Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Woan-Yuh Tarn | + 2850 word(s) | 2850 | 2021-11-30 04:50:38 | | | |
| 2 | Lindsay Dong | + 270 word(s) | 3120 | 2022-01-20 04:53:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tarn, W. Antisense Oligonucleotide-Based Therapy of Viral Infections. Encyclopedia. Available online: https://encyclopedia.pub/entry/18528 (accessed on 14 January 2026).
Tarn W. Antisense Oligonucleotide-Based Therapy of Viral Infections. Encyclopedia. Available at: https://encyclopedia.pub/entry/18528. Accessed January 14, 2026.
Tarn, Woan-Yuh. "Antisense Oligonucleotide-Based Therapy of Viral Infections" Encyclopedia, https://encyclopedia.pub/entry/18528 (accessed January 14, 2026).
Tarn, W. (2022, January 20). Antisense Oligonucleotide-Based Therapy of Viral Infections. In Encyclopedia. https://encyclopedia.pub/entry/18528
Tarn, Woan-Yuh. "Antisense Oligonucleotide-Based Therapy of Viral Infections." Encyclopedia. Web. 20 January, 2022.
Copy Citation
Antisense oligonucleotide (ASO) technology exploits a single-strand short oligonucleotide to either cause target RNA degradation or sterically block the binding of cellular factors or machineries to the target RNA. Chemical modification or bioconjugation of ASOs can enhance both its pharmacokinetic and pharmacodynamic performance, and it enables customization for a specific clinical purpose. ASO-based therapies have been used for treatment of genetic disorders, cancer and viral infections. In particular, ASOs can be rapidly developed for newly emerging virus and their reemerging variants.
virus
antisense oligonucleotide
1. Development and Delivery of Therapeutic ASOs
ASO is a single-stranded DNA of 12–25 nucleotides in length targeting diseases-associated transcripts [1][2][3][4]. The first ASO that inhibits the replication of Rous sarcoma virus and cell transformation was reported in 1978 [5]. ASOs modulate target gene expression via one of the following mechanisms [1][2][3][4]. (1) ASO forms a hybrid with the target RNA, inducing RNA cleavage by RNase H, which is an endonuclease cleaving the RNA strand of a DNA-RNA duplex. (2) ASO base-pairs with a functional cis-element of the target RNA and hence blocks access of cellular machinery to the RNA. Such steric-blocking ASOs may cause splice isoform switch or translation inhibition or may alter the stability of the target RNA (Figure 1).
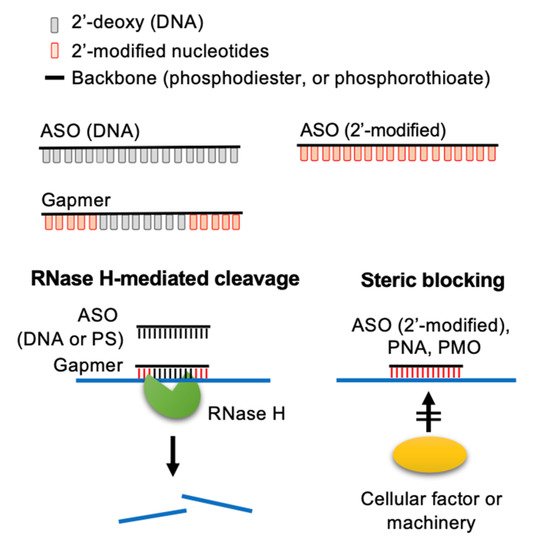
Figure 1. Molecular mechanisms of action of ASOs. ASOs can modulate the expression of target RNAs via two different mechanisms. Conventionally, ASOs cause RNase H-mediated cleavage of the target RNA. Additionally, 2′-O-modified ASOs and neutral DNA mimics (PMOs and PNAs) act as a steric-blocker to prevent the access of cellular factors to the target RNA. Adapted from [3], Springer Nature Limited, 2020.
1.1. Modifications of ASOs
Chemical modifications have been developed to improve the pharmacokinetic properties of ASOs, including stability, specificity, and membrane permeability, and minimize their cytotoxicity. Three generations of ASOs have been broadly classified with respect to the types of modifications [6][7][8]. In the first-generation ASOs, one of the non-bridging oxygen atoms in the phosphodiester bond are replaced, resulting in a phosphoramidate, methylphosphonate, or phosphorothioate (PS) linkage (Figure 2A, PS). The PS linkage improves membrane penetration of ASOs and does not interfere with RNase H-mediated RNA cleavage [6][7][8]. PS-ASOs can bind proteins in plasma, thereby preventing rapid clearance of the ASOs from the circulatory system [9][10]. PS chirality (Rp and Sp) may influence the pharmacological properties of ASOs, such as the stability and RNase H1 cleavage patterns [11][12]. The second-generation ASOs are modified with an alkyl moiety, such as a methyl (2′-OMe) or methoxyethyl (2′-MOE) group at the 2′ position of the ribose (Figure 2A). These ASOs have greater affinity for their target RNAs and lesser cytotoxicity but cannot recruit RNase H1 for RNA cleavage [13][14]. The third-generation ASOs include locked nucleic acid (LNA), peptide nucleic acid (PNA), and phosphorodiamidate morpholino oligomer (PMO) (Figure 2A).
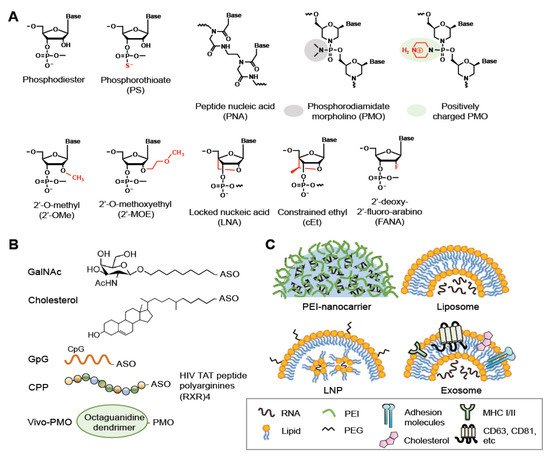
Figure 2. Modifications, bioconjugations and delivery vehicles of ASOs. (A) Structures of backbone or sugar-modified ASOs as well as PNA and PMO oligomers. (B) Bioconjugates of ASOs include GalNac, Cholesterol, CpG DNA and CPP (R, arginine; X, 6-aminohexanoic acid). Vivo-PMO is an PMO covalently linked to an octa-guanidine dendrimer. (C) Representative delivery vehicles of ASOs include EPI-nanocarrier, liposome, LNP, and exosome. Adapted from [3], Spring Nature Limited, 2020; [8], MDPI, 2021.
To improve potency and efficacy, chimeric ASOs have been developed having different modifications in the base, phosphodiester linkage, and deoxyribose moiety. Gapmers have been designed to consist of a central short region of deoxyribonucleotides flanked by a stretch of ribonucleotides in which the ribose ring is modified with 2′-OMe, 2′-MOE or LNA [6][7][8][15]. Therefore, a gapmer can induce RNase H-mediated cleavage of the target RNA with a relatively greater binding affinity and specificity than conventional ASOs (Figure 1). Using a cell-based assay, a gapmer targeting the internal ribosome entry site of HCV exhibited potent antiviral activity (50% effective concentration, 4 nM) [16]. Mipomersen, which targets apolipoprotein B-100 mRNA, is an FDA-approved drug to treat familial hypercholesterolemia; it is a gapmer consisting of a 5′methyl (m5)-C/U-containing PS-ASO “gap” and 2′-MOE nucleotides at both ends [17][18]. Many ASOs act as a steric blocker (Figure 1).
1.2. Bioconjugations of ASOs
Nucleic acid-based drugs generally enter cells via endocytosis; most of these therapeutics must be formulated as a bioconjugate to facilitate receptor-mediated endocytosis and/or increase their lipophilicity [19][20][21] (Figure 2B). For example, conjugation of an siRNA with anandamide, folate, or cholesterol enables efficient uptake of the siRNA by cells, likely via the corresponding conjugate-specific receptor [22]. Notably, a PS backbone assists the membrane translocation of ASOs; thus, bioconjugation and/or delivery agents are often dispensable for PS-ASOs [9][10]. Therapeutic oligonucleotides also can be conjugated to certain ligands that bind cell type-specific receptors. For example, glycoproteins terminating with N-acetylgalactosamine (GalNAc) can be recognized by the asialoglycoprotein receptor (ASGPR), which exists primarily on the cell-surface of hepatocytes [23]. Therefore, conjugation with GalNAc can promote the uptake of siRNAs and ASOs by hepatocytes via ASGPR-mediated endocytosis. GalNAc-2′-MOE-ASOs targeting the mRNA encoding the liver glucagon receptor have been designed for treatment of type 2 diabetes [24]. CpG dinucleotides can lead to the uptake of conjugated oligonucleotides by dendritic cells or macrophages that express innate immune receptors [25]. Besides the aforementioned conjugates, a number of cell-penetrating peptides (CPPs) have been developed to enhance drug delivery, including polycationic HIV-1 Tat peptide, the hydrophobic residue-containing peptide penetratin that is derived from the Drosophila antennapedia homeodomain, and artificial poly-arginine peptides [26]. CPPs may undergo endocytosis or directly penetrate cells [26]. Composite CPPs consisting of penetratin and 6-aminohexanoic-spaced oligo-arginine (RXR) have been used to enhance the efficiency of delivery of charge-neutral PMOs or PNAs to cells in vivo or in culture [27]. Various conditionally activatable CPPs have been designed for selective delivery. For example, a pH-sensitive transportan CPP bearing lysine-to-histidine substitutions can enter cells under acidic conditions, such as the tumor microenvironment [28]. Finally, because guanidinium groups of arginine-rich peptides are critical for peptide translocation across the plasma membrane, a synthetic octa-guanidine dendrimer has been conjugated to PMOs, and such conjugates are called vivo-PMOs [29]. Vivo-PMOs, although widely used for transient gene silencing in vitro, cause coagulation owing to dendrimer clustering in animals; supplementation of Vivo-PMO with anticoagulants may counteract its toxicity [30].
1.3. Vehicle-Mediated Delivery of ASOs
Oligonucleotide bioconjugates offer the potential for enhanced drug delivery, but recent advances in nanotechnology have further benefited the transport of therapeutic ASOs across biological barriers and improved their pharmacokinetics in circulating blood. Several nanoparticle-mediated delivery systems have been developed [3][6][19][20][21] (Figure 2C).
Cationic polymer nanocarriers are formed via ionic interactions between negatively charged ASOs and positively charged macromolecules such as polyethylenimine (PEI). PEI promotes cellular delivery of ASOs but is somewhat cytotoxic. Modification with phospholipid (such as dioleoylphosphatidylethanolamine, DOPE) or copolymerization with polyethylene glycol (PEG) can enhance the efficiency of PEI in ASO delivery and reduce its cytotoxicity [31]. Moreover, bioconjugation with cell-binding ligands such as transferrin, antibodies, or carbohydrates can facilitate receptor-mediated uptake of nanocarriers [6].
LNPs are nanoparticles mainly constructed with lipids. Among them, liposomes are spherical vesicles comprising single or multiple lipid bilayers. ASOs can be carried in the aqueous space encapsulated by artificial liposomes. Most liposomes formulated for RNA delivery comprise both cationic lipids and neutral lipids such as DOPE; such a lipid combination enhances the transfection efficiency and reduces the cytotoxicity of liposomes [32]. At present, LNPs represent a highly potent RNA delivery vehicle; for such LNPs, nucleic acids are organized in inverse lipid micelles inside the nanoparticle [33].
Exosomes are naturally secreted extracellular vesicles that transfer macromolecules between cells [34]. For drug delivery, exosomes have both advantages and drawbacks. For example, they have inherent anti-inflammatory properties, can traverse biological membranes such as the blood-brain barrier, and can be produced in an autologous manner, but they are heterogenous and uneasy for large-scale production [35]. Exosomes can be modified through chemical methods or genetic engineering. Fusion of green fluorescent protein to the exosome surface protein CD63 allows tracking of the exosome and monitoring of cargo delivery [36]. Coating of exosomes with cationic lipids and a pH-sensitive amphipathic peptide can enhance cellular uptake and fusion with endosomes and subsequent cargo release [37].
2. ASOs Targeting Viruses
The development of more efficacious treatments against various viral diseases from acute to persistent infection is still in high demand. Among different nucleic acid-based therapies, ASOs directly act on viral genomic RNA or transcripts (Figure 3) and can be rationally designed for any new virus (or variants) or a reemergent virus.
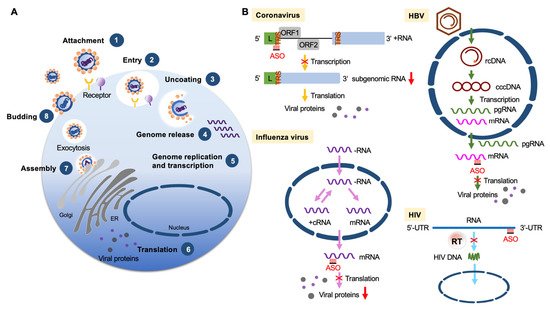
Figure 3. ASOs targeting viruses. (A) Diagram shows viral life cycle from viral attachment and entry into host cells (1, 2), genome release from the capsid (3, 4), genome replication, transcription and protein expression (5, 6), and viral assembly and release (7, 8). (B) ASO-based antiviral strategies. Examples are given for four different types of viruses. Coronavirus (positive-strand RNA virus): ASOs target the transcription regulatory sequence (TRS) of the RNA genome (+RNA) to reduce viral subgenomic RNA production. Influenza (negative-strand RNA virus): ASOs target viral mRNAs to reduce the production of viral nucleoprotein and matrix protein. HBV (partially double-stranded DNA virus): ASOs target a conserved sequence of viral mRNAs to reduce the translation of viral proteins. HIV (retrovirus): ASOs bind to the viral genome to interfere with reverse transcription and hence reduce viral DNA production. Abbreviations: L, leader sequence; cRNA, complementary RNA; rcDNA, relaxed circular DNA; cccDNA, covalently closed circular DNA; pgRNA, pregenomic RNA.
2.1. Coronaviruses
The COVID-19 pandemic has prompted the rapid development of therapeutic strategies against SARS-CoV-2. A combination of cryo-electron microcopy and molecular modeling has revealed the tertiary structure of the frameshift stimulation element of SARS-CoV-2. LNA-modified ASOs targeting the structure of this element can disrupt translational frameshifting and hence inhibit viral replication [38]. Another report showed that a 2′-OMe/SP-ASO conjugated with four 2′-5′-oligoadenylates that can induce RNase L-mediated cleavage and degradation of the SARS-CoV-2 envelop and spike RNAs can effectively inhibit viral propagation in pseudovirus infection models [39].
2.2. Dengue Virus
Dengue infection occurs in tropical and subtropical areas and causes fever and flu-like symptoms. Dengue viruses have a 10.7 kb positive-strand RNA genome encoding three structural proteins and seven nonstructural proteins. The 5′ and 3′ untranslated regions, respectively, fold into conserved structures that are essential for viral viability. The 5′-most stem-loop acts as a promoter of viral RNA replication. RNA replication involves genome cyclization, which is mediated by the interaction between the complementary 5′ and 3′ cyclization sequences [40]. Arginine-rich CPP-PMOs that respectively target the 5′ or 3′-terminal stem-loop or 3′ cyclization sequence of the Dengue genome can inhibit viral replication and decrease viral titer in cultured cells [41][42]. Analogously, a recent study showed that 3′ stem-loop-targeting vivo-PMOs can potently inhibit Dengue replication in dendritic cells that are primary target cells of dengue infection [43].
2.3. Respiratory Syncytial Virus (RSV)
RSV causes lower respiratory tract disease that most often affects children and older individuals. RSV has a negative-strand RNA genome of ~15 kb. After entry into the host cell, the viral nucleocapsid and polymerase are delivered into the cytoplasm. Viral RNA-dependent RNA polymerase (RdRp) transcribes the viral genome into mRNAs that encode viral proteins and synthesizes the antigenome, which serves as the template for genome synthesis [44]. ASOs that induce RNase H-mediated cleavage/degradation of RSV genomic RNA can inhibit RSV replication [45]. A CPP-conjugated PS-PMO could inhibit RSV replication in mice by suppressing the translation of RSV L mRNA [46]. The intranasal route is a rational choice for delivery of antiviral drugs against respiratory infections. One study has demonstrated that intranasal administration of siRNAs that knock down RSV phosphoprotein expression can effectively reduce RSV infection and prevent pulmonary pathology in mice [47].
2.4. Influenza
Influenza viruses cause a contagious respiratory illness ranging from mild to severe. The influenza genome comprises eight negative-strand RNA segments, each of which encodes one or two proteins. In contrast to most RNA viruses, the influenza RNAs are transcribed and replicated by viral RdRp in the nucleus. For viral synthesis, RdRp uses a cap-snatching mechanism to prime transcription [48]. Notably, all eight viral RNAs contain conserved sequences respectively at their 5’ and 3’ termini [48]. Although neuraminidase inhibitors are the most frequently used anti-influenza drugs, other antiviral strategies are still necessary [49]. CPP-conjugated PMOs targeting the 3′ conserved region of the nucleocapsid mRNA can reduce the viral titer [50]. Using titanium dioxide (TiO2) as a nanocarrier, polylysine-linked ASOs targeting the same conserved region exhibited potent antiviral activity with little cytotoxicity [51].
2.5. Ebola Virus
Ebola virus is a rare but deadly virus that causes coagulation abnormalities, leading to hemorrhagic fever. A recent outbreak occurred in West Africa from 2014 to 2016. Ebola and its relative of the Marburg virus belong to the Filoviridae family, and these viruses have a negative-strand RNA genome of 19 kb encoding seven proteins. Among them, VP24 and VP35 antagonize the innate antiviral immune response via multiple pathways and are responsible for the extreme virulence of Ebola virus [52]. Essentially, VP24 inhibits the activation of interferon-stimulated genes by preventing nuclear import of a key transcription factor STAT1, whereas VP35 interacts with double-stranded RNA ends to prevent sensing by cellular pattern recognition receptors such as retinoic acid inducible gene-I (RIG-I) [53][54]. Positively charged PMOs targeting VP35 mRNA could protect mice from infection-induced lethality [55]. Moreover, targeting both VP24 and VP35 achieved postexposure efficacy against Ebola virus in nonhuman primates, indicating positively charged PMOs as effective therapeutic agents [56].
2.6. HBV
HBV, a prototype virus of the Hepadnaviridae family, has a 3.2 kb partially double-stranded, relaxed circular DNA genome [57]. After infection, the viral genome is converted to covalently closed circular DNA in the nucleus, and this DNA serves as the template for synthesis of pregenomic RNA and subgenomic viral transcripts. Viral replication occurs by reverse transcription of pregenomic RNA. Chronic HBV infection may lead to liver failure and liver cancer. Interferon and nucleoside/nucleotide analogs are the most commonly used therapeutics [58]. Developing new therapeutic strategies is still necessary, however, owing to drug resistance. In a pioneering study, a PS-ASO complementary to the HBV polyadenylation signal sequence complexed to a ASGPR ligand and polylysine reduced viral surface antigen (HBsAg) expression and blocked HBV replication in cultured cells [59]. Recently, GalNAc-conjugated LNA-ASOs that can destroy viral mRNAs via RNase-H-mediated degradation showed a significant HBsAg reduction in HBV-infected mice [60]. Notably, the uncapped 5′-triphosphate of RNA can activate the RIG-I-dependent antiviral type-I interferon response [61], and a recent study demonstrated that 5′-triphosphate-modified siRNAs can target HBV and meanwhile elicit an antiviral immune response [62].
2.7. HIV
HIV causes life-threatening immunodeficiency syndrome and thus has long attracted innovative development of antiviral drugs. HIV is a lentivirus that infects and subsequently depletes CD4+ helper T cells. Upon entry into these T cells, the viral RNA genome is reverse transcribed into DNA by viral reverse transcriptase. The resulting viral double-stranded DNA is integrated into the host genome by the viral integrase and host factors [63]. Many anti-HIV drugs have been developed to target viral enzymes (such as reverse transcriptase, integrase and protease) or prevent viral entry by blocking the T-cell receptors CD4 or CCR5 [64]. Numerous nucleic acid-based drugs have also been designed for HIV treatment. For example, early studies showed that an siRNA targeting the HIV genome or HIV Gag-p24 could inhibit viral replication [65][66].
3. ASOs Targeting Host Factors
3.1. Niemann-Pick C1 (NPC1)
NPC1 is a multi-transmembrane protein essential for cholesterol transport from late endosomes and lysosomes and regulates cellular lipid homeostasis [67]. NPC1 mutations cause accumulation of cholesterol and other lipids in various tissues. The lysosomal accumulation of lipids in Niemann-Pick type C disease with NPC1 mutations leads to neurological impairments and progressive neurodegeneration [68]. Moreover, NPC1 and NPC1-like protein participate in the infection by various viruses. NPC1 serves as a fusion receptor for filovirus. Ebola virus is internalized via a micropinocytosis-like process and is subsequently transported to late endosomes [69]. The Ebola virus glycoprotein (GP) is required for virion/cellular membrane fusion. Upon proteolytic processing of the GP1 subunit, its receptor-binding domain interacts with endosomal NPC1 for viral entry and subsequent release of the viral nucleoprotein in the cytoplasm followed by replication of viral genomic RNA [69]. Fibroblasts derived from patients with Niemann-Pick type C disease are resistant to filovirus infection [70]. LNA-PS-modified ASOs targeting NPC1 mRNA can interfere with the cellular entry of a filovirus glycoprotein-pseudotyped virus [71].
3.2. Raf-1
The serine/threonine kinase Raf1 is a downstream effector of RAS in the mitogen-activated protein kinase pathway. Ras/Raf/MEK/ERK signaling regulates numerous cellular processes such as proliferation and differentiation in response to extracellular stimuli [72]. A number of viruses exploit this pathway to modulate their infectious cycle. HCV activates Raf-1 by its core protein and thereby regulates hepatocyte growth and differentiation [73]. Conversely, Raf-1 participates in HCV replication via its interaction with the viral nonstructural protein 5A in the replication complex [74]. Thus, inhibition of Raf-1 attenuates viral replication. Notably, activation of the Ras/Raf/MEK pathway downregulates the expression of interferon-stimulated genes that are critical for the innate immune response. Therefore, HCV infection attenuates interferon signaling by activating Raf-1 and hence benefits viral propagation [75].
3.3. The Heat-Shock Protein MRJ
Heat-shock proteins function as molecular chaperones to maintain proteostasis [76]. A number of viruses modulate the cellular heat-shock response or take advantage of cellular heat-shock proteins to overcome host environmental challenges and complete their life cycle [77][78]. An early study revealed that the human heat-shock protein DNAJB6 (also termed MRJ) is critical for nuclear import of the HIV-2 preintegration complex via its interaction with viral Vpx protein [79]. Notably, MRJ has two splice isoforms that exert different effects on viral infection [78]. Isoform switching from the C-terminally truncated MRJ-S to full-length MRJ-L occurs during monocyte differentiation into macrophages, which are target cells for HIV [80]. Accordingly, individuals with a higher level of MRJ-L in macrophages are more susceptible to HIV infection [81]. MRJ-L possibly facilitates the nuclear import of the HIV preintegration complex via its C-terminal nuclear localization signal [81]. Similarly, it promotes nuclear entry of the cytomegalovirus primase [82]. MRJ-L is also essential for subgenomic mRNA production and viral propagation of RSV even though the RSV life cycle is completed in the cytoplasm [80].
3.4. miR-122
miRNAs regulate gene expression at the post-transcriptional level via binding to the 3′ untranslated region of target mRNAs. Therefore, miRNAs can regulate the pathogenesis of a broad range of viruses; most of them downregulate viral translation and replication [83]. However, viruses can modulate the expression of host miRNAs, most of which are involved in antiviral innate immunity. Notably, HCV infection upregulates miR-122, which is abundant in hepatocytes and regulates liver homeostasis [84]. miR-122 in turn binds to sites upstream of the internal ribosome entry site in the 5′ non-coding region of the viral RNA genome and hence increases viral RNA stability and upregulates viral RNA translation and replication [85][86]. Host factors that are involved in miRNA biogenesis, such as Dicer, TRBP and Ago2, also contribute to viral RNA accumulation [87].
3.5. Other Host Factors Targeted by ASOs
ASGPR is a candidate receptor for HBV, and its major subunit ASGPR1 is upregulated in cells of HBV-infected patients. ASOs targeting ASGPR1 mRNA in HBV-infected human hepatocellular carcinoma cells can reduce the level of viral antigens and DNA [88]. A subcellular proteomic screen implicated the involvement of programmed cell death 5 (PDCD5) during influenza virus infection. PDCD5 may suppress tumors and pathogenic T cells by inhibiting cell proliferation and inducing apoptosis. Knockdown of PDCD5 mitigated influenza HIN1 propagation in cultured cells [89]. A subsequent study showed that PDCD5-targeting ASOs can downregulate PDCD5 in lung tissue and hence protect mice from influenza virus infection [90].
References
- Mansoor, M.; Melendez, A.J. Advances in antisense oligonucleotide development for target identification, validation, and as novel therapeutics. Gene Regul. Syst. Biol. 2008, 2, 275–295.
- Smith, C.I.E.; Zain, R. Therapeutic Oligonucleotides: State of the Art. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 605–630.
- Roberts, T.C.; Langer, R.; Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020, 19, 673–694.
- Quemener, A.M.; Bachelot, L.; Forestier, A.; Donnou-Fournet, E.; Gilot, D.; Galibert, M.-D. The powerful world of antisense oligonucleotides: From bench to bedside. WIREs RNA 2020, 11, e1594.
- Zamecnik, P.C.; Stephenson, M.L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl Acad. Sci. USA 1978, 75, 280–284.
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X.H. Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 2021, 20, 427–453.
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643.
- Adachi, H.; Hengesbach, M.; Yu, Y.-T.; Morais, P. From Antisense RNA to RNA Modification: Therapeutic Potential of RNA-Based Technologies. Biomedicines 2021, 9, 550.
- Crooke, S.T.; Wang, S.; Vickers, T.A.; Shen, W.; Liang, X.H. Cellular uptake and trafficking of antisense oligonucleotides. Nat. Biotechnol. 2017, 35, 230–237.
- Crooke, S.T.; Vickers, T.A.; Liang, X.-h. Phosphorothioate modified oligonucleotide–protein interactions. Nucleic Acids Res. 2020, 48, 5235–5253.
- Iwamoto, N.; Butler, D.C.D.; Svrzikapa, N.; Mohapatra, S.; Zlatev, I.; Sah, D.W.Y.; Meena; Standley, S.M.; Lu, G.; Apponi, L.H.; et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat. Biotechnol. 2017, 35, 845–851.
- Østergaard, M.E.; De Hoyos, C.L.; Wan, W.B.; Shen, W.; Low, A.; Berdeja, A.; Vasquez, G.; Murray, S.; Migawa, M.T.; Liang, X.H.; et al. Understanding the effect of controlling phosphorothioate chirality in the DNA gap on the potency and safety of gapmer antisense oligonucleotides. Nucleic Acids Res. 2020, 48, 1691–1700.
- Sproat, B.S.; Lamond, A.I.; Beijer, B.; Neuner, P.; Ryder, U. Highly efficient chemical synthesis of 2′-O-methyloligoribonucleotides and tetrabiotinylated derivatives; novel probes that are resistant to degradation by RNA or DNA specific nucleases. Nucleic Acids Res. 1989, 17, 3373–3386.
- Agrawal, S.; Jiang, Z.; Zhao, Q.; Shaw, D.; Cai, Q.; Roskey, A.; Channavajjala, L.; Saxinger, C.; Zhang, R. Mixed-backbone oligonucleotides as second generation antisense oligonucleotides: In vitro and in vivo studies. Proc. Natl. Acad. Sci. USA 1997, 94, 2620–2625.
- Chan, L.; Yokota, T. Development and Clinical Applications of Antisense Oligonucleotide Gapmers. Methods Mol. Biol. 2020, 2176, 21–47.
- Laxton, C.; Brady, K.; Moschos, S.; Turnpenny, P.; Rawal, J.; Pryde, D.C.; Sidders, B.; Corbau, R.; Pickford, C.; Murray, E.J. Selection, optimization, and pharmacokinetic properties of a novel, potent antiviral locked nucleic acid-based antisense oligomer targeting hepatitis C virus internal ribosome entry site. Antimicrob. Agents Chemother. 2011, 55, 3105–3114.
- Crooke, S.T.; Geary, R.S. Clinical pharmacological properties of mipomersen (Kynamro), a second generation antisense inhibitor of apolipoprotein B. Br. J. Clin. Pharmacol. 2013, 76, 269–276.
- Parham, J.S.; Goldberg, A.C. Mipomersen and its use in familial hypercholesterolemia. Expert Opin. Pharmacother. 2019, 20, 127–131.
- Gagliardi, M.; Ashizawa, A.T. The Challenges and Strategies of Antisense Oligonucleotide Drug Delivery. Biomedicines 2021, 9, 433.
- Hammond, S.M.; Aartsma-Rus, A.; Alves, S.; Borgos, S.E.; Buijsen, R.A.M.; Collin, R.W.J.; Covello, G.; Denti, M.A.; Desviat, L.R.; Echevarría, L.; et al. Delivery of oligonucleotide-based therapeutics: Challenges and opportunities. EMBO Mol. Med. 2021, 13, e13243.
- Ibba, M.L.; Ciccone, G.; Esposito, C.L.; Catuogno, S.; Giangrande, P.H. Advances in mRNA non-viral delivery approaches. Adv. Drug Deliv. Rev. 2021, 177, 113930.
- Hawner, M.; Ducho, C. Cellular Targeting of Oligonucleotides by Conjugation with Small Molecules. Molecules 2020, 25, 5963.
- Spiess, M. The asialoglycoprotein receptor: A model for endocytic transport receptors. Biochemistry 1990, 29, 10009–10018.
- Morgan, E.S.; Tai, L.J.; Pham, N.C.; Overman, J.K.; Watts, L.M.; Smith, A.; Jung, S.W.; Gajdošík, M.; Krššák, M.; Krebs, M.; et al. Antisense Inhibition of Glucagon Receptor by IONIS-GCGR(Rx) Improves Type 2 Diabetes Without Increase in Hepatic Glycogen Content in Patients with Type 2 Diabetes on Stable Metformin Therapy. Diabetes Care 2019, 42, 585–593.
- Kortylewski, M.; Swiderski, P.; Herrmann, A.; Wang, L.; Kowolik, C.; Kujawski, M.; Lee, H.; Scuto, A.; Liu, Y.; Yang, C.; et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat. Biotechnol. 2009, 27, 925–932.
- McClorey, G.; Banerjee, S. Cell-Penetrating Peptides to Enhance Delivery of Oligonucleotide-Based Therapeutics. Biomedicines 2018, 6, 51.
- Gait, M.J.; Arzumanov, A.A.; McClorey, G.; Godfrey, C.; Betts, C.; Hammond, S.; Wood, M.J.A. Cell-Penetrating Peptide Conjugates of Steric Blocking Oligonucleotides as Therapeutics for Neuromuscular Diseases from a Historical Perspective to Current Prospects of Treatment. Nucleic Acid Ther. 2019, 29, 1–12.
- Zhang, W.; Song, J.; Zhang, B.; Liu, L.; Wang, K.; Wang, R. Design of acid-activated cell penetrating peptide for delivery of active molecules into cancer cells. Bioconjug. Chem. 2011, 22, 1410–1415.
- Morcos, P.A.; Li, Y.; Jiang, S. Vivo-Morpholinos: A non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. BioTechniques 2008, 45, 613, 614, 616, 618 passim.
- Ferguson, D.P.; Dangott, L.J.; Lightfoot, J.T. Lessons learned from vivo-morpholinos: How to avoid vivo-morpholino toxicity. BioTechniques 2014, 56, 251–256.
- Lutz, G.J.; Sirsi, S.R.; Williams, J.H. PEG-PEI copolymers for oligonucleotide delivery to cells and tissues. Methods Mol. Biol. 2008, 433, 141–158.
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176.
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles-From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015.
- Familtseva, A.; Jeremic, N.; Tyagi, S.C. Exosomes: Cell-created drug delivery systems. Mol. Cell. Biochem. 2019, 459, 1–6.
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, e99263.
- Levy, D.; Do, M.A.; Brown, A.; Asano, K.; Diebold, D.; Chen, H.; Zhang, J.; Lawler, B.; Lu, B. Genetic labeling of extracellular vesicles for studying biogenesis and uptake in living mammalian cells. Methods Enzymol. 2020, 645, 1–14.
- Nakase, I.; Futaki, S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 2015, 5, 10112.
- Zhang, K.; Zheludev, I.N.; Hagey, R.J.; Haslecker, R.; Hou, Y.J.; Kretsch, R.; Pintilie, G.D.; Rangan, R.; Kladwang, W.; Li, S.; et al. Cryo-EM and antisense targeting of the 28-kDa frameshift stimulation element from the SARS-CoV-2 RNA genome. Nat. Struct. Mol. Biol. 2021, 28, 747–754.
- Su, X.; Ma, W.; Feng, D.; Cheng, B.; Wang, Q.; Guo, Z.; Zhou, D.; Tang, X. Efficient Inhibition of SARS-CoV-2 Using Chimeric Antisense Oligonucleotides through RNase L Activation*. Angew Chem. Int. Ed. Engl. 2021, 60, 21662–21667.
- Ramos-Lorente, S.; Romero-López, C.; Berzal-Herranz, A. Information Encoded by the Flavivirus Genomes beyond the Nucleotide Sequence. Int. J. Mol. Sci. 2021, 22, 3738.
- Kinney, R.M.; Huang, C.Y.; Rose, B.C.; Kroeker, A.D.; Dreher, T.W.; Iversen, P.L.; Stein, D.A. Inhibition of dengue virus serotypes 1 to 4 in vero cell cultures with morpholino oligomers. J. Virol. 2005, 79, 5116–5128.
- Holden, K.L.; Stein, D.A.; Pierson, T.C.; Ahmed, A.A.; Clyde, K.; Iversen, P.L.; Harris, E. Inhibition of dengue virus translation and RNA synthesis by a morpholino oligomer targeted to the top of the terminal 3′ stem-loop structure. Virology 2006, 344, 439–452.
- Phumesin, P.; Junking, M.; Panya, A.; Yongpitakwattana, P.; Noisakran, S.; Limjindaporn, T.; Yenchitsomanus, P.T. Inhibition of dengue virus replication in monocyte-derived dendritic cells by vivo-morpholino oligomers. Virus Res. 2019, 260, 123–128.
- Battles, M.B.; McLellan, J.S. Respiratory syncytial virus entry and how to block it. Nat. Rev. Microbiol. 2019, 17, 233–245.
- Jairath, S.; Vargas, P.B.; Hamlin, H.A.; Field, A.K.; Kilkuskie, R.E. Inhibition of respiratory syncytial virus replication by antisense oligodeoxyribonucleotides. Antivir. Res. 1997, 33, 201–213.
- Lai, S.H.; Stein, D.A.; Guerrero-Plata, A.; Liao, S.L.; Ivanciuc, T.; Hong, C.; Iversen, P.L.; Casola, A.; Garofalo, R.P. Inhibition of respiratory syncytial virus infections with morpholino oligomers in cell cultures and in mice. Mol. Ther. 2008, 16, 1120–1128.
- Bitko, V.; Musiyenko, A.; Shulyayeva, O.; Barik, S. Inhibition of respiratory viruses by nasally administered siRNA. Nat. Med. 2005, 11, 50–55.
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis Primers 2018, 4, 3.
- Jefferson, T.; Doshi, P. Multisystem failure: The story of anti-influenza drugs. BMJ 2014, 348, g2263.
- Ge, Q.; Pastey, M.; Kobasa, D.; Puthavathana, P.; Lupfer, C.; Bestwick, R.K.; Iversen, P.L.; Chen, J.; Stein, D.A. Inhibition of multiple subtypes of influenza A virus in cell cultures with morpholino oligomers. Antimicrob. Agents Chemother. 2006, 50, 3724–3733.
- Levina, A.S.; Repkova, M.N.; Ismagilov, Z.R.; Shikina, N.V.; Malygin, E.G.; Mazurkova, N.A.; Zinov’ev, V.V.; Evdokimov, A.A.; Baiborodin, S.I.; Zarytova, V.F. High-performance method for specific effect on nucleic acids in cells using TiO2~DNA nanocomposites. Sci. Rep. 2012, 2, 756.
- Hastie, K.M.; Bale, S.; Kimberlin, C.R.; Saphire, E.O. Hiding the evidence: Two strategies for innate immune evasion by hemorrhagic fever viruses. Curr. Opin. Virol. 2012, 2, 151–156.
- Reid, S.P.; Leung, L.W.; Hartman, A.L.; Martinez, O.; Shaw, M.L.; Carbonnelle, C.; Volchkov, V.E.; Nichol, S.T.; Basler, C.F. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J. Virol. 2006, 80, 5156–5167.
- Kimberlin, C.R.; Bornholdt, Z.A.; Li, S.; Woods, V.L., Jr.; MacRae, I.J.; Saphire, E.O. Ebolavirus VP35 uses a bimodal strategy to bind dsRNA for innate immune suppression. Proc. Natl. Acad. Sci. USA 2010, 107, 314–319.
- Swenson, D.L.; Warfield, K.L.; Warren, T.K.; Lovejoy, C.; Hassinger, J.N.; Ruthel, G.; Blouch, R.E.; Moulton, H.M.; Weller, D.D.; Iversen, P.L.; et al. Chemical modifications of antisense morpholino oligomers enhance their efficacy against Ebola virus infection. Antimicrob. Agents Chemother. 2009, 53, 2089–2099.
- Warren, T.K.; Warfield, K.L.; Wells, J.; Swenson, D.L.; Donner, K.S.; Van Tongeren, S.A.; Garza, N.L.; Dong, L.; Mourich, D.V.; Crumley, S.; et al. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat. Med. 2010, 16, 991–994.
- Tsukuda, S.; Watashi, K. Hepatitis B virus biology and life cycle. Antivir. Res. 2020, 182, 104925.
- Tang, L.S.Y.; Covert, E.; Wilson, E.; Kottilil, S. Chronic Hepatitis B Infection: A Review. JAMA 2018, 319, 1802–1813.
- Nakazono, K.; Ito, Y.; Wu, C.H.; Wu, G.Y. Inhibition of hepatitis B virus replication by targeted pretreatment of complexed antisense DNA in vitro. Hepatology 1996, 23, 1297–1303.
- Javanbakht, H.; Mueller, H.; Walther, J.; Zhou, X.; Lopez, A.; Pattupara, T.; Blaising, J.; Pedersen, L.; Albæk, N.; Jackerott, M.; et al. Liver-Targeted Anti-HBV Single-Stranded Oligonucleotides with Locked Nucleic Acid Potently Reduce HBV Gene Expression In Vivo. Mol. Ther. Nucleic Acids 2018, 11, 441–454.
- Chen, X.; Qian, Y.; Yan, F.; Tu, J.; Yang, X.; Xing, Y.; Chen, Z. 5′-triphosphate-siRNA activates RIG-I-dependent type I interferon production and enhances inhibition of hepatitis B virus replication in HepG2.2.15 cells. Eur. J. Pharmacol. 2013, 721, 86–95.
- Han, Q.; Hou, Z.; Yin, C.; Zhang, C.; Zhang, J. 5′-triphosphate siRNA targeting HBx elicits a potent anti-HBV immune response in pAAV-HBV transfected mice. Antivir. Res. 2019, 161, 36–45.
- Li, G.; De Clercq, E. HIV Genome-Wide Protein Associations: A Review of 30 Years of Research. Microbiol. Mol. Biol. Rev. 2016, 80, 679–731.
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747.
- Jacque, J.M.; Triques, K.; Stevenson, M. Modulation of HIV-1 replication by RNA interference. Nature 2002, 418, 435–438.
- Novina, C.D.; Murray, M.F.; Dykxhoorn, D.M.; Beresford, P.J.; Riess, J.; Lee, S.K.; Collman, R.G.; Lieberman, J.; Shankar, P.; Sharp, P.A. siRNA-directed inhibition of HIV-1 infection. Nat. Med. 2002, 8, 681–686.
- Pfeffer, S.R. NPC intracellular cholesterol transporter 1 (NPC1)-mediated cholesterol export from lysosomes. J. Biol. Chem. 2019, 294, 1706–1709.
- Musalkova, D.; Majer, F.; Kuchar, L.; Luksan, O.; Asfaw, B.; Vlaskova, H.; Storkanova, G.; Reboun, M.; Poupetova, H.; Jahnova, H.; et al. Transcript, protein, metabolite and cellular studies in skin fibroblasts demonstrate variable pathogenic impacts of NPC1 mutations. Orphanet J. Rare Dis. 2020, 15, 85.
- Mulherkar, N.; Raaben, M.; de la Torre, J.C.; Whelan, S.P.; Chandran, K. The Ebola virus glycoprotein mediates entry via a non-classical dynamin-dependent macropinocytic pathway. Virology 2011, 419, 72–83.
- Carette, J.E.; Raaben, M.; Wong, A.C.; Herbert, A.S.; Obernosterer, G.; Mulherkar, N.; Kuehne, A.I.; Kranzusch, P.J.; Griffin, A.M.; Ruthel, G.; et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 2011, 477, 340–343.
- Chery, J.; Petri, A.; Wagschal, A.; Lim, S.Y.; Cunningham, J.; Vasudevan, S.; Kauppinen, S.; Näär, A.M. Development of Locked Nucleic Acid Antisense Oligonucleotides Targeting Ebola Viral Proteins and Host Factor Niemann-Pick C1. Nucleic Acid Ther. 2018, 28, 273–284.
- Chang, F.; Steelman, L.S.; Shelton, J.G.; Lee, J.T.; Navolanic, P.M.; Blalock, W.L.; Franklin, R.; McCubrey, J.A. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review). Int. J. Oncol. 2003, 22, 469–480.
- Aoki, H.; Hayashi, J.; Moriyama, M.; Arakawa, Y.; Hino, O. Hepatitis C virus core protein interacts with 14-3-3 protein and activates the kinase Raf-1. J. Virol. 2000, 74, 1736–1741.
- Bürckstümmer, T.; Kriegs, M.; Lupberger, J.; Pauli, E.K.; Schmittel, S.; Hildt, E. Raf-1 kinase associates with Hepatitis C virus NS5A and regulates viral replication. FEBS Lett. 2006, 580, 575–580.
- Zhang, Q.; Gong, R.; Qu, J.; Zhou, Y.; Liu, W.; Chen, M.; Liu, Y.; Zhu, Y.; Wu, J. Activation of the Ras/Raf/MEK pathway facilitates hepatitis C virus replication via attenuation of the interferon-JAK-STAT pathway. J. Virol. 2012, 86, 1544–1554.
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332.
- Neckers, L.; Tatu, U. Molecular chaperones in pathogen virulence: Emerging new targets for therapy. Cell Host Microbe 2008, 4, 519–527.
- Ko, S.H.; Huang, L.M.; Tarn, W.Y. The Host Heat Shock Protein MRJ/DNAJB6 Modulates Virus Infection. Front. Microbiol. 2019, 10, 2885.
- Cheng, X.; Belshan, M.; Ratner, L. Hsp40 facilitates nuclear import of the human immunodeficiency virus type 2 Vpx-mediated preintegration complex. J. Virol. 2008, 82, 1229–1237.
- Ko, S.H.; Liau, Y.J.; Chi, Y.H.; Lai, M.J.; Chiang, Y.P.; Lu, C.Y.; Chang, L.Y.; Tarn, W.Y.; Huang, L.M. Interference of DNAJB6/MRJ Isoform Switch by Morpholino Inhibits Replication of HIV-1 and RSV. Mol. Ther. Nucleic Acids 2019, 14, 251–261.
- Chiang, Y.P.; Sheng, W.H.; Shao, P.L.; Chi, Y.H.; Chen, Y.M.; Huang, S.W.; Shih, H.M.; Chang, L.Y.; Lu, C.Y.; Chang, S.C.; et al. Large Isoform of Mammalian Relative of DnaJ is a Major Determinant of Human Susceptibility to HIV-1 Infection. EBioMedicine 2014, 1, 126–132.
- Pei, Y.; Fu, W.; Yang, E.; Shen, A.; Chen, Y.C.; Gong, H.; Chen, J.; Huang, J.; Xiao, G.; Liu, F. A Hsp40 chaperone protein interacts with and modulates the cellular distribution of the primase protein of human cytomegalovirus. PLoS Pathog. 2012, 8, e1002968.
- Trobaugh, D.W.; Klimstra, W.B. MicroRNA Regulation of RNA Virus Replication and Pathogenesis. Trends Mol. Med. 2017, 23, 80–93.
- Tsai, W.C.; Hsu, S.D.; Hsu, C.S.; Lai, T.C.; Chen, S.J.; Shen, R.; Huang, Y.; Chen, H.C.; Lee, C.H.; Tsai, T.F.; et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J. Clin. Inv. 2012, 122, 2884–2897.
- Jopling, C.L.; Yi, M.; Lancaster, A.M.; Lemon, S.M.; Sarnow, P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 2005, 309, 1577–1581.
- García-Sastre, A.; Evans, M.J. miR-122 is more than a shield for the hepatitis C virus genome. Proc. Natl. Acad. Sci. USA 2013, 110, 1571–1572.
- Shimakami, T.; Yamane, D.; Jangra, R.K.; Kempf, B.J.; Spaniel, C.; Barton, D.J.; Lemon, S.M. Stabilization of hepatitis C virus RNA by an Ago2-miR-122 complex. Proc. Natl. Acad. Sci. USA 2012, 109, 941–946.
- Yang, J.; Bo, X.C.; Ding, X.R.; Dai, J.M.; Zhang, M.L.; Wang, X.H.; Wang, S.Q. Antisense oligonucleotides targeted against asialoglycoprotein receptor 1 block human hepatitis B virus replication. J. Viral Hepat. 2006, 13, 158–165.
- Zhao, H.; Yang, J.; Li, K.; Ding, X.; Lin, R.; Ma, Y.; Liu, J.; Zhong, Z.; Qian, X.; Bo, X.; et al. Proteomic analysis at the subcellular level for host targets against influenza A virus (H1N1). Antivir. Res. 2013, 100, 673–687.
- Li, K.; Zhou, Z.; Wang, Y.O.; Liu, J.; Zhao, H.B.; Yang, J.; Wang, S.Q. Pretreatment of mice with oligonucleotide prop5 protects them from influenza virus infections. Viruses 2014, 6, 573–581.
More
Information
Subjects:
Virology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




