen, S. Increased irradiance reduces food quality of sea ice algae. Mar. Ecol. Prog. Ser. 2010, 411, 49–60.
- Sackett, O.; Petrou, K.; Reedy, B.; De Grazia, A.; Hill, R.; Doblin, M.; Beardall, J.; Ralph, P.; Heraud, P. Phenotypic plasticity of southern ocean diatoms: Key to success in the sea ice habitat? PLoS ONE 2013, 8, e81185.
- Sackett, O.; Petrou, K.; Reedy, B.; Hill, R.; Doblin, M.; Beardall, J.; Ralph, P.; Heraud, P. Snapshot prediction of carbon productivity, carbon and protein content in a Southern Ocean diatom using FTIR spectroscopy. ISME J. 2015, 10, 416–426.
- Pogorzelec, N.; Mundy, C.; Findlay, C.; Campbell, K.; Diaz, A.; Ehn, J.; Rysgaard, S.; Gough, K. FTIR imaging analysis of cell content in sea-ice diatom taxa during a spring bloom in the lower northwest passage of the Canadian Arctic. Mar. Ecol. Prog. Ser. 2017, 569, 77–88.
- Sheehan, C.; Nielsen, D.A.; Petrou, K. Macromolecular composition, productivity and dimethylsulfoniopropionate in Antarctic pelagic and sympagic microalgal communities. Mar. Ecol. Prog. Ser. 2020, 640, 45–61.
- Finkel, Z.V.; Follows, M.J.; Liefer, J.; Brown, C.M.; Benner, I.; Irwin, A.J. Phylogenetic Diversity in the Macromolecular Composition of Microalgae. PLoS ONE 2016, 11, e0155977.
- Thomas, D.N.; Dieckmann, G.S. Antarctic sea ice—A habitat for extremophiles. Science 2002, 295, 641–644.
- Morgan-Kiss, R.M.; Priscu, J.C.; Pocock, T.; Gudynaite-Savitch, L.; Huner, N.P.A. Adaptation and acclimation of photosynthetic microorganisms to permanently cold environments. Microbiol. Mol. Biol. Rev. 2006, 70, 222–252.
- Finkel, Z.V.; Follows, M.J.; Irwin, A.J. Size-scaling of macromolecules and chemical energy content in the eukaryotic microalgae. J. Plankton Res. 2016, 38, 1151–1162.
- Van Oijen, T.; Van Leeuwe, M.A.; Granum, E.; Weissing, F.J.; Bellerby, R.G.J.; Gieskes, W.W.C.; De Baar, H.J.W. Light rather than iron controls photosynthate production and allocation in Southern Ocean phytoplankton populations during austral autumn. J. Plankton Res. 2004, 26, 885–900.
- Ruess, L.; Müller-Navarra, D.C. Essential Biomolecules in Food Webs. Front. Ecol. Evol. 2019, 7, 269.
- Scott, J.M. Effect of growth rate of the food alga on the growth/ingestion efficiency of a marine herbivore. J. Mar. Biol. Assoc. UK 1980, 60, 681–702.
- Lindqvist, K.; Lignell, R. Intracellular partitioning of 14c02 in phytoplankton during a growth season in the northern Baltic. Mar. Ecol. Prog. Ser. 1997, 152, 41–50.
- Søreide, J.E.; Leu, E.; Berge, J.; Graeve, M.; Falk-Petersen, S. Timing of blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Glob. Chang. Biol. 2010, 16, 3154–3163.
- Leu, E.; Søreide, J.; Hessen, D.; Falk-Petersen, S.; Berge, J. Consequences of changing sea-ice cover for primary and secondary producers in the European Arctic shelf seas: Timing, quantity, and quality. Prog. Oceanogr. 2011, 90, 18–32.
- Swalethorp, R.; Kjellerup, S.; Dünweber, M.; Nielsen, T.G.; Møller, E.F.; Rysgaard, S.; Hansen, B. Grazing, egg production, and biochemical evidence of differences in the life strategies of Calanus finmarchicus, C. glacialis and C. hyperboreus in Disko Bay, western Greenland. Mar. Ecol. Prog. Ser. 2011, 429, 125–144.
- Kim, B.K.; Lee, J.H.; Yun, M.S.; Joo, H.; Song, H.J.; Yang, E.J.; Chung, K.H.; Kang, S.-H.; Lee, S.H. High lipid composition of particulate organic matter in the northern Chukchi Sea, 2011. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 120, 72–81.
- Yun, M.S.; Lee, D.B.; Kim, B.K.; Kang, J.J.; Lee, J.H.; Yang, E.J.; Park, W.G.; Chung, K.H.; Lee, S.H. Comparison of phytoplankton macromolecular compositions and zooplankton proximate compositions in the northern Chukchi Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 120, 82–90.
- Smith, W.O.; Nelson, D.M.; DiTullio, G.R.; Leventer, A. Temporal and spatial patterns in the Ross Sea: Phytoplankton biomass, elemental composition, productivity and growth rates. J. Geophys. Res. Space Phys. 1996, 101, 18455–18465.
- Fabiano, M.; Danovaro, R.; Povero, P. Vertical distribution and biochemical composition of pico- and microparticulate organic matter in the Ross Sea (Antarctica). In Oceanography of the Ross Sea Antarctica; Springer: Milano, Italy, 1999; pp. 233–246.
- Kim, B.K.; Lee, J.H.; Joo, H.; Song, H.J.; Yang, E.J.; Lee, S.H. Macromolecular compositions of phytoplankton in the Amundsen Sea, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 123, 42–49.
- Nelson, D.M.; Smith, W.O. Phytoplankton bloom dynamics of the western Ross Sea ice edge—II. Mesoscale cycling of nitrogen and silicon. Deep Sea Res. Part A Oceanogr. Res. Pap. 1986, 33, 1389–1412.
- Pollard, R.; Tréguer, P.; Read, J. Quantifying nutrient supply to the Southern Ocean. J. Geophys. Res. Space Phys. 2006, 111, C0501.
- Kim, B.K.; Lee, S.; Ha, S.-Y.; Jung, J.; Kim, T.W.; Yang, E.J.; Jo, N.; Lim, Y.J.; Park, J. Vertical distributions of macromolecular composition of particulate organic matter in the water column of the amundsen sea polynya during the summer in 2014. J. Geophys. Res. Oceans 2018, 123, 1393–1405.
- Alderkamp, A.-C.; Buma, A.G.J.; van Rijssel, M. The carbohydrates of Phaeocystis and their degradation in the microbial food web. Biogeochemistry 2007, 83, 99–118.
- Hitchcock, G.L. A comparative study of the size-dependent organic composition of marine diatoms and dinoflagellates. J. Plankton Res. 1982, 4, 363–377.
- Stehfest, K.; Toepel, J.; Wilhelm, C. The application of micro-FTIR spectroscopy to analyze nutrient stress-related change in biomass composition of phytoplankton algae. Plant Physiol. Biochem. 2005, 43, 717–726.
- Duncan, R.J.; Nielsen, D.A.; Sheehan, C.E.; Deppeler, S.; Hancock, A.M.; Schulz, K.G.; Davidson, A.T.; Petrou, K. Ocean acidification alters the nutritional value of Antarctic diatoms. New Phytol. 2022, in press.
- An, M.; Mou, S.; Zhang, X.; Ye, N.; Zheng, Z.; Cao, S.; Xu, D.; Fan, X.; Wang, Y.; Miao, J. Temperature regulates fatty acid desaturases at a transcriptional level and modulates the fatty acid profile in the Antarctic microalga Chlamydomonas sp. ICE-L. Bioresour. Technol. 2013, 134, 151–157.
- Teoh, M.-L.; Chu, W.-L.; Marchant, H.; Phang, S.-M. Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. J. Appl. Phycol. 2004, 16, 421–430.
- Torstensson, A.; Hedblom, M.; Andersson, J.; Andersson, M.X.; Wulff, A. Synergism between elevated pCO2 and temperature on the Antarctic sea ice diatom Nitzschia lecointei. Biogeosciences 2013, 10, 6391–6401.
- Torstensson, A.; Jiménez, C.; Nilsson, A.K.; Wulff, A. Elevated temperature and decreased salinity both affect the biochemical composition of the Antarctic sea-ice diatom Nitzschia lecointei, but not increased pCO2. Polar Biol. 2019, 42, 2149–2164.
- Gleitz, M.; Kirst, G. Photosynthesis-irradiance relationships and carbon metabolism of different ice algal assemblages collected from Weddell Sea pack ice during austral spring (EPOS 1). Polar Biol. 1991, 11, 385–392.
- Smith, R.E.H.; Clement, P.; Head, E. Biosynthesis and photosynthate allocation patterns of arctic ice algae. Limnol. Oceanogr. 1989, 34, 591–605.
- Findlay, C.; Morrison, J.; Mundy, C.J.; Sedlmair, J.; Hirschmugl, C.J.; Gough, K.M. Thermal source Fourier transform infrared microtomography applied to Arctic sea ice diatoms. Analyst 2017, 142, 660–669.
- Lee, S.H.; Whitledge, T.E.; Kang, S.-H. Carbon uptake rates of sea ice algae and phytoplankton under different light intensities in a landfast sea ice zone, Barrow, Alaska. Arctic 2009, 61, 281–291.
- Smith, R.; Cavaletto, J.; Eadie, B.; Gardner, W. Growth and lipid composition of high Arctic ice algae during the spring bloom at Resolute, Northwest Territories, Canada. Mar. Ecol. Prog. Ser. 1993, 97, 19–29.
- Smith, R.E.H.; Clement, P.; Cota, G.F.; Li, W.K.W. Intracellular photosynthate allocation and the control of arctic marine ice algal production1. J. Phycol. 2007, 23, 124–132.
- Smith, R.; Herman, A. In situ patterns of intracellular photosynthate allocation by sea ice algae in the Canadian high arctic. Polar Biol. 1992, 12, 545–551.
- Palmisano, A.C.; Sullivan, C.W. Pathways of photosynthetic carbon assimilation in sea-ice microalgae from McMurdo Sound, Antarctica. Limnol. Oceanogr. 1985, 30, 674–678.
- Cade-Menun, B.J.; Paytan, A. Nutrient temperature and light stress alter phosphorus and carbon forms in culture-grown algae. Mar. Chem. 2010, 121, 27–36.
- Lavoie, D.; Denman, K.; Michel, C. Modeling ice algal growth and decline in a seasonally ice-covered region of the Arctic (Resolute Passage, Canadian Archipelago). J. Geophys. Res. Space Phys. 2005, 110, 1–17.
- Rozanska, M.; Gosselin, M.; Poulin, M.; Wiktor, J.; Michel, C. Influence of environmental factors on the development of bottom ice protist communities during the winter–spring transition. Mar. Ecol. Prog. Ser. 2009, 386, 43–59.
- Vancoppenolle, M.; Bopp, L.; Madec, G.; Dunne, J.; Ilyina, T.; Halloran, P.R.; Steiner, N. Future Arctic Ocean primary productivity from CMIP5 simulations: Uncertain outcome, but consistent mechanisms. Glob. Biogeochem. Cycles 2013, 27, 605–619.
- Slagstad, D.; Wassmann, P.F.J.; Ellingsen, I. Physical constrains and productivity in the future Arctic Ocean. Front. Mar. Sci. 2015, 2, 85.
- Jiang, Y.; Yoshida, T.; Quigg, A. Photosynthetic performance, lipid production and biomass composition in response to nitrogen limitation in marine microalgae. Plant Physiol. Biochem. 2012, 54, 70–77.
- Chen, J.-J.; Li, Y.-R.; Lai, W.-L. Application of experimental design methodology for optimization of biofuel production from microalgae. Biomass Bioenergy 2014, 64, 11–19.
- Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Nitrogen starvation-induced cellular crosstalk of ROS-scavenging antioxidants and phytohormone enhanced the biofuel potential of green microalga Acutodesmus dimorphus. Biotechnol. Biofuels 2017, 10, 60.
- Rehman, Z.U.; Anal, A.K. Enhanced lipid and starch productivity of microalga (Chlorococcum sp. TISTR 8583) with nitrogen limitation following effective pretreatments for biofuel production. Biotechnol. Rep. 2019, 21, e00298.
- Xu, D.; Wang, Y.; Fan, X.; Wang, D.; Ye, N.; Zhang, X.; Mou, S.; Guan, Z.; Zhuang, Z. Long-Term Experiment on Physiological Responses to Synergetic Effects of Ocean Acidification and Photoperiod in the Antarctic Sea Ice Algae Chlamydomonas sp. ICE-L. Environ. Sci. Technol. 2014, 48, 7738–7746.




