Microalgae growing on the underside of sea ice are key primary producers in polar marine environments. Their nutritional status, determined by their macromolecular composition, contributes to the region’s biochemistry and the unique temporal and spatial characteristics of their growth makes them essential for sustaining polar marine food webs. The importance of sea ice microalgae as primary producers in polar marine ecosystems means that ongoing research into climate-change driven macromolecular phenotyping is critical to understanding the implications for the regions biochemical cycling and carbon transfer.
- sea ice
- sympagic microalgae
- trophic transfer
- sea ice microalgae
- polar
- arctic
- antarctic
1. Introduction
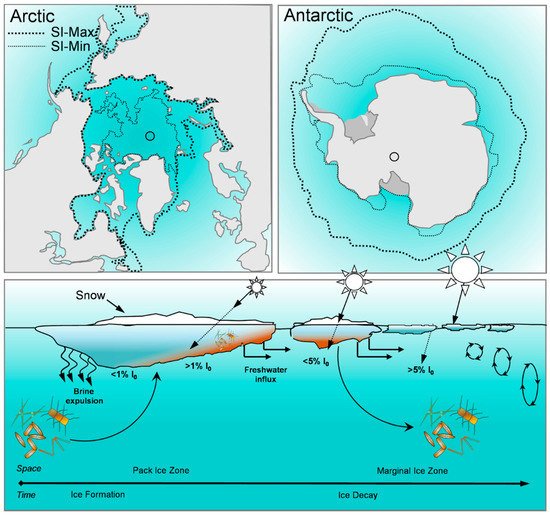
2. Biomolecular Composition of Sea Ice Algae from Polar Regions
| Study | Taxa | Location | Latitude, Longitude | Sampling Date | Biomolecules Investigated | |
|---|---|---|---|---|---|---|
| Antarctica | An et al., 2013 | Chlamydomonas sp. ICE-L | Zhongshan Research Station | 69° S, 77° E | N/A | Fatty acids |
| Cade-Menun & Paytan 2010 | Fragilariopsis curta, Fragilariopsis cylindrus, Nitzschia subcurvata, Phaeocystis Antarctica, Thalassiosira weissflogii, Dunaliella tertiolecta, Synechoccus sp. | Culture | N/A | N/A | Lipid, protein, carbohydrate |
|
| Gleitz & Kirst 1991 | Diatom-dominated mixed community, primarily Nitzschia sp., Chaetoceros sp., Navicula sp., Corethron sp., Rhizosolenia sp., Amphiprora sp., Dactyliosolen sp., Synedropsis sp., Tropidoneis and Phaeocystis pouchetii | Weddell Sea | 58–63° S, 55–45° W |
1988/1989 | Lipid, amino acid, carbohydrate | |
| Mock & Kroon 2002a | Fragilariopsis curta, Navicula gelida var.-antarctica, Nitzschia medioconstricta | Weddell Sea | 70°02′ S, 06°00′ W |
March–May 1999 | Lipid, protein | |
| Mock & Kroon 2002b | Fragilariopsis curta, Navicula gelida var.-antarctica, Nitzschia medioconstricta | Weddell Sea | 70°02′ S, 06°00′ W |
March–May 1999 | Lipid, protein | |
| Palmisano & Sullivan 1985 | Diatom-dominated mixed community, primarily Pleurosigma sp., Nitzschia stellata, Berkeleya sp., Amphiprora kuferathii, Phaeocystis sp. and small centrics. | McMurdo Sound | 77° S, 166° W |
November–December 1983 |
Lipid, protein, polysaccharide | |
| Teoh et al., 2004 | Chlamydomonas sp. and Navicula sp. | Windmill Islands | 66°17′ S, 110°29′ E |
N/A | Lipid, protein, carbohydrate, fatty acids | |
| Sackett et al., 2013 | Fragilariopsis cylindrus, Chaetoceros simplex and Pseudo-nitzschia subcurvata | Southern Ocean and Prydz Bay | 66° S, 147° E, 68° S, 73° E |
N/A | Lipid, protein, carbohydrate, fatty acids, amino acids | |
| Xu et al., 2014 | Chlamydomonas sp. ICE-L | Zhongshan Research Station | 69° S, 77° E | N/A | Lipid, fatty acids | |
| Arctic | Lee et al., 2008a | Mixed community dominated by large chain-forming diatoms | Barrow, Alaska | 71°20′ N, 156°39′ W |
April–June 2003 | Lipid, protein, polysaccharide |
| Lee et al., 2008b | Mixed community dominated by large chain-forming diatoms | Barrow, Alaska | 71°20′ N, 156°39′ W |
February–June 2003 |
Lipid, protein, polysaccharide | |
| Leu et al., 2006b | Thalassiosira antarctica var. borealis | Ny-Ålesund, Svalbard | 78°55′ N, 11°56′ E |
May–June 2004 | Fatty acids | |
| Leu et al., 2010 | Diatom-dominated mixed community, primarily Nitzschia frigida, Navicula septentrionalis and Fragilariopsis cylindrus. | Ripfjorden, Svalbard | 80° N, 22° E |
March–July 2007 | Fatty acids | |
| Lund-Hansen et al., 2020 | Mixed diatom-dominated community. Primarily Nitzschia frigida, Nitzschia longissima and Thalassiosira sp. | Kangerlussuaq West Greenland | 66°57′ N, 50°57′ W | March 2016 | Fatty acids | |
| Mock & Gradinger 2000 | Mixed community dominated by Nitzschia sp., Fragilariopsis sp. and Chaetoceros sp. | Barents Sea | 77°10′ N, 34°04′ E |
May–June 1997 | Lipid, protein, polysaccharides | |
| Pogorzelec et al., 2017 | Nitzschia frigida, pennate ribbon colonies and Attheya sp. | Dease Strait, Nunavut, Canada | 69°1′ N, 105°19′ W |
March–May 2014 | Lipid, protein | |
| Smith et al., 1987 | Mixed community | Resolute Passage, Canada | 74°41′ N, 95°50′ W |
April–June 1985 | Lipid, protein, polysaccharides | |
| Smith et al., 1989 | Diatom-dominated mixed community, primarily Nitzschia frigida and Nitzschia grunowii | Central Canadian Arctic | 74°40′ N, 94°54′ W |
April–May 1985; 1986 | Lipid, protein, amino acid, polysaccharide | |
| Smith et al., 1993 | Diatom-dominated mixed community | Resolute Passage, Canada | 74°41′ N, 95°50′ W |
March–June 1989 | Lipid | |
| Smith & Herman 1992 | Diatom-dominated mixed community | Resolute Passage, Canada | 74°41′ N, 95°50′ W |
May 1987, May–June 1988 |
Lipid, protein, polysaccharide | |
| Søreide et al., 2010 | Diatom-dominated mixed community | Ripfjorden, Svalbard | 80°27′ N, 22°29′ E |
March–July 2007 | Fatty acids | |
| Torstensson et al., 2013 | Nitzschia lecointei | Amundsen Sea | N/A | January 2011 | Fatty acids | |
| Torstensson et al., 2019 | Nitzschia lecointei | Amundsen Sea | N/A | N/A | Lipid, protein carbohydrate, fatty acids |
One of the strongest determinants of biomolecular composition is taxonomic composition. Phylogenetically distinct microalgal groups have been shown to vary in their proportional allocation of biomolecules. Diatoms (Orcophyta: Bacillariophyceae), for example, generally have higher lipid and lower carbohydrate content than other microalgal phyla, such as the Chlorophytes and Haptophytes [61]. More specifically, pennate diatoms within the sea ice have been shown to have higher lipid, fatty acid, and carbohydrate content than the centric diatoms from the same community [60]. Similarly, diatoms with a smaller cell volume (such as pennate diatoms) have been shown to have higher carbohydrate content than larger volume diatoms [79]. At the taxonomic level of species, differentiation is more subtle, but nevertheless has been shown [57,60,80,81]. However, by far most knowledge on species-specific biomolecular profiles is derived from single-species culture studies, providing a poor representation of what may be true for natural mixed communities. It is therefore important that studies on natural communities start to discriminate biomolecular profiles of individual taxa within a community if we are to improve our understanding of taxonomic biomolecular diversity.
3. Environmental Factors That Influence Bioomolecular Composition
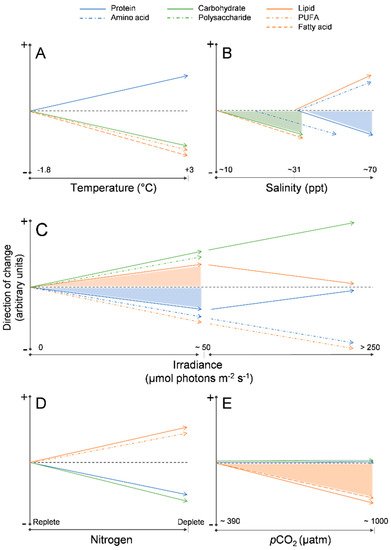
Temperature: At the ice–water interface, temperature generally remains around −1.8 °C throughout spring, however as sea surface temperatures rise with the onset of ocean warming [50], it is possible that warmer temperatures will alter the biochemical composition of the algae, drive an earlier ice melt, and ultimately inhibit sea ice microalgal growth completely [11,43]. Limited work has been completed on the effects of temperature on the biochemical composition of sea ice algae, but from these few studies some patterns have emerged. Extreme subzero temperatures (−20 °C) have been associated with a decline in fatty acids, especially PUFA content [88]. A decline in fatty acids has been observed also under moderate temperature increases (from −1.8 °C to 3 °C), as well as in temperature increases well beyond the natural range (~15 °C) [86,88,89,90] (Figure 2A). In several Antarctic sea-ice diatoms, an increase in relative protein content and decrease in carbohydrate content have been observed with exposure to warmer (up to 3 °C) temperatures [90], including temperatures well above (~20 °C) the natural range for sea ice microalgae [86].
This entry is adapted from the peer-reviewed paper 10.3390/geosciences12010038
